Abstract
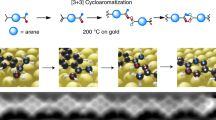
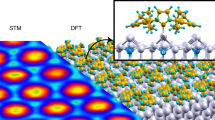
N-Heterocyclic carbenes (NHCs) are established ligands for metal complexes and surfaces. Here we go beyond monomeric NHCs and report on the synthesis of NHC polymers on gold surfaces, consisting of ballbot-type repeating units bound to single Au adatoms. We designed, synthesized and deposited precursors containing different halogens on gold surfaces under ultrahigh vacuum. Conformational, electronic and charge transport properties were assessed by combining low-temperature scanning tunneling microscopy, non-contact atomic force microscopy, X-ray photoelectron spectroscopy, first-principles calculations and reactive force field simulations. The confirmed ballbot-type nature of the NHCs explains the high surface mobility of the incommensurate NHC polymers, which is prerequisite for their desired spatial alignment. The delicate balance between mobility and polymerization rate allows essential parameters for controlling polymer directionality to be derived. These polymers open up new opportunities in the fields of nanoelectronics, surface functionalization and catalysis.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data supporting the findings of this work are available within this paper or its Supplementary Information.
References
Schoenbaum, C. A., Schwartz, D. K. & Medlin, J. W. Controlling the surface environment of heterogeneous catalysts using self-assembled monolayers. Acc. Chem. Res. 47, 1438–1445 (2014).
Mallat, T., Orglmeister, E. & Baiker, A. Asymmetric catalysis at chiral metal surfaces. Chem. Rev. 107, 4863–4890 (2007).
Love, J. C., Estroff, L. A., Kriebel, J. K., Nuzzo, R. G. & Whitesides, G. M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 105, 1103–1169 (2005).
Judd, C. J. et al. Structural characterisation of molecular conformation and the incorporation of adatoms in an on-surface Ullmann-type reaction. Commun. Chem. 3, 166 (2020).
Grossmann, L. et al. Evolution of adsorption heights in the on-surface synthesis and decoupling of covalent organic networks on Ag(111) by normal-incidence X-ray standing wave. Nanoscale Horiz. 7, 51–62 (2022).
Grill, L. & Hecht, S. Covalent on-surface polymerization. Nat. Chem. 12, 115–130 (2020).
Ruffieux, P. et al. On-surface synthesis of graphene nanoribbons with zigzag edge topology. Nature 531, 489–493 (2016).
Bourissou, D., Guerret, O., Gabbaï, F. P. & Bertrand, G. Stable carbenes. Chem. Rev. 100, 39–92 (2000).
Hopkinson, M. N., Richter, C., Schedler, M. & Glorius, F. An overview of N-heterocyclic carbenes. Nature 510, 485–496 (2014).
Bellotti, P., Koy, M., Hopkinson, M. N. & Glorius, F. Recent advances in the chemistry and applications of N-heterocyclic carbenes. Nat. Rev. Chem. 5, 711–725 (2021).
Crudden, C. M. et al. Ultra stable self-assembled monolayers of N-heterocyclic carbenes on gold. Nat. Chem. 6, 409–414 (2014).
Zhukhovitskiy, A. V., MacLeod, M. J. & Johnson, J. A. Carbene ligands in surface chemistry: from stabilization of discrete elemental allotropes to modification of nanoscale and bulk substrates. Chem. Rev. 115, 11503–11532 (2015).
Crudden, C. M. et al. Simple direct formation of self-assembled N-heterocyclic carbene monolayers on gold and their application in biosensing. Nat. Commun. 7, 12654 (2016).
Wu, C.-Y. et al. High-spatial-resolution mapping of catalytic reactions on single particles. Nature 541, 511–515 (2017).
Smith, C. A. et al. N-Heterocyclic carbenes in materials chemistry. Chem. Rev. 119, 4986–5056 (2019).
Koy, M., Bellotti, P., Das, M. & Glorius, F. N-Heterocyclic carbenes as tunable ligands for catalytic metal surfaces. Nat. Catal. 4, 352–363 (2021).
Wang, G. et al. Ballbot-type motion of N-heterocyclic carbenes on gold surfaces. Nat. Chem. 9, 152–156 (2017).
Ranganath, K. V. S., Kloesges, J., Schaefer, A. H. & Glorius, F. Asymmetric nanocatalysis: N-heterocyclic carbenes as chiral modifiers of Fe3O4/Pd nanoparticles. Angew. Chem. Int. Ed. 49, 7786–7789 (2010).
Ernst, J. B. et al. Molecular adsorbates switch on heterogeneous catalysis: induction of reactivity by N-heterocyclic carbenes. J. Am. Chem. Soc. 139, 9144–9147 (2017).
Kaeffer, N., Liu, H.-J., Lo, H.-K., Fedorov, A. & Coperet, C. An N-heterocyclic carbene ligand promotes highly selective alkyne semihydrogenation with copper nanoparticles supported on passivated silica. Chem. Sci. 9, 5366–5371 (2018).
van Dongen, S. F. M., Elemans, J. A. A. W., Rowan, A. E. & Nolte, R. J. M. Processive catalysis. Angew. Chem. Int. Ed. 53, 11420–11428 (2014).
Inayeh, A. et al. Self-assembly of N-heterocyclic carbenes on Au(111). Nat. Commun. 12, 4034 (2021).
Ren, J. et al. A unidirectional surface-anchored N-heterocyclic carbene rotor. Nano Lett. 20, 5922–5928 (2020).
Man, R. W. Y. et al. Ultrastable gold nanoparticles modified by bidentate N-heterocyclic carbene ligands. J. Am. Chem. Soc. 140, 1576–1579 (2018).
Mata, J. A., Poyatos, M. & Peris, E. Structural and catalytic properties of chelating bis- and tris-N-heterocyclic carbenes. Coord. Chem. Rev. 251, 841–859 (2007).
Cao, Z. et al. Chelating N-heterocyclic carbene ligands enable tuning of electrocatalytic CO2 reduction to formate and carbon monoxide: surface organometallic chemistry. Angew. Chem. Int. Ed. 57, 4981–4985 (2018).
Clair, S. & de Oteyza, D. G. Controlling a chemical coupling reaction on a surface: tools and strategies for on-surface synthesis. Chem. Rev. 119, 4717–4776 (2019).
Di Giovannantonio, M. et al. Insight into organometallic intermediate and its evolution to covalent bonding in surface-confined ullmann polymerization. ACS Nano 7, 8190–8198 (2013).
Pawlak, R. et al. Bottom-up synthesis of nitrogen-doped porous graphene nanoribbons. J. Am. Chem. Soc. 142, 12568–12573 (2020).
Eichhorn, J. et al. On-surface Ullmann coupling: the influence of kinetic reaction parameters on the morphology and quality of covalent networks. ACS Nano 8, 7880–7889 (2014).
Fritton, M. et al. The role of kinetics versus thermodynamics in surface-assisted Ullmann coupling on gold and silver surfaces. J. Am. Chem. Soc. 141, 4824–4832 (2019).
Simonov, K. A. et al. From graphene nanoribbons on Cu(111) to nanographene on Cu(110): critical role of substrate structure in the bottom-up fabrication strategy. ACS Nano 9, 8997–9011 (2015).
Di Giovannantonio, M. et al. Mechanistic picture and kinetic analysis of surface-confined Ullmann polymerization. J. Am. Chem. Soc. 138, 16696–16702 (2016).
Gross, L., Mohn, F., Moll, N., Liljeroth, P. & Meyer, G. The chemical structure of a molecule resolved by atomic force microscopy. Science 325, 1110–1114 (2009).
Mönig, H. et al. Quantitative assessment of intermolecular interactions by atomic force microscopy imaging using copper oxide tips. Nat. Nanotechnol. 13, 371–375 (2018).
Bakker, A. et al. Elucidating the binding modes of N-heterocyclic carbenes on a gold surface. J. Am. Chem. Soc. 140, 11889–11892 (2018).
Kawai, S. et al. Direct quantitative measurement of the C=O center dot center dot center dot H-C bond by atomic force microscopy. Sci. Adv. 3, e1603258 (2017).
Ohmann, R. et al. Supramolecular rotor and translator at work: on-surface movement of single atoms. ACS Nano 9, 8394–8400 (2015).
Schuler, B. et al. Adsorption geometry determination of single molecules by atomic force microscopy. Phys. Rev. Lett. 111, 106103 (2013).
Huber, F. et al. Chemical bond formation showing a transition from physisorption to chemisorption. Science 366, 235–238 (2019).
Ternes, M., Lutz, C. P., Hirjibehedin, C. F., Giessibl, F. J. & Heinrich, A. J. The force needed to move an atom on a surface. Science 319, 1066–1069 (2008).
Meyer, J. et al. Tuning the formation of discrete coordination nanostructures. Chem. Commun. 51, 12621–12624 (2015).
Robles, R. et al. Supramolecular chemistry based on 4-acetylbiphenyl on Au(111). Phys. Chem. Chem. Phys. 22, 15208–15213 (2020).
Shi, Z. & Lin, N. Porphyrin-based two-dimensional coordination Kagome lattice self-assembled on a Au(111) surface. J. Am. Chem. Soc. 131, 5376–5377 (2009).
Pan, Y. et al. Effect of lattice-gas atoms on the adsorption behaviour of thioether molecules. Phys. Chem. Chem. Phys. 14, 10987–10993 (2012).
Yang, Z. et al. Orbital redistribution in molecular nanostructures mediated by metal–organic bonds. ACS Nano 8, 10715–10722 (2014).
Jaklevic, R. C. & Elie, L. Scanning-tunneling-microscope observation of surface diffusion on an atomic scale: Au on Au(111). Phys. Rev. Lett. 60, 120–123 (1988).
Liu, J. et al. Iodine-doping-induced electronic structure tuning of atomic cobalt for enhanced hydrogen evolution electrocatalysis. ACS Nano 15, 18125–18134 (2021).
Eder, G. et al. Solution preparation of two-dimensional covalently linked networks by polymerization of 1,3,5-tri(4-iodophenyl)benzene on Au(111). ACS Nano 7, 3014–3021 (2013).
Bieri, M. et al. Two-dimensional polymer formation on surfaces: insight into the roles of precursor mobility and reactivity. J. Am. Chem. Soc. 132, 16669–16676 (2010).
Lipton-Duffin, J. A., Ivasenko, O., Perepichka, D. F. & Rosei, F. Synthesis of polyphenylene molecular wires by surface-confined polymerization. Small 5, 592–597 (2009).
Li, H. K. et al. Nanoparticle PCR: nanogold-assisted PCR with enhanced specificity. Angew. Chem. Int. Ed. 44, 5100–5103 (2005).
Franz, M. et al. Controlled growth of ordered monolayers of N-heterocyclic carbenes on silicon. Nat. Chem. 13, 828–835 (2021).
Doud, E. A. et al. In situ formation of N-heterocyclic carbene-bound single-molecule junctions. J. Am. Chem. Soc. 140, 8944–8949 (2018).
Zhang, H. et al. Surface supported gold-organic hybrids: on-surface synthesis and surface directed orientation. Small 10, 1361–1368 (2014).
Amirjalayer, S., Bakker, A., Freitag, M., Glorius, F. & Fuchs, H. Cooperation of N-heterocyclic carbenes on a gold surface. Angew. Chem. Int. Ed. 59, 21230–21235 (2020).
Linden, S. et al. Electronic structure of spatially aligned graphene nanoribbons on Au(788). Phys. Rev. Lett. 108, 216801 (2012).
Bita, I. et al. Graphoepitaxy of self-assembled block copolymers on two-dimensional periodic patterned templates. Science 321, 939–943 (2008).
Hapala, P., Temirov, R., Tautz, F. S. & Jelinek, P. Origin of high-resolution IETS-STM images of organic molecules with functionalized tips. Phys. Rev. Lett. 113, 226101 (2014).
Schulze Lammers, B. et al. Benchmarking atomically defined AFM tips for chemical-selective imaging. Nanoscale 13, 13617–13623 (2021).
Hapala, P. et al. Mechanism of high-resolution STM/AFM imaging with functionalized tips. Phys. Rev. B 90, 085421 (2014).
Acknowledgements
F.G. and N.L.D. acknowledge financial support from the Deutsche Forschungsgemeinschaft (SFB 858 and SFB 1459), as do H.F. (SFB 858, FU 299/18-1) and H.M. (MO 2345/4-1, 519972808). Q.D. acknowledges financial support from the National Natural Science Foundation of China (U2032206) and the Strategic Priority Research Program of Chinese Academy of Sciences (XDB36000000). H.-J.G. acknowledges financial support from the National Natural Science Foundation of China (61888102) and the National Key Research and Development Program of China (2019YFA0308500). L.H. acknowledges financial support from the National Key Research and Development Program of China (2018YFA0305800). Computations were carried out using the PALMA II HPC cluster provided by the University of Münster.
Author information
Authors and Affiliations
Contributions
F.G. and H.F. initiated the project. F.G., H.F., H.M., M.K. and J.R. designed the experiments and coordinated the study. M.K., A.N. and C.G. designed and synthesized the molecules. J.R. and B.S.L. performed the STM measurements and analysed them with H.M. and Q.D.; H.O., M.N. and N.L.D. performed DFT and ReaxFF calculations. B.S.L., H.M. and J.R. did the nc-AFM(CuOx- tips)/XPS experiments and interpreted the data. Q.Z., L.H., Y.X., J.R. and H.-J.G. did the nc-AFM experiment using CO-functionalized tips. J.R. wrote the manuscript together with M.K., H.O., Q.D., H.M., N.L.D., H.F. and F.G. All the authors read and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Katharina Kaiser, Shigeki Kawai and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures, discussion and tables.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ren, J., Koy, M., Osthues, H. et al. On-surface synthesis of ballbot-type N-heterocyclic carbene polymers. Nat. Chem. 15, 1737–1744 (2023). https://doi.org/10.1038/s41557-023-01310-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01310-1