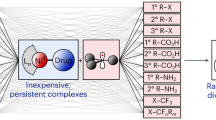
Abstract
Anchoring molecular catalysts on electrode surfaces combines the high selectivity and activity of molecular systems with the practicality of heterogeneous systems. Molecular catalysts, however, are far less stable than traditional heterogeneous electrocatalysts, and therefore a method to easily replace anchored molecular catalysts that have degraded could make such electrosynthetic systems more attractive. Here we applied a non-covalent ‘click’ chemistry approach to reversibly bind molecular electrocatalysts to electrode surfaces through host–guest complexation with surface-anchored cyclodextrins. The host–guest interaction is remarkably strong and enables the flow of electrons between the electrode and the guest catalyst. Electrosynthesis in both organic and aqueous media was demonstrated on metal oxide electrodes, with stability on the order of hours. The catalytic surfaces can be recycled by controlled release of the guest from the host cavities and the readsorption of fresh guest.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The source data for the figures in the main text and Supplementary Information are available on DataDryad (https://doi.org/10.5061/dryad.6t1g1jwxr). Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 1996726 (2), 1996727 (3) and 1976728 (4). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Brazzolotto, D. et al. Nickel-centred proton reduction catalysis in a model of [NiFe] hydrogenase. Nat. Chem. 8, 1054–1060 (2016).
Ren, S. et al. Molecular electrocatalysts can mediate fast, selective CO2 reduction in a flow cell. Science 365, 367–369 (2019).
Nam, D. H. et al. Molecular enhancement of heterogeneous CO2 reduction. Nat. Mater. 19, 266–276 (2020).
Rosser, T. E., Gross, M. A., Lai, Y. H. & Reisner, E. Precious-metal free photoelectrochemical water splitting with immobilised molecular Ni and Fe redox catalysts. Chem. Sci. 7, 4024–4035 (2016).
Sun, L., Reddu, V., Fisher, A. C. & Wang, X. Electrocatalytic reduction of carbon dioxide: opportunities with heterogeneous molecular catalysts. Energy Environ. Sci. 13, 374–403 (2020).
Wang, M. et al. CO2 electrochemical catalytic reduction with a highly active cobalt phthalocyanine. Nat. Commun. 10, 3602 (2019).
Zhang, B. & Sun, L. Artificial photosynthesis: opportunities and challenges of molecular catalysts. Chem. Soc. Rev. 48, 2216–2264 (2019).
Materna, K. L., Crabtree, R. H. & Brudvig, G. W. Anchoring groups for photocatalytic water oxidation on metal oxide surfaces. Chem. Soc. Rev. 46, 6099–6110 (2017).
Hanna, C. M., Luu, A. & Yang, J. Y. Proton-coupled electron transfer at anthraquinone modified indium tin oxide electrodes. ACS Appl. Energy Mater. 2, 59–65 (2019).
Creus, J. et al. A million turnover molecular anode for catalytic water oxidation. Angew. Chem. Int. Ed. 55, 15382–15386 (2016).
Ashford, D. L. et al. Water oxidation by an electropolymerized catalyst on derivatized mesoporous metal oxide electrodes. J. Am. Chem. Soc. 136, 6578–6581 (2014).
Liu, Y. & McCrory, C. C. L. Modulating the mechanism of electrocatalytic CO2 reduction by cobalt phthalocyanine through polymer coordination and encapsulation. Nat. Commun. 10, 1683 (2019).
Krawicz, A. et al. Photofunctional construct that interfaces molecular cobalt-based catalysts for H2 production to a visible-light-absorbing semiconductor. J. Am. Chem. Soc. 135, 11861–11868 (2013).
Schreiber, C. L. & Smith, B. D. Molecular conjugation using non-covalent click chemistry. Nat. Rev. Chem. 3, 393–400 (2019).
Rojas, M. T., Kaifer, A. E., Königer, R. & Stoddart, J. F. Supported monolayers containing preformed binding sites. Synthesis and interfacial binding properties of a thiolated β-cyclodextrin derivative. J. Am. Chem. Soc. 117, 336–343 (1995).
Beulen, M. W. J. et al. Host–guest interactions at self-assembled monolayers of cyclodextrins on gold. Chem. Eur. J. 6, 1176–1183 (2000).
Beulen, M. W. J. et al. Self-assembled monolayers of heptapodant β-cyclodextrins on gold. Langmuir 14, 6424–6429 (1998).
Méndez-Ardoy, A., Steentjes, T., Kudernac, T. & Huskens, J. Self-assembled monolayers on gold of β-cyclodextrin adsorbates with different anchoring groups. Langmuir 30, 3467–3476 (2014).
Liu, W., Zhang, Y. & Gao, X. Interfacial supramolecular self-assembled monolayers of C60 by thiolated β-cyclodextrin on gold surfaces via monoanionic C60. J. Am. Chem. Soc. 129, 4973–4980 (2007).
Freitag, M. & Galoppini, E. Molecular host–guest complexes: shielding of guests on semiconductor surfaces. Energy Environ. Sci. 4, 2482–2494 (2011).
Freitag, M. & Galoppini, E. Cucurbituril complexes of viologens bound to TiO2 films. Langmuir 26, 8262–8269 (2010).
Li, H. et al. Visible-light-driven water oxidation on a photoanode by supramolecular assembly of photosensitizer and catalyst. ChemPlusChem 81, 1056–1059 (2016).
Kim, H. J., Lee, M. H., Mutihac, L., Vicens, J. & Kim, J. S. Host–guest sensing by calixarenes on the surfaces. Chem. Soc. Rev. 41, 1173–1190 (2012).
Murray, J., Kim, K., Ogoshi, T., Yao, W. & Gibb, B. C. The aqueous supramolecular chemistry of cucurbit[n]urils, pillar[n]arenes and deep-cavity cavitands. Chem. Soc. Rev. 46, 2479–2496 (2017).
Méndez-Ardoy, A. et al. Electron-transfer rates in host–guest assemblies at β-cyclodextrin monolayers. Langmuir 33, 8614–8623 (2017).
Al-Soufi, W. et al. Fluorescence correlation spectroscopy, a tool to investigate supramolecular dynamics: inclusion complexes of pyronines with cyclodextrin. J. Am. Chem. Soc. 127, 8775–8784 (2005).
Domi, Y., Yoshinaga, Y., Shimazu, K. & Porter, M. D. Characterization and optimization of mixed thiol-derivatized β-cyclodextrin/pentanethiol monolayers with high-density guest-accessible cavities. Langmuir 25, 8094–8100 (2009).
Perl, A. et al. Gradient-driven motion of multivalent ligand molecules along a surface functionalized with multiple receptors. Nat. Chem. 3, 317–322 (2011).
Chang, B. Y., Hong, S. Y., Yoo, J. S. & Park, S. M. Determination of electron transfer kinetic parameters by Fourier transform electrochemical impedance spectroscopic analysis. J. Phys. Chem. B 110, 19386–19392 (2006).
Lee, J.-Y. & Park, S.-M. Electrochemistry of guest molecules in thiolated cyclodextrin self-assembled monolayers: an implication for size-selective sensors. J. Phys. Chem. B 102, 9940–9945 (1998).
Habibzadeh, F., Miller, S. L., Hamann, T. W. & Smith, M. R. Homogeneous electrocatalytic oxidation of ammonia to N2 under mild conditions. Proc. Natl Acad. Sci. USA 116, 2849–2853 (2019).
Nakajima, K., Toda, H., Sakata, K. & Nishibayashi, Y. Ruthenium-catalysed oxidative conversion of ammonia into dinitrogen. Nat. Chem. 11, 702–709 (2019).
Dunn, P. L., Johnson, S. I., Kaminsky, W. & Bullock, R. M. Diversion of catalytic C–N bond formation to catalytic oxidation of NH3 through modification of the hydrogen atom abstractor. J. Am. Chem. Soc. 142, 3361–3365 (2020).
Adli, N. M., Zhang, H., Mukherjee, S. & Wu, G. Review—ammonia oxidation electrocatalysis for hydrogen generation and fuel cells. J. Electrochem. Soc. 165, J3130–J3147 (2018).
Sévery, L., Siol, S. & Tilley, S. Design of molecular water oxidation catalysts stabilized by ultrathin inorganic overlayers—is active site protection necessary? Inorganics 6, 105 (2018).
Hamai, S. Inclusion of methyl 2-naphthalenecarboxylate and dimethyl 2,3-, 2,6-, and 2,7-naphthalenedicarboxylates by cyclodextrins in aqueous solution. Bull. Chem. Soc. Jpn 83, 1489–1500 (2010).
Organero, J. A., Tormo, L. & Douhal, A. Caging ultrafast proton transfer and twisting motion of 1-hyroxyl-2-acetonapthone. Chem. Phys. Lett. 363, 409–414 (2002).
Hutter, J., Iannuzzi, M., Schiffmann, F. & Vandevondele, J. CP2K: atomistic simulations of condensed matter systems. Wiley Interdiscip. Rev. Comput. Mol. Sci. 4, 15–25 (2014).
Jackson, M. N. et al. Strong electronic coupling of molecular sites to graphitic electrodes via pyrazine conjugation. J. Am. Chem. Soc. 140, 1004–1010 (2018).
Veerbeek, J., Méndez-Ardoy, A. & Huskens, J. Electrochemistry of redox-active guest molecules at β-cyclodextrin-functionalized silicon electrodes. ChemElectroChem 4, 1470–1477 (2017).
Cherevko, S., Topalov, A. A., Zeradjanin, A. R., Katsounaros, I. & Mayrhofer, K. J. J. Gold dissolution: towards understanding of noble metal corrosion. RSC Adv. 3, 16516–16527 (2013).
Widrig, C. A., Chung, C. & Porter, M. D. The electrochemical desorption of n-alkanethiol monolayers from polycrystalline Au and Ag electrodes. J. Electroanal. Chem. 310, 335–359 (1991).
Wong, E. H. J., May, G. L. & Wilde, C. P. Oxidative desorption of thiols as a route to controlled formation of binary self assembled monolayer surfaces. Electrochim. Acta 109, 67–74 (2013).
Hashmi, A. S. K. & Hutchings, G. J. Gold catalysis. Angew. Chem. Int. Ed. 45, 7896–7936 (2006).
Bangle, R., Sampaio, R. N., Troian-Gautier, L. & Meyer, G. J. Surface grafting of Ru(ii) diazonium-based sensitizers on metal oxides enhances alkaline stability for solar energy conversion. ACS Appl. Mater. Interfaces 10, 3121–3132 (2018).
Ide, A. et al. Monitoring bisphosphonate surface functionalization and acid stability of hierarchically porous titanium zirconium oxides. Langmuir 27, 12985–12995 (2011).
Biggs, C. I., Edmondson, S. & Gibson, M. I. Thiol-ene immobilisation of carbohydrates onto glass slides as a simple alternative to gold–thiol monolayers, amines or lipid binding. Biomater. Sci. 3, 175–181 (2015).
Wang, W. & Kaifer, A. E. Transfer of cationic cucurbit[7]uril inclusion complexes from water to non-aqueous solvents. Supramol. Chem. 22, 710–716 (2010).
Connors, K. A. The stability of cyclodextrin complexes in solution. Chem. Rev. 97, 1325–1358 (2002).
Seah, M. P., Gilmore, I. S. & Beamson, G. XPS: binding energy calibration of electron spectrometers 5—re‐evaluation of the reference energies. Surf. Interface Anal. 26, 642–649 (1998).
Zabka, W.-D. et al. Functionalization and passivation of ultrathin alumina films of defined sub-nanometer thickness with self-assembled monolayers. J. Phys. Condens. Matter 30, 424002 (2018).
Tanuma, S., Powell, C. J. & Penn, D. R. Calculations of electron inelastic mean free paths. V. Data for 14 organic compounds over the 50–2000 eV range. Surf. Interface Anal. 21, 165–176 (1994).
Scofield, J. H. Hartree–Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectros. Relat. Phenom. 8, 129–137 (1976).
Goedecker, S. & Teter, M. Separable dual-space Gaussian pseudopotentials. Phys. Rev. B 54, 1703–1710 (1996).
VandeVondele, J. & Hutter, J. Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases. J. Chem. Phys. 127, 114105 (2007).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Sabatini, R., Gorni, T. & De Gironcoli, S. Nonlocal van der Waals density functional made simple and efficient. Phys. Rev. B 87, 041108 (2013).
Heyd, J., Scuseria, G. E. & Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 118, 8207–8215 (2003).
Acknowledgements
S.D.T. thanks the University of Zurich, the University Research Priority Program LightChEC and the Swiss National Science Foundation (PYAPP2 160586) for funding. J.O. also acknowledges funding from the LightChEC. G.T., C.C., F.B.N. and M.I. thank the Swiss National Supercomputing Centre (CSCS) for generous resources under the Project IDs uzh1 and s965. C.C. thanks the INSPIRE potential master fellowship supported by the SNSF NCCR-MARVEL. G.T. thanks the Swiss National Science Foundation (Sinergia Grant No. CRSII2_160801). The authors thank T. Fox for the measurements of the solid-state NMR spectra. T. Moehl is thanked for assistance and fitting of the impedance data.
Author information
Authors and Affiliations
Contributions
L.S. and S.D.T. conceived the project. L.S. performed the synthesis, electrochemical and catalytic experiments. I.T. assisted with the synthesis and electrochemical experiments. J.S. and R.Z. conducted and evaluated the TERS experiments. M.T. and J.O. conducted and evaluated the XPS and STM experiments. O.B. measured and refined the crystal structures. G.T., C.C., F.B.N. and M.I. designed, conducted and evaluated the calculations. L.S. and S.D.T. wrote the manuscript. All the authors contributed to discussions of the results and revisions of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks Arnold Rheingold, Javier Concepcion and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–36, Experimental procedures and NMR spectra.
Supplementary Data 1
Cif file for complex 2.
Supplementary Data 2
Cif file for complex 3.
Supplementary Data 3
Cif file for complex 4.
Rights and permissions
About this article
Cite this article
Sévery, L., Szczerbiński, J., Taskin, M. et al. Immobilization of molecular catalysts on electrode surfaces using host–guest interactions. Nat. Chem. 13, 523–529 (2021). https://doi.org/10.1038/s41557-021-00652-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00652-y
This article is cited by
-
Spatiotemporal control for integrated catalysis
Nature Reviews Methods Primers (2023)
-
Non-covalent ligand-oxide interaction promotes oxygen evolution
Nature Communications (2023)
-
Supramolecular tuning of supported metal phthalocyanine catalysts for hydrogen peroxide electrosynthesis
Nature Catalysis (2023)
-
Adaptive insertion of a hydrophobic anchor into a poly(ethylene glycol) host for programmable surface functionalization
Nature Chemistry (2023)
-
Metal-like molecules
Nature Catalysis (2022)