Abstract
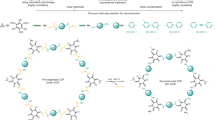
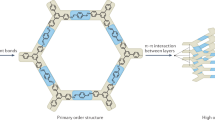
Porous covalent organic frameworks (COFs) enable the realization of functional materials with molecular precision. Past research has typically focused on generating rigid frameworks where structural and optoelectronic properties are static. Here we report dynamic two-dimensional (2D) COFs that can open and close their pores upon uptake or removal of guests while retaining their crystalline long-range order. Constructing dynamic, yet crystalline and robust frameworks requires a well-controlled degree of flexibility. We have achieved this through a ‘wine rack’ design where rigid π-stacked columns of perylene diimides are interconnected by non-stacked, flexible bridges. The resulting COFs show stepwise phase transformations between their respective contracted-pore and open-pore conformations with up to 40% increase in unit-cell volume. This variable geometry provides a handle for introducing stimuli-responsive optoelectronic properties. We illustrate this by demonstrating switchable optical absorption and emission characteristics, which approximate ‘null-aggregates’ with monomer-like behaviour in the contracted COFs. This work provides a design strategy for dynamic 2D COFs that are potentially useful for realizing stimuli-responsive materials.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within this Article and its Supplementary Information. Structure models of the COFs are available in the Supplementary Information. Toluene vapour sorption isotherms are included in the Supplementary Information; these follow the adsorption information file (.aif) standard67. Source data are provided with this paper.
References
Côte, A. P. et al. Porous, crystalline, covalent organic frameworks. Science 310, 1166–1170 (2005).
Uribe-Romo, F. J. et al. A crystalline imine-linked 3-D porous covalent organic framework. J. Am. Chem. Soc. 131, 4570–4571 (2009).
Liu, Y. et al. Weaving of organic threads into a crystalline covalent organic framework. Science 351, 365–369 (2016).
Wang, X. et al. Sulfone-containing covalent organic frameworks for photocatalytic hydrogen evolution from water. Nat. Chem. 10, 1180–1189 (2018).
Jin, S. et al. Creation of superheterojunction polymers via direct polycondensation: segregated and bicontinuous donor-acceptor π‑columnar arrays in covalent organic frameworks for long-lived charge separation. J. Am. Chem. Soc. 137, 7817–7827 (2015).
Bessinger, D., Muggli, K., Beetz, M., Auras, F. & Bein, T. Fast-switching vis-IR electrochromic covalent organic frameworks. J. Am. Chem. Soc. 143, 7351–7357 (2021).
Furukawa, H. & Yaghi, O. M. Storage of hydrogen, methane and carbon dioxide in highly porous covalent organic frameworks for clean energy applications. J. Am. Chem. Soc. 131, 8875–8883 (2009).
Vyas, V. S. et al. A tunable azine covalent organic framework platform for visible light-induced hydrogen generation. Nat. Commun. 6, 8508 (2015).
Calik, M. et al. Extraction of photogenerated electrons and holes from a covalent organic framework integrated heterojunction. J. Am. Chem. Soc. 136, 17802–17807 (2014).
Bessinger, D., Ascherl, L., Auras, F. & Bein, T. Spectrally switchable photodetection with near-infrared-absorbing covalent organic frameworks. J. Am. Chem. Soc. 139, 12035–12042 (2017).
Ascherl, L. et al. Perylene-based covalent organic frameworks for acid vapor sensing. J. Am. Chem. Soc. 141, 15693–15699 (2019).
Ascherl, L. et al. Molecular docking sites designed for the generation of highly crystalline covalent organic frameworks. Nat. Chem. 8, 310–316 (2016).
Ma, T. et al. Single-crystal X-ray diffraction structures of covalent organic frameworks. Science 361, 48–52 (2018).
Jin, E. et al. Two-dimensional sp2 carbon-conjugated covalent organic frameworks. Science 357, 673–676 (2017).
Ma, Y.-X. et al. A dynamic three-dimensional covalent organic framework. J. Am. Chem. Soc. 139, 4995–4998 (2017).
Kang, C. et al. Covalent organic framework atropisomers with multiple gas-triggered structural flexibilities. Nat. Mater. 22, 636–643 (2023).
Zhao, C. et al. Urea-linked covalent organic frameworks. J. Am. Chem. Soc. 140, 16438–16441 (2018).
Kang, C. et al. Interlayer shifting in two-dimensional covalent organic frameworks. J. Am. Chem. Soc. 142, 12995–13002 (2020).
Liu, C., Wang, Z., Zhang, L. & Dong, Z. Soft 2D covalent organic framework with compacted honeycomb topology. J. Am. Chem. Soc. 144, 18784–18789 (2022).
Kang, C. et al. Tunable interlayer shifting in two-dimensional covalent organic frameworks triggered by CO2 sorption. J. Am. Chem. Soc. 144, 20363–20371 (2022).
Li, Y., Sui, J., Cui, L.-S. & Jiang, H.-L. Hydrogen bonding regulated flexibility and disorder in hydrazone-linked covalent organic frameworks. J. Am. Chem. Soc. 145, 1359–1366 (2023).
Zhou, Z.-B., Sun, H.-H., Qi, Q.-Y. & Zhao, X. Gradually tuning the flexibility of two-dimensional covalent organic frameworks via stepwise structural transformation and their flexibility-dependent properties. Angew. Chem. Int. Ed. 62, e202305131 (2023).
Kitagawa, S. & Uemura, K. Dynamic porous properties of coordination polymers inspired by hydrogen bonds. Chem. Soc. Rev. 34, 109–119 (2005).
Evans, J. D., Bon, V., Senkovska, I., Lee, H.-C. & Kaskel, S. Four-dimensional metal–organic frameworks. Nat. Commun. 11, 2690 (2020).
Schneemann, A. et al. Flexible metal-organic frameworks. Chem. Soc. Rev. 43, 6062–6096 (2014).
Krause, S. et al. A pressure-amplifying framework material with negative gas adsorption transitions. Nature 532, 348–352 (2016).
Fernandez, C. A. et al. An electrically switchable metal-organic framework. Sci. Rep. 4, 6114 (2014).
Krause, S. et al. Cooperative light-induced breathing of soft porous crystals via azobenzene buckling. Nat. Commun. 13, 1951 (2022).
Krause, S., Hosono, N. & Kitagawa, S. Chemistry of soft porous crystals: structural dynamics and gas adsorption properties. Angew. Chem. Int. Ed. 59, 15325–15341 (2020).
Carrington, E. J. et al. Solvent-switchable continuous-breathing behaviour in a diamondoid metal–organic framework and its influence on CO2 versus CH4 selectivity. Nat. Chem. 9, 882–889 (2017).
Knebel, A. et al. Defibrillation of soft porous metal-organic frameworks with electric fields. Science 358, 347–351 (2017).
Mason, J. A. et al. Methane storage in flexible metal–organic frameworks with intrinsic thermal management. Nature 527, 357–361 (2015).
Yanai, N. et al. Gas detection by structural variations of fluorescent guest molecules in a flexible porous coordination polymer. Nat. Mater. 10, 787–793 (2011).
Halder, G. J., Kepert, C. J., Moubaraki, B., Murray, K. S. & Cashion, J. D. Guest-dependent spin crossover in a nanoporous molecular framework material. Science 298, 1762–1765 (2002).
Chen, Y. et al. Guest-dependent dynamics in a 3D covalent organic framework. J. Am. Chem. Soc. 141, 3298–3303 (2019).
Sun, T., Wei, L., Chen, Y., Ma, Y. & Zhang, Y.-B. Atomic-level characterization of dynamics of a 3D covalent organic framework by cryo-electron diffraction tomography. J. Am. Chem. Soc. 141, 10962–10966 (2019).
Zhu, Q. et al. 3D cage COFs: a dynamic three-dimensional covalent organic framework with high-connectivity organic cage nodes. J. Am. Chem. Soc. 142, 16842–16848 (2020).
Liu, X. et al. A crystalline three-dimensional covalent organic framework with flexible building blocks. J. Am. Chem. Soc. 143, 2123–2129 (2021).
Wei, L. et al. Guest-adaptive molecular sensing in a dynamic 3D covalent organic framework. Nat. Commun. 13, 7936 (2022).
Yu, B. et al. Linkage conversions in single-crystalline covalent organic frameworks. Nat. Chem. 16, 114–121 (2024).
Zeng, T. et al. Atomic observation and structural evolution of covalent organic framework rotamers. Proc. Natl Acad. Sci. USA 121, e2320237121 (2024).
Eaton, S. W. et al. Singlet exciton fission in polycrystalline thin films of a slip-stacked perylenediimide. J. Am. Chem. Soc. 135, 14701–14712 (2013).
Rabbani, M. G. et al. A 2D mesoporous imine-linked covalent organic framework for high pressure gas storage applications. Chem. Eur. J. 19, 3324–3328 (2013).
Auras, F. et al. Synchronized offset stacking: a concept for growing large-domain and highly crystalline 2D covalent organic frameworks. J. Am. Chem. Soc. 138, 16703–16710 (2016).
Chen, X. et al. Towards covalent organic frameworks with predesignable and aligned open docking sites. Chem. Commun. 50, 6161–6163 (2014).
Ascherl, L. et al. Solvatochromic covalent organic frameworks. Nat. Commun. 9, 3802 (2018).
Hartnett, P. E. et al. Effects of crystalline perylenediimide acceptor morphology on optoelectronic properties and device performance. Chem. Mater. 28, 3928–3936 (2016).
Hunter, C. A. & Sanders, J. K. M. The nature of π–π interactions. J. Am. Chem. Soc. 112, 5525–5534 (1990).
Rice, C. M. et al. Following the unusual breathing behaviour of 17O-enriched mixed-metal (Al,Ga)-MIL-53 using NMR crystallography. Phys. Chem. Chem. Phys. 22, 14514–14526 (2020).
Pallach, R. et al. Frustrated flexibility in metal–organic frameworks. Nat. Commun. 12, 4097 (2021).
Hestand, N. J. & Spano, F. C. Molecular aggregate photophysics beyond the kasha model: novel design principles for organic materials. Acc. Chem. Res. 50, 341–350 (2017).
Kaiser, T. E., Wang, H., Stepanenko, V. & Würthner, F. Supramolecular construction of fluorescent J-aggregates based on hydrogen-bonded perylene dyes. Angew. Chem. Int. Ed. 46, 5541–5544 (2007).
Kaufmann, C., Bialas, D., Stolte, M. & Würthner, F. Discrete π‑stacks of perylene bisimide dyes within folda-dimers: insight into long- and short-range exciton coupling. J. Am. Chem. Soc. 140, 9986–9995 (2018).
Veldman, D. et al. Triplet formation involving a polar transition state in a well-defined intramolecular perylenediimide dimeric aggregate. J. Phys. Chem. A 112, 5846–5857 (2008).
Oleson, A. et al. Perylene diimide-based Hj- and hJ-aggregates: the prospect of exciton band shape engineering in organic materials. J. Phys. Chem. C 123, 20567–20578 (2019).
Kaiser, T. E., Stepanenko, V. & Würthner, F. Fluorescent J-aggregates of core-substituted perylene bisimides: studies on structure-property relationship, nucleation-elongation mechanism, and sergeants-and-soldiers principle. J. Am. Chem. Soc. 131, 6719–6732 (2009).
Li, H. et al. Supramolecular alternating donor–acceptor assembly toward intercalated covalent organic frameworks. J. Am. Chem. Soc. 142, 3712–3717 (2020).
Chupas, P. J., Chapman, K. W. & Lee, P. L. Applications of an amorphous silicon-based area detector for high-resolution, high-sensitivity and fast time-resolved pair distribution function measurements. J. Appl. Crystallogr. 40, 463–470 (2007).
Chupas, P. J. et al. Rapid-acquisition pair distribution function (RA-PDF) analysis. J. Appl. Crystallogr. 36, 1342–1347 (2003).
Chupas, P. J. et al. A versatile sample-environment cell for non-ambient X-ray scattering experiments. J. Appl. Crystallogr. 41, 822–824 (2008).
Toby, B. H. & Von Dreele, R. B. GSAS-II: the genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 46, 544–549 (2013).
Juhás, P., Davis, T., Farrow, C. L. & Billinge, S. J. L. PDFgetX3: a rapid and highly automatable program for processing powder diffraction data into total scattering pair distribution functions. J. Appl. Crystallogr. 46, 560–566 (2013).
Farrow, C. L. et al. PDFfit2 and PDFgui: computer programs for studying nanostructure in crystals. J. Phys. Condens. Matter 19, 335219 (2007).
Coelho, A. A. TOPAS and TOPAS-Academic: an optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Crystallogr. 51, 210–218 (2018).
O’Nolan, D. et al. A multimodal analytical toolkit to resolve correlated reaction pathways: the case of nanoparticle formation in zeolites. Chem. Sci. 12, 13836–13847 (2021).
Chen, Z. et al. Node distortion as a tunable mechanism for negative thermal expansion in metal-organic frameworks. J. Am. Chem. Soc. 145, 268–276 (2023).
Evans, J. D., Bon, V., Senkovska, I. & Kaskel, S. A universal standard archive file for adsorption data. Langmuir 37, 4222–4226 (2021).
Acknowledgements
This project received funding from the European Research Council (ERC) under the European Union’s Seventh Framework Programme (grant agreement no. 321339, T.B.) and the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 670405, R.H.F.). We acknowledge support from the Free State of Bavaria (research network ‘Solar Technologies Go Hybrid’) and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany´s Excellence Strategy–EXC 2089/1–390776260 (T.B.). F.A. acknowledges funding from DFG project no. 525243720. S. Krause acknowledges funding from the Alexander von Humboldt Foundation as well as the Fonds der Chemischen Industrie. S. Kaskel acknowledges funding from the ERC under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 742743) and DFG CRC 1415 ‘Chemistry of Synthetic Two-Dimensional Materials’. This work was supported as part of GENESIS: a Next Generation Synthesis Centre, an Energy Frontier Research Centre funded by the United States Department of Energy (US DOE), Office of Science, Basic Energy Sciences under award no. DESC0019212 (K.W.C.). This research used resources at beamline 11-ID-B of the Advanced Photon Source, a US DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357.
Author information
Authors and Affiliations
Contributions
F.A. and L.A. designed and synthesized the materials, analysed the results and wrote the manuscript. V.B., S. Krause and S. Kaskel conducted the sorption analyses and in situ PXRD experiments. S.M.V. and K.W.C. performed the synchrotron-based experiments. M.D. performed the TEM analysis. D.B. and S.R. carried out supplementary syntheses and materials characterization. F.A. and R.H.F. conducted and analysed the optical spectroscopy experiments. F.A. and T.B. supervised the project. All authors discussed the results and contributed to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Francoise Mystere Amombo Noa, Yue-Biao Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Dynamic phase transformations of the buPDI-1P COF monitored with synchrotron radiation (λ = 0.2116 Å).
The sample was exposed to a stream of either toluene-saturated or dry helium. (a) Dynamic phase transformations measured with a time resolution of 12 s per scan. Bottom: Toluene uptake triggers the stepwise transformation from the cp to the ip and the op phases. Top: Desorption of the toluene in dry helium triggers the transformation back to the ip phase. As shown in the toluene sorption experiments, the cp/ip phase transformation occurs at very low toluene concentration and thus was not observed in the time window of this desorption experiment. The phase transitions are apparent from stepwise shifts of the 110 reflection from 0.82° (cp phase) to 0.77° (ip phase) and 0.65° (op phase), respectively. Since the COF does not expand notably along its b axis, the position of the 020 reflection at 0.55° remains virtually unchanged. (b) Non-negative matrix factorization (NMF) analysis of the phase transformations during toluene adsorption confirms that the data set is well represented by three components (these are purely mathematical components). These three components are very close to the simulated patterns for the cp, ip, and op phases (peak intensities differ for some reflections because solvent molecules located in the pores are not included in the simulations). (c) The relative populations of the NMF components showing the evolution and devolution of phases are in very good agreement with the PXRD data shown in (a). Component 1 (representing the cp phase) converts within 40 s to component 2 (ip phase). This component converts to component 3 (op phase) after 80 s of toluene exposure.
Extended Data Fig. 2 Comparison of the buPDI-1P COF structures when loaded with different solvents.
(a) Comparison of the XRD patterns of the solvent-loaded buPDI-1P COF. While the peak intensities vary depending on the respective solvent (structure factors differ considerably depending on the solvent in the pores), the peak positions are almost identical. Only the position of the 110 reflection around 4.5° (marked with a dashed line) shifts slightly, whereas the position of the 020 reflection at 4.0° remains constant. This indicates a small variation in the a axis length ranging from 28.0 Å (heptane) to 29.8 Å for the bulkier mesitylene and 1,2-dichlorobenzene. The XRD pattern of the toluene-loaded COF was recorded with synchrotron radiation under a controlled atmosphere and converted to Cu Kα angles for comparison. (b) Comparison of the XRD patterns of the buPDI-1P COF at intermediate solvent loading (achieved by slow drying of the solvent-loaded sample in air, except for toluene). The XRD pattern of the toluene-loaded COF was recorded with synchrotron radiation under a controlled atmosphere and converted to Cu Kα angles for comparison. To our surprise, we observe two different intermediate pore phases (denoted as ip and ip*), depending on the solvent. For all solvents, the respective intermediate phase forms from the op phase and converts to the cp phase when the solvent is further removed. No phase transformations between these two intermediate phases are observed. (c) The less bulky heptane, isopropanol, toluene and cyclohexane produce a fairly contracted ip phase where the butyl chains adopt a side-by-side configuration with maximal overlap. (d) The very bulky mesitylene and 1,2-dichlorobenzene enforce a different ip* phase where the butyl chains are in a side-by-side configuration, but with less overlap. This phase is more expanded and hence provides a larger accessible porosity (Connolly surface is shown in light blue). (e) Change of selected unit cell parameters during the phase transformations. The expansion is mostly along the projection of the a axis perpendicular to the pores, that is, a · sin(β), while the b axis remains nearly constant. Filled symbols: toluene, empty symbols: mesitylene. While the op phases are virtually the same, the ip* phase of the mesitylene-filled COF has a substantially larger a axis and unit cell volume.
Extended Data Fig. 3 Structure analysis and solvent-induced phase transformations of the prPDI-1P COF.
(a) PXRD analysis of the dry prPDI-1P COF. Pawley refinement (red line) provides a very good fit to the PXRD data (black dots). Rwp = 5.8% (w/o background), Rp = 3.8%. Inset: Force-field optimised structure model of the COF. In this phase, the COF is strongly contracted along the a axis. Its porosity is very limited and not accessible to N2 or larger molecules (Connolly surface shown in light blue). Apart from its negligible porosity, the dry prPDI-1P COF closely resembles the buPDI-1P COF ip phase where the alkyl chains are straight but arranged in a side-by-side configuration. Note: A fully contracted cp phase is not observed for this COF. (b) PXRD analysis of the toluene-loaded prPDI-1P COF. Pawley refinement (red line) provides a good fit to the PXRD data (black dots). Rwp = 18% (w/o background), Rp = 5.8%. Inset: Force-field optimised structure model of the COF. In this phase, the COF has an open-pore configuration (Connolly surface shown in light blue) with a head-to-head arrangement of the alkyl chains that closely resembles the buPDI-1P COF op phase. The reflections at 6.5° and 11.1° are due to unreacted prPDI starting material (prPDI has very low solubility). (c,d) Solvent-induced structural transformations monitored by in-situ PXRD during toluene vapour adsorption. The points on the adsorption isotherm correspond to the PXRD patterns shown in (d). Toluene uptake causes a phase transformation to the op phase around p/p0 = 0.1. The last XRD pattern, collected under vacuum after solvent desorption, confirms the full reversibility of the phase transformation. The reflections at 6.5° and 11.1° are due to unreacted starting material. In this COF, the PDIs are slip-stacked with 3.1 Å offset along the long molecular axis, which translates into a bridge-to-bridge distance of 4.1 Å.
Extended Data Fig. 4 Structure analysis and solvent-induced phase transformations of the hexPDI-1P COF.
(a) PXRD analysis of the dry hexPDI-1P COF. Rietveld refinement (red line) using the structure model shown in the inset provides a very good fit to the PXRD data (black dots) with only small differences between experimental and the refined patterns. Rwp = 9.1% (w/o background), Rp = 10.7%. Inset: The COF is strongly contracted along the a axis. Its porosity is very limited and not accessible to N2 or larger molecules (Connolly surface shown in light blue). The dry hexPDI-1P COF closely resembles the buPDI-1P COF cp phase where the alkyl chains are bent sidewards to allow for a closer packing of the PDI columns. (b) PXRD analysis of the toluene-loaded hexPDI-1P COF. Pawley refinement (red line) provides a good fit to the PXRD data (black dots). Rwp = 14% (w/o background), Rp = 3.2%. Inset: Force-field optimised structure model of the COF. In this phase, the COF has an open-pore configuration (Connolly surface shown in light blue). The hexyl chains are straight and adopt a side-by side configuration that closely resembles the buPDI-1P COF ip phase. Note: An op phase where the alkyl chains adopt a head-to-head configuration is not observed for this COF, even at high toluene loading. This is most likely due to steric reasons (the hexyl chains are too long). (c,d) Solvent-induced structural transformations monitored by in-situ PXRD during toluene vapour adsorption. The points on the adsorption and desorption isotherms correspond to the PXRD patterns shown in (d). Toluene uptake causes a phase transformation to the ip phase around p/p0 = 0.05. No further phase transformations are observed at higher toluene loading. The COF returns to the cp phase upon desorption of the solvent. In this COF, the PDIs are slip-stacked with 3.2 Å offset along the long molecular axis, which translates into a bridge-to-bridge distance of 4.2 Å.
Supplementary information
Supplementary Information
Supplementary Figs. 1–11, Sections 1–11 and references.
Supplementary Data 1
Structure models of the PDI COFs.
Supplementary Data 2
Toluene physisorption data of the PDI COFs.
Source data
Source Data Fig. 2
Source data of all plots in Fig. 2.
Source Data Fig. 3
Source data of all plots in Fig. 3.
Source Data Fig. 4
Source data of all plots in Fig. 4.
Source Data Extended Data Fig. 1
Source data of all plots in Extended Data Fig. 1.
Source Data Extended Data Fig. 2
Source data of all plots in Extended Data Fig. 2.
Source Data Extended Data Fig. 3
Source data of all plots in Extended Data Fig. 3.
Source Data Extended Data Fig. 4
Source data of all plots in Extended Data Fig. 4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Auras, F., Ascherl, L., Bon, V. et al. Dynamic two-dimensional covalent organic frameworks. Nat. Chem. (2024). https://doi.org/10.1038/s41557-024-01527-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41557-024-01527-8