Abstract
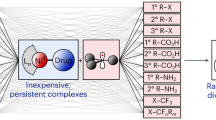
The development of crystalline porous materials with high chemical stability is of paramount importance for their practical application. Here, we report the synthesis of polyarylether-based covalent organic frameworks (PAE-COFs) with high crystallinity, porosity and chemical stability, including towards water, owing to the inert nature of their polyarylether-based building blocks. The PAE-COFs are synthesized through nucleophilic aromatic substitution reactions between ortho-difluoro benzene and catechol building units, which form ether linkages. The resulting materials are shown to be stable against harsh chemical environments including boiling water, strong acids and bases, and oxidation and reduction conditions. Their stability surpasses the performance of other known crystalline porous materials such as zeolites, metal–organic frameworks and covalent organic frameworks. We also demonstrate the post-synthetic functionalization of these materials with carboxyl or amino functional groups. The functionalized PAE-COFs combine porosity, high stability and recyclability. A preliminary application of these materials is demonstrated with the removal of antibiotics from water over a wide pH range.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the Article and its Supplementary Information or from the corresponding author upon reasonable request.
References
Côté, A. P. et al. Porous, crystalline, covalent organic frameworks. Science 310, 1166–1170 (2005).
Colson, J. W. & Dichtel, W. R. Rationally synthesized two-dimensional polymers. Nat. Chem. 5, 453–465 (2013).
Huang, N., Wang, P. & Jiang, D. L. Covalent organic frameworks: a materials platform for structural and functional designs. Nat. Rev. Mater. 1, 1–19 (2016).
Diercks, C. S. & Yaghi, O. M. The atom, the molecule, and the covalent organic framework. Science 355, 923–930 (2017).
Ding, S. Y. & Wang, W. Covalent organic frameworks (COFs): from design to applications. Chem. Soc. Rev. 42, 548–568 (2013).
Jin, Y. H., Hu, Y. M. & Zhang, W. Tessellated multiporous two-dimensional covalent organic frameworks. Nat. Rev. Chem. 1, 0056 (2017).
Kuhn, P., Antonietti, M. & Thomas, A. Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew. Chem. Int. Ed. 47, 3450–3453 (2008).
Furukawa, H. & Yaghi, O. M. Storage of hydrogen, methane, and carbon dioxide in highly porous covalent organic frameworks for clean energy applications. J. Am. Chem. Soc. 131, 8875–8883 (2009).
Guan, X. Y. et al. Fast, ambient temperature and pressure ionothermal synthesis of three-dimensional covalent organic frameworks. J. Am. Chem. Soc. 140, 4494–4498 (2018).
Ding, S. Y. et al. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki–Miyaura coupling reaction. J. Am. Chem. Soc. 133, 19816–19922 (2011).
Fang, Q. R. et al. 3D microporous base-functionalized covalent organic frameworks for size-selective catalysis. Angew. Chem. Int. Ed. 53, 2878–2882 (2014).
Li, H. et al. 3D covalent organic frameworks with dual linkages for bifunctional cascade catalysis. J. Am. Soc. Chem. 138, 14783–14788 (2016).
Han, X. et al. Chiral covalent organic frameworks with high chemical stability for heterogeneous asymmetric catalysis. J. Am. Chem. Soc. 139, 8693–8697 (2017).
Wan, S., Guo, J., Kim, J., Ihee, H. & Jiang, D. L. A belt-shaped, blue luminescent, and semiconducting covalent organic framework. Angew. Chem. Int. Ed. 47, 8826–8830 (2008).
Bertrand, G. H. V., Michaelis, V. K., Ong, T. C., Griffin, R. G. & Dincă, M. Thiophene-based covalent organic frameworks. Proc. Natl Acad. Sci. USA 110, 4923–4928 (2013).
Calik, M. et al. Extraction of photogenerated electrons and holes from a covalent organic framework integrated heterojunction. J. Am. Chem. Soc. 136, 17802–17807 (2014).
Fang, Q. R. et al. 3D porous crystalline polyimide covalent organic frameworks for drug delivery. J. Am. Chem. Soc. 137, 8352–8355 (2015).
Du, Y. et al. Ionic covalent organic frameworks with spiroborate linkage. Angew. Chem. Int. Ed. 55, 1737–1741 (2016).
Wang, S. et al. Exfoliation of covalent organic frameworks into few-layer redox-active nanosheets as cathode materials for lithium-ion batteries. J. Am. Chem. Soc. 139, 4258–4261 (2017).
Sun, Q. et al. Postsynthetically modified covalent organic frameworks for efficient and effective mercury removal. J. Am. Chem. Soc. 139, 2786–2793 (2017).
Li, Z. L. et al. Three-dimensional ionic covalent organic frameworks for rapid, reversible and selective ion exchange. J. Am. Chem. Soc. 139, 17771–17774 (2017).
Lu, Q. Y. et al. Postsynthetic functionalization of three-dimensional covalent organic framework for selective extraction of lanthanide ions. Angew. Chem. Int. Ed. 57, 6042–6048 (2018).
Kandambeth, S. et al. Self-templated chemically stable hollow spherical covalent organic framework. Nat. Commun. 6, 6786 (2015).
Mallick, A., Lukose, B., Mane, M. V., Heine, T. & Banerjee, R. Construction of crystalline 2D covalent organic frameworks with remarkable chemical (acid/base) stability via a combined reversible and irreversible route. J. Am. Chem. Soc. 134, 19524–19527 (2012).
Xu, H., Gao, J. & Jiang, D. L. Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts. Nat. Chem. 7, 905–912 (2015).
Wei, P. F. et al. Benzoxazole-linked ultrastable covalent organic frameworks for photocatalysis. J. Am. Chem. Soc. 140, 4623–4631 (2018).
Hergenrother, P. M. The use, design, synthesis, and properties of high performance/high temperature polymers: an overview. High Perform. Polym. 15, 3–45 (2003).
Gao, Y. et al. Comparison of PEM properties of copoly(aryl ether ether nitrile)s containing sulfonic acid bonded to naphthalene in structurally different ways. Macromolecules 40, 1512–1520 (2007).
Gotham, K. & Turner, S. Poly(ether sulphone) as an engineering material. Polymer 15, 665–670 (1974).
McKeown, N. B. & Budd, P. M. Polymers of intrinsic microporosity (PIMs): organic materials for membrane separations, heterogeneous catalysis and hydrogen storage. Chem. Soc. Rev. 35, 675–683 (2006).
Materials Studio v.7.0 (Accelrys Inc, 2013); http://www.3dsbiovia.com/products/collaborative-science/biovia-materials-studio/
Huang, N., Wang, P., Addicoat, M. A., Heine, T. & Jiang, D. L. Ionic covalent organic frameworks: design of a charged interface aligned on 1D channel walls and its unusual electrostatic functions. Angew. Chem. Int. Ed. 56, 4982–4986 (2017).
Du, N. et al. Polymer nanosieve membranes for CO2-capture applications. Nat. Mater. 10, 372–375 (2011).
Henry, W. Experiments on the quantity of gases absorbed by water, at different temperatures, and under different pressures. Philos. Trans. R. Soc. Lond. 93, 29–42 (1803).
Fang, Q. R. et al. Designed synthesis of large-pore crystalline polyimide covalent organic frameworks. Nat. Commun. 5, 4503 (2014).
Guo, J. et al. Conjugated organic framework with three-dimensionally ordered stable structure and delocalized π clouds. Nat. Commun. 4, 2736 (2013).
Halder, A. et al. Ultrastable imine-based covalent organic frameworks for sulfuric acid recovery: an effect of interlayer hydrogen bonding. Angew. Chem. Int. Ed. 57, 5797–5802 (2018).
Katz, M. J. et al. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 49, 9449–9451 (2013).
Pan, Y. C., Liu, Y. Y., Zeng, G. F., Zhao, L. & Lai, Z. P. Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystalsin an aqueous system. Chem. Commun. 47, 2071–2073 (2011).
Stephen, S. Y., Samuel, M. F., Jonathan, P. H., Charmant, A. & Williams, I. D. A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n. Science 283, 1148–1151 (1999).
Eddaoudi, M. et al. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 295, 469–472 (2002).
Du, N., Robertson, G. P., Song, J., Pinnau, I. & Guiver, M. D. High-performance carboxylated polymers of intrinsic microporosity (PIMs) with tunable gas transport properties. Macromolecules 42, 6038–6043 (2009).
Peng, C. et al. Diverse macroscopic helical motions of microribbons driven by electrons. Chem. Commun. 53, 2578–2581 (2017).
Sholl, D. S. & Lively, R. P. Seven chemical separations to change the world. Nature 533, 316–322 (2016).
Ali, I. New generation adsorbents for water treatment. Chem. Rev. 112, 5073–5091 (2012).
Wang, T. et al. Adsorptive removal of antibiotics from water using magnetic ion exchange resin. J. Environ. Sci. 52, 111–117 (2017).
Wang, Y., Pan, X., Wang, J., Hou, P. & Qiang, Z. J. Adsorption behavior and mechanisms of norfloxacin onto porous resins and carbon nanotube. Chem. Eng. J. 179, 112–118 (2012).
Ali, M. M. & Ahmed, M. J. Adsorption behavior of doxycycline antibiotic on NaY zeolite from wheat (Triticum aestivum) straws ash. J. Inst. Chem. Eng. 81, 218–224 (2017).
Singh, S. et al. Nanocuboidal-shaped zirconium based metal organic framework for the enhanced adsorptive removal of nonsteroidal anti-inflammatory drug, ketorolac tromethamine, from aqueous phase. New J. Chem. 42, 1921–1930 (2018).
Jade v.5.0 (Materials Data Inc, 1999); https://materialsdata.com/prodjd.html
Kaye, S. S., Dailly, A., Yaghi, O. M. & Long, J. R. Impact of preparation and handling on the hydrogen storage properties of Zn4O(1,4-benzenedicarboxylate)3 (MOF-5). J. Am. Chem. Soc. 129, 14176–14177 (2007).
Acknowledgements
Q.F., V.V., S.Q. and M.X. acknowledge support from the National Natural Science Foundation of China (21571079, 21621001, 21390394, 21571076 and 21571078), the ‘111’ project (B07016 and B17020), Guangdong and Zhuhai Science and Technology Department Project (2012D0501990028), the programme for JLU Science and Technology Innovative Research Team and Sino-French joint laboratory. Q.F. and V.V. acknowledge support from the Thousand Talents programme (China).
Author information
Authors and Affiliations
Contributions
Q.F., V.V., Y.Y. and S.Q. were responsible for the overall design, direction and supervision of the project. X.G. performed the experimental work. H.L. and Y.M. took SEM images and helped with the TGA and PXRD tests. M.X. was in charge of other physical measurements. All authors discussed the results and contributed to the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, supplementary synthetic procedures and supplementary experimental data
Rights and permissions
About this article
Cite this article
Guan, X., Li, H., Ma, Y. et al. Chemically stable polyarylether-based covalent organic frameworks. Nat. Chem. 11, 587–594 (2019). https://doi.org/10.1038/s41557-019-0238-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-019-0238-5
This article is cited by
-
Linkage conversions in single-crystalline covalent organic frameworks
Nature Chemistry (2024)
-
A general large-scale synthesis approach for crystalline porous materials
Nature Communications (2023)
-
A sweat-responsive covalent organic framework film for material-based liveness detection and sweat pore analysis
Nature Communications (2023)
-
Supramolecular framework membrane for precise sieving of small molecules, nanoparticles and proteins
Nature Communications (2023)
-
Precise fabrication of ternary ordered covalent organic frameworks for photocatalysis
Science China Chemistry (2023)