Abstract
Intracellular surveillance for systemic microbial components during homeostasis and infections governs host physiology and immunity. However, a long-standing question is how circulating microbial ligands become accessible to intracellular receptors. Here we show a role for host-derived extracellular vesicles (EVs) in this process; human and murine plasma-derived and cell culture-derived EVs have an intrinsic capacity to bind bacterial lipopolysaccharide (LPS). Remarkably, circulating host EVs capture blood-borne LPS in vivo, and the LPS-laden EVs confer cytosolic access for LPS, triggering non-canonical inflammasome activation of gasdermin D and pyroptosis. Mechanistically, the interaction between the lipid bilayer of EVs and the lipid A of LPS underlies EV capture of LPS, and the intracellular transfer of LPS by EVs is mediated by CD14. Overall, this study demonstrates that EVs capture and escort systemic LPS to the cytosol licensing inflammasome responses, uncovering EVs as a previously unrecognized link between systemic microbial ligands and intracellular surveillance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Uncropped immunoblot images for this article are included in the Supplementary Information. Source data are provided with this paper. All other data supporting the findings of the paper are available from the corresponding author upon reasonable request.
References
Gabanyi, I. et al. Bacterial sensing via neuronal Nod2 regulates appetite and body temperature. Science 376, eabj3986 (2022).
Clarke, T. B. et al. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 16, 228–231 (2010).
Farrokhi, V. et al. Bacterial lipodipeptide, Lipid 654, is a microbiome-associated biomarker for multiple sclerosis. Clin. Transl. Immunol. 2, e8 (2013).
Huang, Z. et al. Antibody neutralization of microbiota-derived circulating peptidoglycan dampens inflammation and ameliorates autoimmunity. Nat. Microbiol. 4, 766–773 (2019).
Moltke von, J., Ayres, J. S., Kofoed, E. M., Chavarría-Smith, J. & Vance, R. E. Recognition of bacteria by inflammasomes. Annu Rev. Immunol. 31, 73–106 (2013).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 (2010).
Poltorak, A. et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 (1998).
Rathinam, V. A. K., Zhao, Y. & Shao, F. Innate immunity to intracellular LPS. Nat. Immunol. 20, 527–533 (2019).
Kayagaki, N. et al. Non-canonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013).
Hagar, J. A., Powell, D. A., Aachoui, Y., Ernst, R. K. & Miao, E. A. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253 (2013).
Shi, J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187 (2014).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015).
Vanaja, S. K. et al. Bacterial outer membrane vesicles mediate cytosolic localization of LPS and caspase-11 activation. Cell 165, 1106–1119 (2016).
Luo, Z. et al. Variation in blood microbial lipopolysaccharide (LPS) contributes to immune reconstitution in response to suppressive antiretroviral therapy in HIV. EBioMedicine 80, 104037 (2022).
Mohammad, S. & Thiemermann, C. Role of metabolic endotoxemia in systemic inflammation and potential interventions. Front. Immunol. 11, 594150 (2021).
Kell, D. B. & Pretorius, E. On the translocation of bacteria and their lipopolysaccharides between blood and peripheral locations in chronic, inflammatory diseases: the central roles of LPS and LPS-induced cell death. Integr. Biol. 7, 1339–1377 (2015).
Prins, J. M., Deventer van, S. J., Kuijper, E. J. & Speelman, P. Clinical relevance of antibiotic-induced endotoxin release. Antimicrob. Agents Chemother. 38, 1211–1218 (1994).
Matsuura, M. Structural modifications of bacterial lipopolysaccharide that facilitate Gram-negative bacteria evasion of host innate immunity. Front Immunol. 4, 109 (2013).
Fukui, H. Endotoxin and other microbial translocation markers in the blood: a clue to understand leaky gut syndrome. Cell. Mol. Med. Open Access https://doi.org/10.21767/2573-5365.100023 (2016).
Vasudevan, S. O., Russo, A. J., Kumari, P., Vanaja, S. K. & Rathinam, V. A. A TLR4-independent critical role for CD14 in intracellular LPS sensing. Cell Rep. 39, 110755 (2022).
Colombo, M., Raposo, G. & Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30, 1–35 (2014).
Jeppesen, D. K. et al. Reassessment of exosome composition. Cell 177, 428–445.e18 (2019).
Kalluri, R. & LeBleu, V. S. The biology, function, and biomedical applications of exosomes. Science 367, eaau6977 (2020).
O’Brien, K., Breyne, K., Ughetto, S., Laurent, L. C. & Breakefield, X. O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 21, 585–606 (2020).
Kim, D.-K. et al. EVpedia: a community web portal for extracellular vesicles research. Bioinformatics 31, 933–939 (2015).
Wang, N. et al. Circulating exosomes contain protein biomarkers of metastatic non‐small‐cell lung cancer. Cancer Sci. 109, 1701–1709 (2018).
Li, W. et al. LPS induces active HMGB1 release from hepatocytes into exosomes through the coordinated activities of TLR4 and caspase-11/GSDMD signaling. Front. Immunol. 11, 229 (2020).
Cosme, J., Guo, H., Hadipour-Lakmehsari, S., Emili, A. & Gramolini, A. O. Hypoxia-induced changes in the fibroblast secretome, exosome, and whole-cell proteome using cultured, cardiac-derived cells isolated from neonatal mice. J. Proteome Res. 16, 2836–2847 (2017).
Jin, M., Drwal, G., Bourgeois, T., Saltz, J. & Wu, H. M. Distinct proteome features of plasma microparticles. Proteomics 5, 1940–1952 (2005).
Kugeratski, F. G. et al. Quantitative proteomics identifies the core proteome of exosomes with syntenin-1 as the highest abundant protein and a putative universal biomarker. Nat. Cell Biol. 23, 631–641 (2021).
Budden, C. F. et al. Inflammasome‐induced extracellular vesicles harbour distinct RNA signatures and alter bystander macrophage responses. J. Extracell. Vesicles 10, e12127 (2021).
Lobb, R. J. et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 4, 27031 (2015).
Mathieu, M., Martin-Jaular, L., Lavieu, G. & Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 21, 9–17 (2019).
Jeppesen, D. K., Zhang, Q., Franklin, J. L. & Coffey, R. J. Extracellular vesicles and nanoparticles: emerging complexities. Trends Cell Biol. https://doi.org/10.1016/j.tcb.2023.01.002 (2023).
Cheng, K. T. et al. Caspase-11–mediated endothelial pyroptosis underlies endotoxemia-induced lung injury. J. Clin. Invest. 127, 4124–4135 (2017).
Kumari, P., Russo, A. J., Wright, S. S., Muthupalani, S. & Rathinam, V. A. Hierarchical cell-type-specific functions of caspase-11 in LPS shock and antibacterial host defense. Cell Rep. 35, 109012 (2021).
Russo, A. J. et al. Intracellular immune sensing promotes inflammation via gasdermin D-driven release of a lectin alarmin. Nat. Immunol. 22, 154–165 (2021).
Gong, T., Liu, L., Jiang, W. & Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 20, 95–112 (2020).
Catalano, M. & O’Driscoll, L. Inhibiting extracellular vesicles formation and release: a review of EV inhibitors. J. Extracell. Vesicles 9, 1703244 (2020).
Zhang, H., Lu, J., Liu, J., Zhang, G. & Lu, A. Advances in the discovery of exosome inhibitors in cancer. J. Enzyme Inhib. Med. Chem. 35, 1322–1330 (2020).
Lu, M. et al. Comparison of exosome-mimicking liposomes with conventional liposomes for intracellular delivery of siRNA. Int. J. Pharm. 550, 100–113 (2018).
Raetz, C. R. H., Reynolds, C. M., Trent, M. S. & Bishop, R. E. Lipid A modification systems in Gram-negative bacteria. Annu. Rev. Biochem. 76, 295–329 (2007).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007).
Zanoni, I. et al. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell 147, 868–880 (2011).
Wright, S. D., Ramos, R. A., Tobias, P. S., Ulevitch, R. J. & Mathison, J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249, 1431 1433 (1990).
Kitchens, R. L. & Munford, R. S. CD14-dependent internalization of bacterial lipopolysaccharide (LPS) is strongly influenced by LPS aggregation but not by cellular responses to LPS. J. Immunol. 160, 1920–1928 (1998).
Tan, Y., Zanoni, I., Cullen, T. W., Goodman, A. L. & Kagan, J. C. Mechanisms of Toll-like receptor 4 endocytosis reveal a common immune-evasion strategy used by pathogenic and commensal bacteria. Immunity 43, 909–922 (2015).
Keller, M. D. et al. Decoy exosomes provide protection against bacterial toxins. Nature 579, 260–264 (2020).
Robbins, P. D. & Morelli, A. E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 14, 195–208 (2014).
Théry, C., Ostrowski, M. & Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 9, 581–593 (2009).
Vreugdenhil, A. C. E. et al. Lipopolysaccharide (LPS)-binding protein mediates LPS detoxification by chylomicrons. J. Immunol. 170, 1399–1405 (2003).
Vreugdenhil, A. C., Snoek, A. M., van ′t Veer, C., Greve, J. W. & Buurman, W. A. LPS-binding protein circulates in association with apoB-containing lipoproteins and enhances endotoxin-LDL/VLDL interaction. J. Clin. Invest. 107, 225–234 (2001).
Berbée, J. F. P., Havekes, L. M. & Rensen, P. C. N. Apolipoproteins modulate the inflammatory response to lipopolysaccharide. J. Endotoxin Res. 11, 97–103 (2005).
Yao, Z. et al. Blood-borne lipopolysaccharide is rapidly eliminated by liver sinusoidal endothelial cells via high-density lipoprotein. J. Immunol. 197, 2390–2399 (2016).
Topchiy, E. et al. Lipopolysaccharide is cleared from the circulation by hepatocytes via the low-density lipoprotein receptor. PLoS ONE 11, e0155030 (2016).
Deng, M. et al. The endotoxin delivery protein HMGB1 mediates caspase-11-dependent lethality in sepsis. Immunity 49, 740–753.e7 (2018).
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117 (2011).
Lamkanfi, M. et al. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J. Immunol. 185, 4385–4392 (2010).
Kayagaki, N. et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature 591, 131–136 (2021).
Minihane, A. M. et al. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br. J. Nutr. 114, 999–1012 (2015).
Glaros, T. G. et al. Causes and consequences of low-grade endotoxemia and inflammatory diseases. Front. Biosci. 5, 754–765 (2013).
Geng, S. et al. The persistence of low-grade inflammatory monocytes contributes to aggravated atherosclerosis. Nat. Commun. 7, 13436 (2016).
Pasternak, B. A. et al. Lipopolysaccharide exposure is linked to activation of the acute phase response and growth failure in pediatric Crohn’s disease and murine colitis. Inflamm. Bowel Dis. 16, 856–869 (2010).
Acknowledgements
We thank V. Dixit and K. Fitzgerald for Casp11−/− mice and N. Frank for the HEK-Blue TLR4 reporter cell line. This work was supported by the National Institutes of Health grant nos. R01AI119015 and R01AI148491 (V.A.R.) and R01AI132850 (S.K.V.). V.F.-Á., I.R. and M.B. were supported by the Deutsche Forschungsgemeinschaft (German Research Foundation) under Germany’s Excellence Strategy (EXC 2051: Balance of the Microverse, project number 390713860). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
V.A.R., P.K. and S.K.V. conceived the study. V.A.R., P.K., S.O.V. and S.K.V. designed the experiments and wrote the paper. P.K., S.O.V., A.J.R., S.S.W., V.F.-Á., D.K., E.R.J., I.R., M.B. and J.S.P. performed experiments, analysed data or provided technical or conceptual help. K.F. and A.S. provided biotin–lipid A, and Y.Z. provided EV mimic liposomes.
Corresponding author
Ethics declarations
Competing interests
Y.Z.’s company FormuMax is interested in the sales of exosome-mimicking liposomes. The other authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks Xuetao Cao, Raghu Kalluri and Rongbin Zhou for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Host-derived EVs bind LPS in vivo.
a, Plasma EV levels in wild-type (WT) mice injected with PBS or LPS (10 mg/kg) for indicated times as assessed by nanoparticle tracking analysis (NTA). b, Size distribution of EVs isolated from the plasma of WT mice injected with PBS or LPS (25 mg/kg) 1.5 h post-injection via OptiPrep density gradient method as assessed by NTA. c, Immunoblotting analysis of EVs isolated from the plasma of PBS- or LPS-injected WT mice as in (b). d, Negative staining TEM of EVs isolated from the plasma of PBS or LPS-injected WT mice as in (b). e, LPS content of the EVs isolated from WT mice injected with PBS or LPS as assessed by the LAL assay (n = 4). f,g, Percentage of FITC-LPS+ve EVs (f) and FITC histogram and mean fluorescence intensity (MFI) of EVs (g) isolated from mice injected with PBS or FITC-LPS (25 mg/kg) 1.5 h post-injection as assessed by ImageStream flow cytometry (n = 3). h, Immunoblotting analysis of EVs isolated by the DIC method using CD9, CD63, and CD81 antibodies-coated magnetic beads from the plasma of PBS- or FITC-LPS (25 mg/kg)-injected WT mice. i,j, LPS content of the DIC-isolated EVs from PBS- or FITC-LPS-injected mice 1.5 h post-injection as assessed by the LAL (n = 3) (i) and HEK-Blue TLR4 reporter cell (n = 3) (j) assays. k,l, Percentage of FITC-LPS+ve EVs (k) and FITC histogram and mean fluorescence intensity (MFI) of EVs (l) isolated by the DIC method from mice injected with PBS or FITC-LPS as assessed by flow cytometry (n = 3). Combined data from two (a) or three (e,f,g,i,j,k,l) or representative data of two (b,c,d,h) independent experiments are shown. Data are shown as mean±s.e.m (a). Each circle represents a mouse, and the horizontal lines represent mean (a, e–g and i–l). P values were determined by one-way ANOVA with Dunnett’s post- test (a) or unpaired two-tailed t-test (e–g and i–l). Scale bar, 50 nm (d).
Extended Data Fig. 2 Microvesicles bind LPS in vivo.
(a–b) Percentage of FITC-LPS+ve microvesicles (MVs; annexin A1+ve) and LPS content of MVs isolated from Casp11−/− mice injected with PBS or FITC-LPS via ultracentrifugation as assessed by ImageStream flow cytometry (a) and the LAL assay (b). Each circle represents pooled sample from two mice. Combined data from two independent experiments are shown.
Extended Data Fig. 3 Plasma EVs bind LPS independent of blood components.
a, LPS content of the EVs isolated by the SEC method from the plasma incubated with PBS or FITC-LPS (500 μg) at 37 °C for 45 min as assessed by the LAL assay (n = 3). b,c, Percentage of FITC-LPS+ve EVs (b) and FITC histogram and mean fluorescence intensity (MFI) of EVs (c) isolated by the SEC method from the plasma incubated with PBS or FITC-LPS in vitro as assessed by ImageStream flow cytometry (n = 3). d–f and h–j, EVs isolated from the plasma by the SEC method were incubated with PBS, FITC-LPS, or biotin-LPS as indicated at 37 °C for 45 min in vitro, and the LPS binding was assessed by the LAL assay (n = 3 or 6 as indicated) (d,h) and ImageStream flow cytometry (n = 3 or 6 as indicated) (e,f,i,j). g, TEM of EVs isolated and treated as described above and stained with anti-FITC-gold particles. Arrows indicate LPS. Combined data from three (a–c and h–j) or six (d–f) experiments or one representative of two experiments (g) are shown. Each circle represents a mouse, and the horizontal lines represent the mean in a–f and h–j. P values were determined by unpaired two-tailed t-test. Scale bar, 50 nm (g).
Extended Data Fig. 4 Human plasma EVs and endothelial and epithelial cell EVs bind LPS.
a–h, EVs isolated from the human plasma by the SEC method were incubated with PBS, FITC-LPS or biotin-LPS as indicated at 37 °C for 45 min, and the LPS binding was assessed by the LAL (a,e) and HEK-Blue TLR4 reporter cell (b,f) assays and ImageStream flow cytometry (c,d,g,h) (n = 3). i–p, EVs isolated from bEnd.3 endothelial (i–l) and HeLa (m–p) cells cultured in serum-free conditions by the UC method were incubated with PBS or FITC-LPS (500 μg) as indicated at 37 °C for 45 min followed by re-purification via SEC, and the LPS binding was assessed by the LAL (i,m) and HEK-Blue TLR4 reporter cell (j,n) assays and ImageStream flow cytometry (k,l,o,p) (n = 3). Combined data from three experiments (a–p) are shown. Each circle represents an independent experiment, and the horizontal lines represent the mean. P values were determined by unpaired two-tailed t-test (a–p).
Extended Data Fig. 5 Intracellular localization of LPS is EV-dependent.
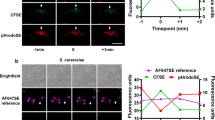
(a–d) Confocal microscopy of Casp11−/− iBMDMs stimulated for 4 h with unlabeled EVs (a), FITC-LPS (b), FITC-LPS-EVs (c), or CellBrite Steady Membrane 488-labeled-EVs (d) and stained with anti-CD45, anti-EEA1 and anti-FITC antibodies to visualize plasma membrane, early endosomes and LPS, respectively. Arrows indicate intracellular localization of EV-bound FITC-LPS (c) or CellBrite 488-labeled-EVs (d). Images representative of two experiments are shown. Scale bar: 5 μm.
Extended Data Fig. 6 LPS-laden host EVs induce endolysosomal membrane disruption.
Confocal images of WT BMDMs stimulated with LPS or LPS-EVs for 6 h and stained for galectin-3 (red), lamp1 (yellow; a), and Rab5 (blue; b). Scale bar: 5 μM. White arrows indicate the colocalization of galectin-3 and lamp1 or Rab5. Images representative of two experiments are shown.
Extended Data Fig. 7 EV-bound LPS activates the noncanonical inflammasome.
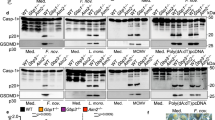
a,b, GSDMD and caspase-4 in lysates (a) and cell death (b) of IFN-γ-primed (10 ng/ml) THP1 monocytes stimulated as indicated for 16 h. c,d, Cleaved IL-1β (p17) and caspase-1 (p20) in the supernatants and indicated proteins in lysates of (c) and IL-1β secretion (d) by IFN-γ-primed WT and Casp11−/− BMDMs stimulated as indicated for 16 h. e–h, GSDMD, caspase-11 and β-actin in lysates (e,g) and cell death (f,h) of IFN-γ-primed indicated BMDMs stimulated as indicated for 16 h. i,j, Indicated proteins in lysates (i) and cell death (j) of IFN-γ-primed RAW macrophages stimulated as indicated for 16 h. k, IL-6 and TNF secretion by indicated BMDMs stimulated with PBS-EVs, LPS-EVs (2.5 μg LPS/ml) or LPS (2.5 μg/ml) for 16 h. l, Plasma IL-6 and TNF in indicated mice administered with LPS-EVs for 6 h (n = 6). m, Survival of poly(I:C)-primed WT and Tlr4−/− mice injected with LPS-EVs (n = 6). n, Baseline IL-1β and IL-18 in the plasma of WT mice injected with DMSO (vehicle) or GW4869 (2.5 μg/g) twice 24 h apart. DMSO-treated LPS-injected mice are shown as controls (n = 6). o, Survival of Tlr4−/− mice injected with DMSO or GW4869 (2.5 μg/g) on days 1–3, primed with poly(I:C) on day 3 and injected with LPS (25 μg/g) 6 h later (n = 5). p, Survival of indicated mice injected with DMSO or GW4869 (2.5 μg/g) on days 1–3 and injected with LPS (25 μg/g) on day 3 (n = 6). q–s, GSDMD, caspase-11 and β-actin in the liver and spleen (q) and IL-18 and IL-1β in the plasma (r,s; n = 6) of WT mice pretreated with DMSO (2.5 μg/g) or Nexinhib20 (25 μg/g) on days 1 and 2 and injected with LPS (25 μg/g) on day 3 for 8 h. Each circle represents a mouse, and the horizontal lines represent mean (l,n,r,s). Each lane represents a mouse (q). Data are presented as mean±s.e.m (b,d,f,h,j,k). Combined data from two (k–p,r,s) or three (b,d,f,h,j) experiments or one experiment representative of two (a,c,e,g,i,q) are shown. P values were determined by one-way ANOVA with Dunnett’s post-test (b,j,n), two-way ANOVA with Sidak’s post-test (d,f,h,k), unpaired two-tailed t-test (l,r,s) or Mantel-Cox test (m,o,p).
Extended Data Fig. 8 EV binding of LPS is not mediated by common LPS binding proteins.
a–d, LPS binding by EVs preincubated with isotype control, anti-HMGB1 or anti-LBP antibodies (Ab) and incubated with FITC-LPS (500 μg) at 37 °C for 45 min as assessed by ImageStream flow cytometry (a,c) and the HEK-Blue TLR4 reporter cell assay (b,d). Combined data from three experiments are shown, and the horizontal lines represent mean. P values were determined by unpaired two-tailed t-test.
Extended Data Fig. 9 EV binding of LPS is not mediated by EV surface proteins.
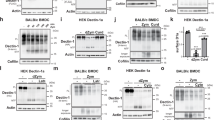
a,b, Immunoblotting (a) and negative staining TEM (b) of unshaved EVs and EVs subjected to surface protein shaving with trypsin (shaved EVs). c,d, Unshaved and shaved EVs incubated with FITC-LPS at 37 °C for 45 min were subjected to NTA (c), and the HEK-Blue TLR4 reporter cell assay (d). e,f, Percentage of FITC-LPS+ve EVs (e) and FITC MFI (f) of EVs isolated from FITC-LPS-injected mice and subjected or not to surface-protein shaving. Combined data from three (c,d) or four (e,f) experiments or one experiment representative of two (a,b) are shown, and horizontal lines represent mean (c–f). P values were determined by unpaired two-tailed t-test.
Extended Data Fig. 10 LPS-bound EVs colocalize with CD14.
a,b, Casp11−/− iBMDMs were stimulated with FITC-LPS-EVs unlabeled (a) or labeled with CellBrite Steady 550 dye (b). After 4 h, cells were stained with anti-CD14, anti-EEA1, and anti-FITC antibodies and subjected to confocal imaging. In (b), light blue arrows indicate colocalization of FITC-LPS-EVs (CellBrite-labeled) with CD14 (top two rows) and their internalization (bottom two rows). Images are representative of two experiments. Scale bar, 5 µm (a,b).
Supplementary information
Supplementary Information
Supplementary Fig. 1.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 1
Uncropped western blots.
Source Data Fig. 2
Uncropped western blots.
Source Data Fig. 4
Uncropped western blots.
Source Data Fig. 5
Uncropped western blots.
Source Data Fig. 7
Uncropped western blots.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 1
Uncropped western blots.
Source Data Extended Data Fig. 7
Uncropped western blots.
Source Data Extended Data Fig. 9
Uncropped western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumari, P., Vasudevan, S.O., Russo, A.J. et al. Host extracellular vesicles confer cytosolic access to systemic LPS licensing non-canonical inflammasome sensing and pyroptosis. Nat Cell Biol 25, 1860–1872 (2023). https://doi.org/10.1038/s41556-023-01269-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-023-01269-8
This article is cited by
-
Cytosolic transfer of circulating LPS by EVs
Nature Cell Biology (2023)