Abstract
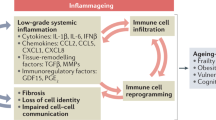
The elucidation of the mechanisms of ageing and the identification of methods to control it have long been anticipated. Recently, two factors associated with ageing—the accumulation of senescent cells and the change in the composition of gut microbiota—have been shown to play key roles in ageing. However, little is known about how these phenomena occur and are related during ageing. Here we show that the persistent presence of commensal bacteria gradually induces cellular senescence in gut germinal centre B cells. Importantly, this reduces both the production and diversity of immunoglobulin A (IgA) antibodies that target gut bacteria, thereby changing the composition of gut microbiota in aged mice. These results have revealed the existence of IgA-mediated crosstalk between the gut microbiota and cellular senescence and thus extend our understanding of the mechanism of gut microbiota changes with age, opening up possibilities for their control.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The SILVA 16S rRNA sequence database (version 138) (https://www.arb-silva.de/) was used for the 16S rRNA gene sequence analysis. scRNA-seq data, IgA repertoire analysis data (the IgA coding region sequence) and microbiome analysis data (the bacterial 16S rRNA gene sequence) generated in this study have been deposited in the DNA Data Bank of Japan (https://www.ddbj.nig.ac.jp) with the accession codes DRA015340, DRA015341 and DRA015346, respectively. In addition, processed data for scRNA-seq have been deposited in the DNA Data Bank of Japan with the accession code E-GEAD-583. Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding author upon reasonable request.
Code availability
The code used for data analysis is publicly available from GitHub (https://github.com/KawamotoShimpei?tab=repositories).
References
DeJong, E. N., Surette, M. G. & Bowdish, D. M. E. The gut microbiota and unhealthy aging: disentangling cause from consequence. Cell Host Microbe 28, 180–189 (2020).
Ghosh, T. S., Shanahan, F. & O’Toole, P. W. The gut microbiome as a modulator of healthy ageing. Nat. Rev. Gastroenterol. Hepatol. 19, 565–584 (2022).
O’Toole, P. W. & Jeffery, I. B. Gut microbiota and aging. Science 350, 1214–1215 (2015).
Wilmanski, T. et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat. Metab. 3, 274–286 (2021).
Fransen, F. et al. Aged gut microbiota contributes to systemical inflammaging after transfer to germ-free mice. Front. Immunol. 8, 1385 (2017).
Smith, P. et al. Regulation of life span by the gut microbiota in the short-lived African turquoise killifish. eLife 6, e27014 (2017).
Boehme, M. et al. Microbiota from young mice counteracts selective age-associated behavioral deficits. Nat. Aging 1, 666–676 (2021).
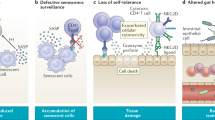
Gorgoulis, V. et al. Cellular senescence: defining a path forward. Cell 179, 813–827 (2019).
He, S. H. & Sharpless, N. E. Senescence in health and disease. Cell 169, 1000–1011 (2017).
Coppe, J. P. et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6, 2853–2868 (2008).
Acosta, J. C. et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133, 1006–1018 (2008).
Kuilman, T. et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133, 1019–1031 (2008).
Krishnamurthty, J. et al. Ink4a/Arf expression is a biomarker of aging. J. Clin. Investig. 114, 1299–1307 (2004).
Yamakoshi, K. et al. Real-time in vivo imaging of p16Ink4a reveals cross talk with p53. J. Cell Biol. 186, 393–407 (2009).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Yoshimoto, S. et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499, 97–101 (2013).
Baker, D. J. et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature 530, 184–189 (2016).
Chan, A. S. L. & Narita, M. Short-term gain, long-term pain: the senescence life cycle and cancer. Genes Dev. 33, 127–143 (2019).
Di Micco, R., Krizhanovsky, V., Baker, D. & di Fagagna, F. D. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 22, 75–95 (2021).
Gasek, N. S., Kuchel, G. A., Kirkland, J. L. & Xu, M. Strategies for targeting senescent cells in human disease. Nat. Aging 1, 870–879 (2021).
Demaria, M. et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31, 722–733 (2014).
Grosse, L. et al. Defined p16High senescent cell types are indispensable for mouse healthspan. Cell Metab. 32, 87–99 (2020).
Okumura, S. et al. Gut bacteria identified in colorectal cancer patients promote tumourigenesis via butyrate secretion. Nat. Commun. 12, 5674 (2021).
Serrano, M., Hannon, G. J. & Beach, D. A new regulatory motif in cell-cycle control causing specific-inhibition of cyclin-D/CDK4. Nature 366, 704–707 (1993).
Hara, E. et al. Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol. Cell. Biol. 16, 859–867 (1996).
Gil, J. & Peters, G. Regulation of the INK4b–ARF–INK4a tumour suppressor locus: all for one or one for all. Nat. Rev. Mol. Cell Biol. 7, 667–677 (2006).
Morgan, D. & Tergaonkar, V. Unraveling B cell trajectories at single cell resolution. Trends Immunol. 43, 210–229 (2022).
Aloisi, F. & Pujol-Borrell, R. Lymphoid neogenesis in chronic inflammatory diseases. Nat. Rev. Immunol. 6, 205–217 (2006).
McDonald, K. G., Leach, M. R., Huang, C., Wang, C. & Newberry, R. D. Aging impacts isolated lymphoid follicle development and function. Immun. Ageing 8, 1 (2011).
Sutherland, D. B., Suzuki, K. & Fagarasan, S. Fostering of advanced mutualism with gut microbiota by immunoglobulin A. Immunol. Rev. 270, 20–31 (2016).
Peterson, D. A., McNulty, N. P., Guruge, J. L. & Gordon, J. I. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe 2, 328–339 (2007).
Macpherson, A. J., Yilmaz, B., Limenitakis, J. P. & Ganal-Vonarburg, S. C. IgA function in relation to the intestinal microbiota. Annu. Rev. Immunol. 36, 359–381 (2018).
Stebegg, M. et al. Heterochronic faecal transplantation boosts gut germinal centres in aged mice. Nat. Commun. 10, 2443 (2019).
Yatsunenko, T. et al. Human gut microbiome viewed across age and geography. Nature 486, 222–227 (2012).
Kau, A. L. et al. Functional characterization of IgA-targeted bacterial taxa from undernourished Malawian children that produce diet-dependent enteropathy. Sci. Transl. Med. 7, 276ra24 (2015).
Takeuchi, S. et al. Intrinsic cooperation between p16INK4a and p21Waf1/Cip1 in the onset of cellular senescence and tumor suppression in vivo. Cancer Res. 70, 9381–9390 (2010).
Pabst, O. & Slack, E. IgA and the intestinal microbiota: the importance of being specific. Mucosal Immunol. 13, 12–21 (2020).
Liu, Y. et al. Expression of p16INK4a in peripheral blood T-cells is a biomarker of human aging. Aging Cell 8, 439–448 (2009).
Liu, Y. et al. Expression of p16INK4a prevents cancer and promotes aging in lymphocytes. Blood 117, 3257–3267 (2011).
Kawamoto, S. et al. Foxp3+ T cells regulate immunoglobulin A selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity 41, 152–165 (2014).
Bergqvist, P. et al. Re-utilization of germinal centers in multiple Peyer’s patches results in highly synchronized, oligoclonal, and affinity-matured gut IgA responses. Mucosal Immunol. 6, 122–135 (2013).
Thevaranjan, N. et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 21, 455–466 (2017).
Fadlallah, J. et al. Microbial ecology perturbation in human IgA deficiency. Sci. Transl. Med. 10, eaan1217 (2018).
Aghamohammadi, A. et al. IgA deficiency: correlation between clinical and immunological phenotypes. J. Clin. Immunol. 29, 130–136 (2009).
Moll, J. M. et al. Gut microbiota perturbation in IgA deficiency is influenced by IgA-autoantibody status. Gastroenterology 160, 2423–2434 (2021).
Nagaishi, T. et al. Immunoglobulin A-specific deficiency induces spontaneous inflammation specifically in the ileum. Gut 71, 487–496 (2022).
Rei, D. et al. Age-associated gut microbiota impair hippocampus-dependent memory in a vagus-dependent manner. JCI Insight 7, e147700 (2022).
D’Amato, A. et al. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- and neurotransmission-related proteins in young recipients. Microbiome 8, 140 (2020).
Cougnoux, A. et al. Bacterial genotoxin colibactin promotes colon tumour growth by inducing a senescence-associated secretory phenotype. Gut 63, 1932–1942 (2014).
Takeuchi, T. et al. Acetate differentially regulates IgA reactivity to commensal bacteria. Nature 595, 560–564 (2021).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019).
Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Turnbaugh, P. J., Baeckhed, F., Fulton, L. & Gordon, J. I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3, 213–223 (2008).
Konishi, Y. et al. Development and evaluation of a colorectal cancer screening method using machine learning-based gut microbiota analysis. Cancer Med. 11, 3194–3206 (2022).
Rognes, T., Flouri, T., Nichols, B., Quince, C. & Mahe, F. VSEARCH: a versatile open source tool for metagenomics. PeerJ 4, e2584 (2016).
Bokulich, N. A. et al. q2-longitudinal: longitudinal and paired-sample analyses of microbiome data. mSystems 3, e00219-18 (2018).
Estaki, M. et al. QIIME 2 enables comprehensive end-to-end analysis of diverse microbiome data and comparative studies with publicly available data. Curr. Protoc. Bioinformatics 70, e100 (2020).
Planer, J. D. et al. Development of the gut microbiota and mucosal IgA responses in twins and gnotobiotic mice. Nature 534, 263–266 (2016).
Bokulich, N. A. et al. q2-sample-classifier: machine-learning tools for microbiome classification and regression. J. Open Res. Softw. 3, 934 (2018).
Mallick, H. et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput. Biol. 17, e1009442 (2021).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902 (2019).
McGinnis, C. S. et al. MULTI-seq: sample multiplexing for single-cell RNA sequencing using lipid-tagged indices. Nat. Methods 16, 619–626 (2019).
Li, H. et al. Mucosal or systemic microbiota exposures shape the B cell repertoire. Nature 584, 274–278 (2020).
Bolotin, D. A. et al. MiXCR: software for comprehensive adaptive immunity profiling. Nat. Methods 12, 380–381 (2015).
Shugay, M. et al. VDJtools: unifying post-analysis of T cell receptor repertoires. PLoS Comput. Biol. 11, e1004503 (2015).
Acknowledgements
We thank D. Okuzaki and D. Motooka (Osaka University) for performing scRNA-seq analysis. We are grateful to members of the E.H. laboratory for helpful discussion during the preparation of this manuscript. This work was supported in part by grants from the Japan Agency for Medical Research and Development under grant numbers JP21gm5010001h0005, JP22gm1710004h0001, JP22zf0127008h0001 and JP22ama221114h0001 (to E.H.), JP22ama121025 (to K.K. and D.M.S.) and JP22gm1010009h0005 (to N.O.), the Japan Science and Technology Agency under grant numbers JPMJMS2022 (to E.H.) and JPMJER1902 (to S.F.), the Japan Society for the Promotion of Science under grant numbers JP22H00457 (to E.H.), JP20K07446 (to S.K.), JP22H03541 (to S.F.) and JP22H03540 (to N.O.), the Naito Foundation (to S.K.), the Food Science Institute Foundation (to S.F.) and the Mitsubishi Foundation (to E.H.). Some of the aged mice were provided by the Foundation for Biomedical Research and Innovation at Kobe through the National BioResource Project of the Ministry of Education, Culture, Sports, Science and Technology in Japan (to S.K.).
Author information
Authors and Affiliations
Contributions
S.K. and E.H. designed the experiments, analysed the data and wrote the manuscript. S.K. and K.U. performed most of the experiments. N.H., Y.S. and T. Matsudaira helped with the immunostaining analysis. M.S. helped to perform the animal experiments. L.T., Y.K., K.K., T. Matsumoto and W.S. helped with the bioinformatics analysis. N.O. analysed the survival curves of p16/p21 DKO mice. T.A., D.M.S. and S.F. analysed the data. E.H. oversaw the projects.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
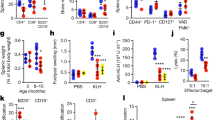
Extended Data Fig. 1 Gut microbiota-dependent accumulation of senescent cells in the ileum of aged female mice.
a-c, Representative images of non-invasive (upper) or ex vivo (lower) BLI of SPF or GF p16-luc mice (female) at 6 or 20 months of age are shown. The colour bars indicate the radiance with minimum and maximum threshold values (a). The bioluminescence intensity emitted from the central abdomen (b) or ileum (c). The areas used for the bioluminescence measurement are shown as the dotted squares (a). The sample size (n) represents the number of biologically independent animals (b, c, n = 5). Data are presented as mean values ± s.e.m. Statistical significance was determined with two-way ANOVA followed by Šídák’s multiple comparisons test (b, c). All experiments were repeated at least twice, independently, with similar results. NS, not significant. Numerical data is available in source data.
Extended Data Fig. 2 Expression of senescence-associated genes in ileal GC B cells of aged mice.
a, UMAP plot gathering all cells from ileal lamina propria from 6 or 20 M SPF or GF p16-luc mice, in which all cells are clustered and color-coded by cell types (upper panel). B cells and activated B cells were further subdivided into three clusters (lower panel). b, UMAP plots showing subdivided B cells collected from 6 M or 20 M SPF or GF p16-luc mice, with Cdkn2a-, Cdkn1a-, Aicda-, Bcl6-, Fas- or Tnfrsf13b-expressing cells represented by red dots. c, Heat map showing differential expression levels of genes classified as SASP factors in these three B-cell clusters classified in the bottom row of panel a. Blue or red intensity indicates a negative or positive z-score, respectively. Note that a series of SASP factors are highly expressed in activated B cells where cells with high expression of Cdkn2a are present.
Extended Data Fig. 3 Establishment of an immunohistochemical staining method for p16INK4a in ageing mice.
a, Immunohistochemical images of isolated lymphoid follicles (ILFs) from the ileum of 15 M WT or p16/p21-DKO mice stained with antibodies against B220 (green) or p16INK4a (red). b, Immunohistochemical images of lungs, mesenteric lymph nodes, spleen, liver, and colon of 3 M or 15 M WT and 15 M p16/21-DKO mice, stained with antibody against p16INK4a (red). Scale bars, 20 μm. All experiments were repeated three times, independently, with similar results.
Extended Data Fig. 4 Accumulation of p16INK4a expressing cells in other tissues with ageing.
a, The bioluminescence imaging with a lowered threshold for the data in Fig. 1a shows an increase in signal with ageing in mesenteric lymph nodes, spleen, liver and colon. The colour bars indicate the radiance with minimum and maximum threshold values. b and c, The bioluminescence intensity emitted from the lungs, mesenteric lymph nodes, spleen, liver, and colon of 6 M or 20 M SPF or GF mice (b) or the expression level of p16INK4a in those tissues (c). The sample size (n) represents the number of biologically independent animals (b, c, n = 5). Data are presented as mean values ± s.e.m. Statistical significance was determined with two-way ANOVA followed by Šídák’s multiple comparisons test (b, c). All experiments were repeated at least twice, independently, with similar results. NS, not significant. Numerical data is available in source data.
Extended Data Fig. 5 Gating strategy of B cells in flow cytometry.
Total lymphocytes isolated from Peyer’s patches or lamina propria of small intestine were gated on a forward scatter (FSC)/side scatter (SSC) plot and then gated on the Zombie-NIR– population to remove dead cells. These cells were further gated for the B cell subsets of interest, namely IgA plasma cells (B220– IgA+), germinal center (GC) B cells (B220+ PNA+ FAS+), or non-GC B cells (B220+ PNA– FAS–). Data were analyzed using FlowJo software, and percentages in the figure represent the frequency in the parent population.
Extended Data Fig. 6 Changes in gut bacterial composition in mice during ageing.
a, An attempt was made to build a model predicting Faith’s phylogenetic diversity (PD) based on various parameters by means of an LME model. Scatter plot of Faith’s PD diversity in each age with linear regression trend lines. Male and female-derived samples are marked in different colours. Of the two parameters ‘Sex’ and ‘Age’, only ‘Age’ was evaluated as a valid parameter (P (>|z|) =0.016*), and finally the equation of LME model was constructed with only ‘Age’ as a valid parameter (P (>|z|) =0.003**). b, Principal Coordinate Analysis (PCoA) plots of the Bray-Curtis distance show changes in gut bacterial composition during ageing process (3, 6, 12, 18, and 24 M). PCoA plots for each age group are shown on the right. The statistical significance judged by PERMANOVA are shown at the top. c, Bar plots showing the phylogenetic composition of the gut microbiota of mice of different ages at genus level. The bacterial genera with statistically significant difference between 3 M and 24 M are marked with an asterisk. Blue (decrease) and red (increase) at 24 M. d, Heat map showing age-related changes of bacterial genera. The degree of blue- or yellow-colour intensity indicates a negative or positive z-score, respectively. The bacterial genera with statistically significant difference between 3 M and 24 M are marked with an asterisk. Blue (decrease) and red (increase) at 24 M. e and f, An age prediction model based on changes in gut microbiota was built using machine learning-based analysis. Scheme of machine learning-based analysis (e). Model building by machine learning and evaluation of model accuracy is repeated 1,000 times (e) and all evaluation results are shown (f). Statistical significance was determined with Wilcoxon matched-paired singed rank test (c, d). * p < 0.05, ** p < 0.01, *** p < 0.005, **** p < 0.001. Numerical data and exact P values are available in source data.
Extended Data Fig. 7 Identification of bacteria with statistically significant changes throughout ageing using cross-nested differential abundance analysis.
Microbiome Multivariable Association with Linear Models (MaAsLin) was used to identify the OTUs that changed statistically significantly with age, considering each random variable (individual, sex, rearing cage, and parent), and 84 OTUs were finally identified as statistically significant OTUs. Heat map showing age-related changes in the 84 OTUs identified as statistically significant bacteria using MaAsLin. The degree of blue- or yellow-colour intensity indicates a negative or positive z-score, respectively. The OTU number and name of the bacterial taxonomy when the corresponding sequence is classified by SILVA are appended to the right-hand side. The OTUs and taxonomies that overlap with the top 50 most important bacteria for building age prediction models for mice using machine learning-based analysis of gut microbiota composition (random forest regression) in Fig. 4d are highlighted by red colour. Note that 35 OTUs (70%) out of the 50 OTUs identified by machine learning overlapped with those identified by MaAsLin.
Extended Data Fig. 8 Survival curves of p16-KO, p21-KO and p16/21-DKO mice.
Survival curves were measured for WT mice (n = 10), p16/p21-DKO mice (n = 19), p16-KO mice (n = 24) and p21-KO mice (n = 13) under SPF environment. Note that p16/p21-DKO, p16-KO and p21-KO mice start to die around 12–15 months of age. In particular, p16/p21-DKO mice showed a severe phenotype, mainly cancer, and all individuals died by 17 months of age.
Extended Data Fig. 9 Graphical model of IgA−mediated crosstalk between the gut microbiota and B cell senescence.
In young mice, GC B cells induced by the gut microbiota are appropriately selected in the PPs and differentiate into IgA plasma cells, secrete sufficient amounts of gut bacteria-specific IgA into the gut lumen to regulate the gut microbiota and maintain symbiosis (left). However, during the ageing process, cellular senescence is induced in the GC B cells of PPs and ILFs by continuous stimulation of the gut microbiota, leading to a decrease in the quantity and quality of IgA produced. This provokes changes in the gut microbiota known as dysbiosis (right).
Supplementary information
Supplementary Tables
Supplementary Table 1. Mouse breeding conditions. Supplementary Table 2. Mouse information used for Fig. 4d. Supplementary Table 3. Primers used in this study.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed western blot.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Unprocessed western blot.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kawamoto, S., Uemura, K., Hori, N. et al. Bacterial induction of B cell senescence promotes age-related changes in the gut microbiota. Nat Cell Biol 25, 865–876 (2023). https://doi.org/10.1038/s41556-023-01145-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-023-01145-5
This article is cited by
-
B cell senescence takes guts
Nature Cell Biology (2023)