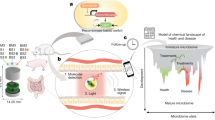
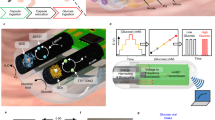
Abstract
Diagnosing and monitoring inflammatory bowel diseases, such as Crohn’s disease, involves the use of endoscopic imaging, biopsies and serology. These infrequent tests cannot, however, identify sudden onsets and severe flare-ups to facilitate early intervention. Hence, about 70% of patients with Crohn’s disease require surgical intestinal resections in their lifetime. Here we report wireless, miniaturized and implantable temperature sensors for the real-time chronic monitoring of disease progression, which we tested for nearly 4 months in a mouse model of Crohn’s-disease-like ileitis. Local measurements of intestinal temperature via intraperitoneally implanted sensors held in place against abdominal muscular tissue via two sutures showed the development of ultradian rhythms at approximately 5 weeks before the visual emergence of inflammatory skip lesions. The ultradian rhythms showed correlations with variations in the concentrations of stress hormones and inflammatory cytokines in blood. Decreasing average temperatures over the span of approximately 23 weeks were accompanied by an increasing percentage of inflammatory species in ileal lesions. These miniaturized temperature sensors may aid the early treatment of inflammatory bowel diseases upon the detection of episodic flare-ups.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. Source data for Figs. 2d, 3b and 4f, as well as representative individual histology images used to produce Figs. 4g and 5d, are available with this paper. A larger number of additional individual histology images used to produce Figs. 4g and 5d are available from the corresponding authors on reasonable request. Source data are provided with this paper.
Code availability
Data analysis (spline fits, FFT, wavelet transform, peak/valley analysis, moving standard deviation) made use of inbuilt functions in MATLAB. All parameters used for analysis are available in Methods.
References
Jairath, V. & Feagan, B. G. Global burden of inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 5, 2–3 (2020).
Kaplan, G. G. & Windsor, J. W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 18, 56–66 (2021).
Kamm, M. A. Rapid changes in epidemiology of inflammatory bowel disease. Lancet 390, 2741–2742 (2017).
Khor, B., Gardet, A. & Xavier, R. J. Genetics and pathogenesis of inflammatory bowel disease. Nature 474, 307–317 (2011).
Torres, J., Mehandru, S., Colombel, J. F. & Peyrin-Biroulet, L. Crohn’s disease. Lancet 389, 1741–1755 (2017).
Santos, M. P. C., Gomes, C. & Torres, J. Familial and ethnic risk in inflammatory bowel disease. Ann. Gastroenterol. 31, 14–23 (2018).
Goertz, R. S., Hensel, S., Wildner, D., Neurath, M. F. & Strobel, D. Bowel wall thickening and hyperemia assessed by high-frequency ultrasound indicate histological inflammation in Crohn’s ileitis. Abdom. Radiol. 46, 1855–1863 (2021).
Brown, E. & Taylor, C. T. Hypoxia-sensitive pathways in intestinal inflammation. J. Physiol. 596, 2985–2989 (2018).
Li, J. et al. Dynamic role of macrophage CX3CR1 expression in inflammatory bowel disease. Immunol. Lett. 232, 39–44 (2021).
Cushing, K. & Higgins, P. D. R. Management of Crohn disease: a review. JAMA 325, 69–80 (2021).
Xiong, S. et al. Reverse translation approach generates a signature of penetrating fibrosis in Crohn’s disease that is associated with anti-TNF response. Gut 71, 1289–1301 (2022).
Hyun, J. G. et al. Anti-interferon-inducible chemokine, CXCL10, reduces colitis by impairing T helper-1 induction and recruitment in mice. Inflamm. Bowel Dis. 11, 799–805 (2005).
Burgmann, T. et al. The Manitoba Inflammatory Bowel Disease Cohort Study: prolonged symptoms before diagnosis—how much is irritable bowel syndrome? Clin. Gastroenterol. Hepatol. 4, 614–620 (2006).
Guglielmo, F. F. et al. Small bowel Crohn disease at CT and MR enterography: imaging atlas and glossary of terms. Radiographics 40, 354–375 (2020).
Calabrese, E., Zorzi, F. & Pallone, F. Ultrasound of the small bowel in Crohn’s disease. Int J. Inflamm. 2012, 964720 (2012).
Stenczel, N. D., Purcarea, M. R., Tribus, L. C. & Oniga, G. H. The role of the intestinal ultrasound in Crohn’s disease diagnosis and monitoring. J. Med. Life 14, 310–315 (2021).
Yin, J. et al. The role of hypoxia-inducible factor 1-alpha in inflammatory bowel disease. Cell Biol. Int. 46, 46–51 (2022).
Pizarro, T. T. et al. SAMP1/YitFc mouse strain: a spontaneous model of Crohn’s disease-like ileitis. Inflamm. Bowel Dis. 17, 2566–2584 (2011).
Bury, M. I. et al. Self-assembling nanofibers inhibit inflammation in a murine model of Crohn’s-disease-like ileitis. Adv. Therap. 4, 2000274 (2021).
Lucke, S. et al. Acute and chronic local inflammatory reaction after implantation of different extracellular porcine dermis collagen matrices in rats. BioMed. Res. Int. 2015, 938059 (2015).
Schwerdt, H. N. et al. Long-term dopamine neurochemical monitoring in primates. Proc. Natl Acad. Sci. USA 114, 13260–13265 (2017).
Chen, S. L. et al. The gut microbiota regulates acute foreign body reaction and tissue repair after biomaterial implantation. Biomaterials 289, 121807 (2022).
Ellison, G. W., Case, J. B. & Regier, P. J. Intestinal surgery in small animals: historical foundations, current thinking, and future horizons. Vet. Surg. 48, 1171–1180 (2019).
Doloff, J. C. et al. The surface topography of silicone breast implants mediates the foreign body response in mice, rabbits and humans. Nat. Biomed. Eng. 5, 1115–1130 (2021).
Park, G. et al. Immunologic and tissue biocompatibility of flexible/stretchable electronics and optoelectronics. Adv. Health. Mater. 3, 515–525 (2014).
Matsuzaki, K. et al. In vivo demonstration of T lymphocyte migration and amelioration of ileitis in intestinal mucosa of SAMP1/Yit mice by the inhibition of MAdCAM-1. Clin. Exp. Immunol. 140, 22–31 (2005).
Lamont, E. W. & Amir, S. in Encyclopedia of Behavioral Neuroscience (eds Koob, G. F., Le Moal, M. & Thompson, R. F.) 257–261 (Academic Press, 2010).
Goh, G. H., Maloney, S. K., Mark, P. J. & Blache, D. Episodic ultradian events—ultradian rhythms. Biology 8 doi, 10.3390/biology8010015 (2019).
Smolensky, M. H., Hermida, R. C., Portaluppi, F., Haus, E. & Reinberg, A. in Hypertension 2nd edn (eds Oparil, S. & Weber, M.A.) 530–542 (W.B. Saunders, 2005).
Arts, L. P. A. & van den Broek, E. L. The fast continuous wavelet transformation (fCWT) for real-time, high-quality, noise-resistant time-frequency analysis. Nat. Comput Sci. 2, 47–58 (2022).
Lou, H. & Ye, Z. [HRV signal analysis based on wavelet transform]. Sheng Wu Yi Xue Gong. Cheng Xue Za Zhi 23, 21–24 (2006).
Addison, P. S. Wavelet transforms and the ECG: a review. Physiol. Meas. 26, R155–R199 (2005).
Rankin, G. B. Extraintestinal and systemic manifestations of inflammatory bowel disease. Med Clin. North Am. 74, 39–50 (1990).
Vavricka, S. R. et al. Extraintestinal manifestations of inflammatory bowel disease. Inflamm. Bowel Dis. 21, 1982–1992 (2015).
Gordon, C. J. The mouse thermoregulatory system: its impact on translating biomedical data to humans. Physiol. Behav. 179, 55–66 (2017).
Aschoff, J. Thermal conductance in mammals and birds—its dependence on body size and circadian phase. Comp. Biochem Phys. A 69, 611–619 (1981).
Oh, S. et al. Simple, miniaturized biosensors for wireless mapping of thermoregulatory responses. Biosens. Bioelectron. 237, 115545 (2023).
Atreya, R. et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat. Med. 6, 583–588 (2000).
Atreya, R. et al. In vivo imaging using fluorescent antibodies to tumor necrosis factor predicts therapeutic response in Crohn’s disease. Nat. Med. 20, 313–318 (2014).
Langer, V. et al. IFN-gamma drives inflammatory bowel disease pathogenesis through VE-cadherin-directed vascular barrier disruption. J. Clin. Invest. 129, 4691–4707 (2019).
Bouma, G. & Strober, W. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 3, 521–533 (2003).
Mao, L., Kitani, A., Strober, W. & Fuss, I. J. The role of NLRP3 and IL-1beta in the pathogenesis of inflammatory bowel disease. Front. Immunol. 9, 2566 (2018).
Liu, S., Russo, P. A., Baldassano, R. N. & Sullivan, K. E. CD68 expression is markedly different in Crohn’s disease and the colitis associated with chronic granulomatous disease. Inflamm. Bowel Dis. 15, 1213–1217 (2009).
Friedrich, M. et al. IL-1-driven stromal-neutrophil interactions define a subset of patients with inflammatory bowel disease that does not respond to therapies. Nat. Med. 27, 1970–1981 (2021).
Padmanabhan, P., Grosse, J., Asad, A. B., Radda, G. K. & Golay, X. Gastrointestinal transit measurements in mice with 99mTc-DTPA-labeled activated charcoal using NanoSPECT-CT. EJNMMI Res. 3, 60 (2013).
Rahman, M. M., Afroz, S., Arthur, S. & Sundaram, U. Mast cell mediated regulation of small intestinal chloride malabsorption in SAMP1/YitFc mouse model of spontaneous chronic ileitis. Cells https://doi.org/10.3390/cells10030697 (2021).
Gracie, D. J., Hamlin, P. J. & Ford, A. C. The influence of the brain–gut axis in inflammatory bowel disease and possible implications for treatment. Lancet Gastroenterol. Hepatol. 4, 632–642 (2019).
Carabotti, M., Scirocco, A., Maselli, M. A. & Severi, C. The gut–brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 28, 203–209 (2015).
Farhadi, A. et al. Heightened responses to stressors in patients with inflammatory bowel disease. Am. J. Gastroenterol. 100, 1796–1804 (2005).
Vidrich, A. et al. Altered epithelial cell lineage allocation and global expansion of the crypt epithelial stem cell population are associated with ileitis in SAMP1/YitFc mice. Am. J. Pathol. 166, 1055–1067 (2005).
Rivera-Nieves, J. et al. Emergence of perianal fistulizing disease in the SAMP1/YitFc mouse, a spontaneous model of chronic ileitis. Gastroenterology 124, 972–982 (2003).
Daynes, R. A. & Jones, D. C. Emerging roles of PPARs in inflammation and immunity. Nat. Rev. Immunol. 2, 748–759 (2002).
Wang, N. et al. Vascular PPARgamma controls circadian variation in blood pressure and heart rate through Bmal1. Cell Metab. 8, 482–491 (2008).
Berger, J. P., Akiyama, T. E. & Meinke, P. T. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol. Sci. 26, 244–251 (2005).
Evans, R. M., Barish, G. D. & Wang, Y. X. PPARs and the complex journey to obesity. Nat. Med. 10, 355–361 (2004).
Sugawara, K. et al. Linkage to peroxisome proliferator-activated receptor-gamma in SAMP1/YitFc mice and in human Crohn’s disease. Gastroenterology 128, 351–360 (2005).
Baumgart, D. C. & Sandborn, W. J. Crohn’s disease. Lancet 380, 1590–1605 (2012).
Favazzo, L. J. et al. The gut microbiome-joint connection: implications in osteoarthritis. Curr. Opin. Rheumatol. 32, 92–101 (2020).
Arora, V. et al. Gut-microbiota modulation: the impact of the gut-microbiota on osteoarthritis. Gene 785, 145619 (2021).
Sharma, S. et al. Location-aware ingestible microdevices for wireless monitoring of gastrointestinal dynamics. Nat. Electron. 6, 242–256 (2023).
Tu, J. B. et al. A wireless patch for the monitoring of C-reactive protein in sweat. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-023-01059-5 (2023).
Wagatsuma, K., Yokoyama, Y. & Nakase, H. Role of biomarkers in the diagnosis and treatment of inflammatory bowel disease. Life https://doi.org/10.3390/life11121375 (2021).
Chen, P. et al. Serum biomarkers for inflammatory bowel disease. Front. Med. 7, 123 (2020).
Winogrodzki, T. et al. TNF DeltaARE pigs: a translational Crohn’s disease model. J. Crohns Colitis 17, 1128–1138 (2023).
Wanner, S. P., Costa, K. A., Soares, A. D., Cardoso, V. N. & Coimbra, C. C. Physical exercise-induced changes in the core body temperature of mice depend more on ambient temperature than on exercise protocol or intensity. Int. J. Biometeorol. 58, 1077–1085 (2014).
Madhvapathy, S. R. et al. Implantable bioelectronic systems for early detection of kidney transplant rejection. Science 381, 1105–1112 (2023).
Zhang, L. N. et al. Physiological and behavioral responses to intermittent starvation in C57BL/6J mice. Physiol. Behav. 105, 376–387 (2012).
Severinsen, T. & Munch, I. C. Body core temperature during food restriction in rats. Acta Physiol. Scand. 165, 299–305 (1999).
Kararli, T. T. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm. Drug Dispos. 16, 351–380 (1995).
Casteleyn, C., Rekecki, A., Van der Aa, A., Simoens, P. & Van den Broeck, W. Surface area assessment of the murine intestinal tract as a prerequisite for oral dose translation from mouse to man. Lab. Anim./ 44, 176–183 (2010).
Yang, Q. et al. Photocurable bioresorbable adhesives as functional interfaces between flexible bioelectronic devices and soft biological tissues. Nat. Mater. 20, 1559–1570 (2021).
Hwang, S. W. et al. A physically transient form of silicon electronics. Science 337, 1640–1644 (2012).
Shih, T. C., Yuan, P., Lin, W. L. & Kou, H. S. Analytical analysis of the Pennes bioheat transfer equation with sinusoidal heat flux condition on skin surface. Med. Eng. Phys. 29, 946–953 (2007).
Yang, Y. et al. Wireless multilateral devices for optogenetic studies of individual and social behaviors. Nat. Neurosci. 24, 1035–1045 (2021).
Castellani, M. P., Rioux, T. P., Castellani, J. W., Potter, A. W. & Xu, X. A geometrically accurate 3 dimensional model of human thermoregulation for transient cold and hot environments. Comput. Biol. Med. 138, 104892 (2021).
Acknowledgements
We thank F. Turek, M. Hotz-Vitaterna and K. Summa for useful discussions; M. Seniw (Simpson Querrey Institute, Northwestern University) for the illustrations in Fig. 1c; and H. M. Arafa, D. Ostojich and J.T. Williams for preliminary efforts in microfabrication and near-field communication device prototypes. This work made use of the MatCI Facility supported by the Materials Research Science and Engineering Center (MRSEC) program of the National Science Foundation (NSF) (DMR-1720139) at the Materials Research Center of Northwestern University, and of the micro/nano-fabrication (NUFAB) facility of Northwestern University’s Atomic and Nanoscale Characterization Experimental Center, which has received support from the SHyNE Resource (NSF ECCS-2025633), the International Institute for Nanotechnology and Northwestern’s MRSEC program. S.R.M and J.L.C. disclose support for the research described in this study from the NSF Graduate Research Fellowship Program (NSF DGE-2234667). The work was supported by the Querrey–Simpson Institute for Bioelectronics.
Author information
Authors and Affiliations
Contributions
S.R.M., M.I.B., A.K.S. and J.A.R. conceived the project. S.R.M. designed the device hardware, software and encapsulation; fabricated devices; calibrated devices; conducted video analysis; and performed benchtop testing/characterization (with assistance from J.L.C.) and temperature data analysis. M.I.B. designed the surgical procedure and conducted surgeries, post-operative animal monitoring/care (with assistance from L.W.W.), motility measurements, histology sample preparation (with assistance from L.W.W.), analysis of quantitative histology (with assistance from L.W.W.), radiograph collection, video collection and blood collection/analysis. R.A. and Y.H. helped with thermal, electromagnetic and mechanical-modelling efforts associated with device operation. S.R.M. and M.I.B. performed data visualization. S.R.M., M.I.B., A.K.S. and J.A.R. analysed the data. S.R.M., M.I.B., A.K.S. and J.A.R. wrote the paper. All authors read and provided comments on the paper. J.A.R. and A.K.S. jointly supervised the work.
Corresponding authors
Ethics declarations
Competing interests
J.A.R., A.K.S., S.R.M., and M.I.B. are co-inventors on a patent related to the technology (US Patent App. 63/604,400) described in this work. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Jonathan Cooper, Wei Gao and Taeyoon Lee for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 FEA modeling of the temperature sensitivity of the device to temperature fluctuations in the intestines.
(a) Temperature profile in the sensor over a 5-hour period to reach steady state temperature when the temperature of the intestines increases by 1 °C (black), remains the same 0 °C (red), and decreases by 1 °C (blue) with respect to the body temperature. The core body temperature was fixed at 37 °C. (b) Steady state temperature change (ΔT = Tintestines – Tcore) profile between the surface of the intestines and the outer skin layer in a mouse for the three cases in (a); the distance between the intestines and skin is assumed to be 5 mm and the thickness of the skin layer is 1 mm. (c) FEA model for (a,b). (d) Sinusoidal temperature profile at the surface of the intestines over a 24-hr period and the corresponding temperature profile in the temperature sensors showing excellent agreement by capturing the thermal fluctuations. (e) A 4th order polynomial temperature profile at the surface of the ‘lesion’ intestines over a 24-hr period and the corresponding temperature profile in the temperature sensors showing excellent agreement by capturing the thermal fluctuations. In both (d,e), the distance between the sensor and intestines is 0.75 mm. (f) (Top) Temperature profile in the sensor as the vertical separation distance between the surface of the intestines and sensor increases from 5 mm to 100 mm, modeling the scenario in a human. The temperature in the intestines is modeled as a sinusoidal wave. As the vertical separation distance increases to 50 mm and 100 mm the sensor is unable to pick up the thermal fluctuations in the intestines. (Bottom) Schematic of the FEA model. (g) Temperature profiles in the sensor as a function of time when the vertical distance between the sensor increases from 0.75 mm to 3.75 mm showing that for all cases the sensor can capture the 4th order polynomial profile of the intestines. (h) Temperature profiles in the sensor as a function of time when the horizontal distance, or lateral misalignment, between the sensor increases from 5 mm to 30 mm. For the cases when the sensor is 20 mm and 30 mm away from the edge of the intestines the sensor is unable to capture the thermal fluctuations at the surface of the intestines. (i) (Top) Thermal profiles for the sensor when placed on top of the lesion region (solid red), normal region (solid blue), and between lesion and normal region (solid black). The thermal profiles are a sinusoidal wave for the normal intestine (dashed blue) and 4th order polynomial for the lesion intestine (dashed red). (Bottom) Schematic of the arrangement for the lesion intestines, normal intestines, and the sensor placement. (j) (Top) Steady state temperature limits at the sensor when the intestine is empty (dashed blue) and with fecal matter (dashed red). The black curve shows the thermal profile when fecal matter enters (temperature increases) and leaves (temperature decreases) the intestine over a period of 8 hrs. (Bottom) Schematic of the model showing the difference between the internal part of the intestine for the empty/fecal cases. (k) Surface plot of the steady state temperature in the sensor based on a parametric sweep of the distance between the sensor and intestine (horizontal axis) and the thermal conductivity of the intestines (vertical axis). (l) Transient heat transfer process over a 12-hr process to reach thermal equilibrium in the intestine’s region.
Extended Data Fig. 2 A DSS-induced model of colitis.
Tintestines as a function of time for individual AKR/J mice subject to intermittent administration of (a, b) 1% and (c, d) 3% by volume DSS in the drinking water over 107–110 days. The red shaded regions indicate the time period for which DSS was administered. Animals were killed on day 107 in panels (b, c) and day 110 in panels (a, d). (e, f) Tintestines as a function of time for individual mice subject to 5% by volume of DSS in the drinking water. The red shaded region indicates the time window for which DSS was administered. Animals were euthanized on day 35. The black arrows in all panels indicate a significant drop in Tintestines after administration of DSS, if applicable.
Supplementary information
Supplementary Information
Supplementary figures, tables and references.
Source data
Source Data for Fig. 2
Source data.
Source Data for Fig. 3
Source data.
Source Data for Fig. 4
Source data and images.
Source Data for Fig. 5
Source data and images.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Madhvapathy, S.R., Bury, M.I., Wang, L.W. et al. Miniaturized implantable temperature sensors for the long-term monitoring of chronic intestinal inflammation. Nat. Biomed. Eng (2024). https://doi.org/10.1038/s41551-024-01183-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-024-01183-w