Abstract
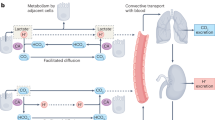
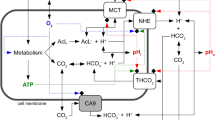
Extracellular pH impacts many molecular, cellular and physiological processes, and hence is tightly regulated. Yet, in tumours, dysregulated cancer cell metabolism and poor vascular perfusion cause the tumour microenvironment to become acidic. Here by leveraging fluorescent pH nanoprobes with a transistor-like activation profile at a pH of 5.3, we show that, in cancer cells, hydronium ions are excreted into a small extracellular region. Such severely polarized acidity (pH <5.3) is primarily caused by the directional co-export of protons and lactate, as we show for a diverse panel of cancer cell types via the genetic knockout or inhibition of monocarboxylate transporters, and also via nanoprobe activation in multiple tumour models in mice. We also observed that such spot acidification in ex vivo stained snap-frozen human squamous cell carcinoma tissue correlated with the expression of monocarboxylate transporters and with the exclusion of cytotoxic T cells. Severely spatially polarized tumour acidity could be leveraged for cancer diagnosis and therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The raw imaging files generated during the study are too large to be publicly shared, yet they are available for research purposes from the corresponding author on reasonable request. De-identified patient-related data are available from the corresponding author, subject to approval from the Institutional Review Board of the University of Texas Southwestern Medical Center. Source data are provided with this paper.
References
Aoi, W. & Marunaka, Y. Importance of pH homeostasis in metabolic health and diseases: crucial role of membrane proton transport. BioMed Res. Int. 2014, 598986 (2014).
Hamm, L. L., Nakhoul, N. & Hering-Smith, K. S. Acid–base homeostasis. Clin. J. Am. Soc. Nephrol. 10, 2232–2242 (2015).
Bonen, A. Lactate transporters (MCT proteins) in heart and skeletal muscles. Med. Sci. Sports Exerc. 32, 778–789 (2000).
Street, D., Bangsbo, J. & Juel, C. Interstitial pH in human skeletal muscle during and after dynamic graded exercise. J. Physiol. 537, 993–998 (2001).
Katsura, K., Ekholm, A., Asplund, B. & Siesjo, B. K. Extracellular pH in the brain during ischemia—relationship to the severity of lactic-acidosis. J. Cereb. Blood Flow. Metab. 11, 597–599 (1991).
Okajima, F. Regulation of inflammation by extracellular acidification and proton-sensing GPCRs. Cell. Signal. 25, 2263–2271 (2013).
Massip-Copiz, M. M. & Santa-Coloma, T. A. Extracellular pH and lung infections in cystic fibrosis. Eur. J. Cell Biol. 97, 402–410 (2018).
Boedtkjer, E. & Pedersen, S. F. The acidic tumor microenvironment as a driver of cancer. Annu. Rev. Physiol. 82, 103–126 (2020).
Corbet, C. & Feron, O. Tumour acidosis: from the passenger to the driver’s seat. Nat. Rev. Cancer 17, 577–593 (2017).
Cardone, R. A., Casavola, V. & Reshkin, S. J. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat. Rev. Cancer 5, 786–795 (2005).
Peppicelli, S., Bianchini, F. & Calorini, L. Extracellular acidity, a ‘reappreciated’ trait of tumor environment driving malignancy: perspectives in diagnosis and therapy. Cancer Metastasis Rev. 33, 823–832 (2014).
Huber, V. et al. Cancer acidity: an ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin. Cancer Biol. 43, 74–89 (2017).
Voegtlin, C. & Kahler, H. The estimation of the hydrogen-ion concentration of the tissues in living animals. Science 75, 362–364 (1932).
Volk, T., Jahde, E., Fortmeyer, H. P., Glusenkamp, K. H. & Rajewsky, M. F. pH in human tumor xenografts—effect of intravenous administration of glucose. Br. J. Cancer 68, 492–500 (1993).
Gillies, R. J., Raghunand, N., Karczmar, G. S. & Bhujwalla, Z. M. MRI of the tumor microenvironment. J. Magn. Reson. Imaging 16, 430–450 (2002).
Savic, L. J. et al. Molecular imaging of extracellular tumor pH to reveal effects of locoregional therapy on liver cancer microenvironment. Clin. Cancer Res. 26, 428–438 (2020).
Garcia-Martin, M. L. et al. Mapping extracellular pH in rat brain gliomas in vivo by 1H magnetic resonance spectroscopic imaging: comparison with maps of metabolites. Cancer Res. 61, 6524–6531 (2001).
Garcia-Martin, M. L. et al. High resolution pHe imaging of rat glioma using pH-dependent relaxivity. Magn. Reson. Med. 55, 309–315 (2006).
Ma, X. et al. Ultra-pH-sensitive nanoprobe library with broad pH tunability and fluorescence emissions. J. Am. Chem. Soc. 136, 11085–11092 (2014).
Feng, Q., Wilhelm, J. & Gao, J. Transistor-like ultra-pH-sensitive polymeric nanoparticles. Acc. Chem. Res. 52, 1485–1495 (2019).
Zhao, T. et al. A transistor-like pH nanoprobe for tumour detection and image-guided surgery. Nat. Biomed. Eng. 1, 0006 (2016).
Voskuil, F. J. et al. Exploiting metabolic acidosis in solid cancers using a tumor-agnostic pH-activatable nanoprobe for fluorescence-guided surgery. Nat. Commun. 11, 3257 (2020).
Gallagher, F. A. et al. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature 453, 940–943 (2008).
Wike-Hooley, J. L., van den Berg, A. P., van der Zee, J. & Reinhold, H. S. Human tumour pH and its variation. Eur. J. Cancer Clin. Oncol. 21, 785–791 (1985).
Li, Y. et al. Molecular basis of cooperativity in pH-triggered supramolecular self-assembly. Nat. Commun. 7, 13214 (2016).
Ma, X. et al. Nanotechnology-enabled delivery of NQO1 bioactivatable drugs. J. Drug Target. 23, 672–680 (2015).
Wang, C. et al. A nanobuffer reporter library for fine-scale imaging and perturbation of endocytic organelles. Nat. Commun. 6, 8524 (2015).
Weinberg, R. The Biology of Cancer (Garland Science, 2013).
Hinck, L. & Nathke, I. Changes in cell and tissue organization in cancer of the breast and colon. Curr. Opin. Cell Biol. 26, 87–95 (2014).
Sahai, E. et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 20, 174–186 (2020).
Glabman, R. A., Choyke, P. L. & Sato, N. Cancer-associated fibroblasts: tumorigenicity and targeting for cancer therapy. Cancers 14, 3906 (2022).
Kim, H. S. et al. Systematic identification of molecular subtype-selective vulnerabilities in non-small-cell lung cancer. Cell 155, 552–566 (2013).
Ly, P. et al. Characterization of aneuploid populations with trisomy 7 and 20 derived from diploid human colonic epithelial cells. Neoplasia 13, 348–357 (2011).
Hensley, C. T. et al. Metabolic heterogeneity in human lung tumors. Cell 164, 681–694 (2016).
Polanski, R. et al. Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer. Clin. Cancer Res. 20, 926–937 (2014).
Huang, G. et al. PET imaging of occult tumours by temporal integration of tumour-acidosis signals from pH-sensitive 64Cu-labelled polymers. Nat. Biomed. Eng. 4, 314–324 (2020).
Brand, A. et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK Cells. Cell Metab. 24, 657–671 (2016).
Warburg, O. The metabolism of carcinoma cells. J. Cancer Res. 9, 148–163 (1925).
Anderson, M., Moshnikova, A., Engelman, D. M., Reshetnyak, Y. K. & Andreev, O. A. Probe for the measurement of cell surface pH in vivo and ex vivo. Proc. Natl Acad. Sci. USA 113, 8177–8181 (2016).
Lindeman, L. R. et al. A comparison of exogenous and endogenous CEST MRI methods for evaluating in vivo pH. Magn. Reson. Med. 79, 2766–2772 (2018).
Grashei, M. et al. Simultaneous magnetic resonance imaging of pH, perfusion and renal filtration using hyperpolarized 13C-labelled Z-OMPD. Nat. Commun. 14, 5060 (2023).
Wang, Z. J. et al. Hyperpolarized 13C MRI: state of the art and future directions. Radiology 291, 273–284 (2019).
Vicente-Manzanares, M. & Sanchez-Madrid, F. Cell polarization: a comparative cell biology and immunological view. Dev. Immunol. 7, 51–65 (2000).
Kopnick, A. L., Jansen, A., Geistlinger, K., Epalle, N. H. & Beitz, E. Basigin drives intracellular accumulation of l-lactate by harvesting protons and substrate anions. PLoS ONE 16, e0249110 (2021).
Kirk, P. et al. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 19, 3896–3904 (2000).
Updegraff, B. L. et al. Transmembrane protease TMPRSS11B promotes lung cancer growth by enhancing lactate export and glycolytic metabolism. Cell Rep. 25, 2223 (2018).
Newman, J. Fluorescence image guided surgical resection of solid tumors using the pH-responsive micellar imaging agent pegsitacianine: a summary of an ongoing phase 2 study. WMIC https://www.xcdsystem.com/wmis/program/QSb7b5D/index.cfm?pgid=2585&sid=22758&abid=86473 (2021).
Wagner, P. Detection of residual peritoneal metastases following cytoreductive surgery using the pH-sensitive micellar imaging agent pegsitacianine: an interim review of an ongoing phase 2 study. WMIC https://www.xcdsystem.com/wmis/program/cJi6upE/index.cfm?pgid=2765&sid=25500&abid=93257 (2022).
Feng, Q. et al. 1329 ultra-pH sensitive nanoparticles increase therapeutic index of IL-2-Fc. J. Immunother. Cancer 10, A1380 (2022).
Johnston, R. J. et al. VISTA is an acidic pH-selective ligand for PSGL-1. Nature 574, 565–570 (2019).
Gaggero, S. et al. IL-2 is inactivated by the acidic pH environment of tumors enabling engineering of a pH-selective mutein. Sci. Immunol. 7, eade5686 (2022).
Feng, Q. et al. Lactate increases stemness of CD8+ T cells to augment anti-tumor immunity. Nat. Commun. 13, 4981 (2022).
Rundqvist, H. et al. Cytotoxic T-cells mediate exercise-induced reductions in tumor growth. eLife 9, e59996 (2020).
Kanamala, M., Wilson, W. R., Yang, M., Palmer, B. D. & Wu, Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: a review. Biomaterials 85, 152–167 (2016).
Zhang, L. et al. Exome sequencing of normal and isogenic transformed human colonic epithelial cells (HCECs) reveals novel genes potentially involved in the early stages of colorectal tumorigenesis. BMC Genomics 16, S8 (2015).
Lin, K.-Y. et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen1. Cancer Res. 56, 21–26 (1996).
Ramirez, R. D. et al. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res. 64, 9027–9034 (2004).
Acknowledgements
We thank J. Shay, J. Minna and T.C. Wu for providing cell lines for this study. We thank Z. Wang, S. Li and P. Shaji for the assistance on polymer synthesis and characterization, and W. Li and T. Vo for the in vivo imaging study and in vitro cell culture assays. The study was supported by the National Institutes of Health grants R01CA211930 (to J.G.) and R01CA13291074 (to B.D.S.), the Cancer Prevention Research Institute of Texas grant RP180343 (to J.G.), and the Mendelson-Young endowment in cancer therapeutics (to J.G.).
Author information
Authors and Affiliations
Contributions
J.G. and Q.F. designed the study. Q.F., T.H., A.G., R.P. and G.H. performed the experiments. B.F. prepared the MCT1 and MCT4 knockout HCC827 cell lines. M.C. performed histology analysis. Q.F., A.G. and Z.B. prepared the manuscript. J.G., B.D.S. and R.J.D. supervised the study. J.G., R.J.D. and B.D.S. edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
B.D.S. and J.G. are scientific co-founders and advisors of OncoNano Medicine, Inc. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Alfredo Quinones-Hinojosa and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of the pH-dependent transition from micelle to unimer state of UPS nanoprobes and proposed models for imaging pH in Matrigel with UPS nanoprobes.
a, Schematic illustration of the pH threshold-dependent phase transition from micelle to unimer state. b, Hydrodynamic diameter of UPS nanoprobes as a function of environmental pH. c-e, DLS analysis of three UPS nanoprobes in the micelle state (pH 7.4) and unimer state (pH below the transition points). f, Schematic illustration of two biological models for acid secretion and detection using UPS nanoprobes. g, Schematic illustration of pH gradient generated from proton diffusion with or without buffering effect.
Extended Data Fig. 2 Extracellular acidification rate (ECAR) of HCC827 lung cancer cells and HBEC3-KT cells.
The ECAR analysis was performed with the Agilent Seahorse platform according to the manufacturer’s standard protocol. n = 10-12 biologically independent replicates from one experiment. Assays were repeated for 3 times independently. Data are shown as means ± SEM. P values were determined by two-tail unpaired t-test.
Extended Data Fig. 3 Distribution of UPS nanoprobe in Matrigel and SPEAR formation by a dual functional always-ON/ON-OFF UPS5.3 nanoprobe.
a, Relative fluorescent intensity as a function of pH for the UPS5.3 with ON-OFF channel (TMR) and always-ON channel (Cy5). b, SPEAR imaging of HCC827 cells with UPS5.3 through ON-OFF channel (TMR) and always-ON channel (Cy5). Data are shown as mean ± s.e.m.
Extended Data Fig. 4 Cellular photostability of SPEAR detection using UPS5.3-TMR nanoprobe.
a, Representative confocal images of SPEAR after repeated scans. b, Alterations in UPS5.3-TMR signal within SPEAR zones (ON) and background zones (OFF), along with the ON/OFF signal ratio. c, QP and RP analysis of SPEAR from 60 scans show consistent measurement using ratio of ON/OFF signal (n = 4 replicates). Data are shown as mean ± s.e.m.
Extended Data Fig. 5 Representative cell images from SPEAR imaging.
a, Confocal images of the time course of SPEAR formation with UPS5.3-TMR in HN5 human head/neck cancer cells. b, Confocal images of SPEAR formation from different cancer and normal cell lines with UPS5.3-TMR at physiological glucose condition (5 mM) after 30-min incubation. Confocal images were acquired on a Zeiss laser confocal microscope (LM710 model).
Extended Data Fig. 6 Extracellular acidification rate (ECAR) and lactate secretion of different cell lines.
a, ECAR of HCC827 cells, HCC827 MCT1 knockout and HCC827 MCT4 knockout cells. The ECAR analysis was performed with the Agilent Seahorse platform according to the manufacturer’s standard protocol. b, Lactate secretion from HCC827 cells measured with a NOVA bioanalyzer. n = 10-12 biologically independent replicates from one experiment (a). Assays were repeated for 3 times independently. n = 6 biologically independent replicates from two independent experiments (b). Data are shown as means ± SEM. P value was determined by one-tail, one-way ANOVA.
Extended Data Fig. 7 L-Lactate but not D-lactate suppresses SPEAR formation.
a, L-Lactate secretion of HCC827 cells measured with NOVA bioanalyzer (n = 5). b, Confocal images and quantification of SPEAR of HCC827 cells treated with D-Lactate (40 mM) or L-Lactate (40 mM). Scale bar: 10 μm. n = 10 from two independent experiments. c, ECAR of HCC827 cells treated with D-Lactate (20 mM) or L-Lactate (20 mM). P values were determined by one-tail one-way ANOVA. Data are shown as mean ± s.e.m. (a,c).
Extended Data Fig. 8 In vivo tumor imaging of SPEAR phenotype by UPS5.3 nanoprobe.
a, Treatment regimen for in vivo tumor imaging with UPS5.3. b, Immunohistochemistry staining of MCT1 in HCC827 wild type tumors and HCC827 MCT1 knockout tumors. Scale bar: 10 μm. c, Representative LICOR Pearl images of tumor bearing mice after intravenously injection of UPS5.3. d, Quantification of tumor fluorescence of HCC827 tumors, HCC827 MCT1 knockout tumors and corresponding normal tissues (n = 8). e, Treatment regimen of AZD3965 or lactate, followed by imaging with UPS5.3. f, Quantification of tumor imaging signals of different treatments in HCC827, TC-1 or B16F10 tumor models (n = 4). P values were determined by one-tail one-way ANOVA or t-test. Data are shown as mean ± s.e.m.
Supplementary information
Supplementary Information
Supplementary figures and tables.
Supplementary Video 1
Rotation of a three-dimensional confocal image (Z-stack) of a polarized cap with a pH below 5.3 at 30 min.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, Q., Bennett, Z., Grichuk, A. et al. Severely polarized extracellular acidity around tumour cells. Nat. Biomed. Eng (2024). https://doi.org/10.1038/s41551-024-01178-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-024-01178-7