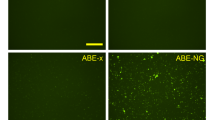
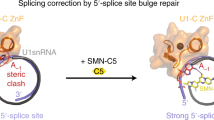
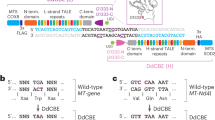
Abstract
Spinal muscular atrophy (SMA) is caused by mutations in SMN1. SMN2 is a paralogous gene with a C•G-to-T•A transition in exon 7, which causes this exon to be skipped in most SMN2 transcripts, and results in low levels of the protein survival motor neuron (SMN). Here we show, in fibroblasts derived from patients with SMA and in a mouse model of SMA that, irrespective of the mutations in SMN1, adenosine base editors can be optimized to target the SMN2 exon-7 mutation or nearby regulatory elements to restore the normal expression of SMN. After optimizing and testing more than 100 guide RNAs and base editors, and leveraging Cas9 variants with high editing fidelity that are tolerant of different protospacer-adjacent motifs, we achieved the reversion of the exon-7 mutation via an A•T-to-G•C edit in up to 99% of fibroblasts, with concomitant increases in the levels of the SMN2 exon-7 transcript and of SMN. Targeting the SMN2 exon-7 mutation via base editing or other CRISPR-based methods may provide long-lasting outcomes to patients with SMA.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Plasmids from this study have been made available through Addgene (https://www.addgene.org/browse/article/28233786/). Primary datasets are available in Supplementary Tables 1, 2, 6 and 7. Sequencing datasets were deposited with the NCBI Sequence Read Archive (PRJNA1014715). The main data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are available for research purposes from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Groen, E. J. N., Talbot, K. & Gillingwater, T. H. Advances in therapy for spinal muscular atrophy: promises and challenges. Nat. Rev. Neurol. 14, 214–224 (2018).
Bergin, A. et al. Identification and characterization of a mouse homologue of the spinal muscular atrophy-determining gene, survival motor neuron. Gene 204, 47–53 (1997).
Ogino, S. & Wilson, R. B. Genetic testing and risk assessment for spinal muscular atrophy (SMA). Hum. Genet. 111, 477–500 (2002).
Crawford, T. O. et al. Evaluation of SMN protein, transcript, and copy number in the biomarkers for spinal muscular atrophy (BforSMA) clinical study. PLoS ONE 7, e33572 (2012).
Mailman, M. D. et al. Molecular analysis of spinal muscular atrophy and modification of the phenotype by SMN2. Genet. Med. 4, 20–26 (2002).
Feldkotter, M., Schwarzer, V., Wirth, R., Wienker, T. F. & Wirth, B. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am. J. Hum. Genet. 70, 358–368 (2002).
Finkel, R. S. et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet 388, 3017–3026 (2016).
Finkel, R. S. et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N. Engl. J. Med. 377, 1723–1732 (2017).
Mercuri, E. et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N. Engl. J. Med. 378, 625–635 (2018).
Sturm, S. et al. A phase 1 healthy male volunteer single escalating dose study of the pharmacokinetics and pharmacodynamics of risdiplam (RG7916, RO7034067), a SMN2 splicing modifier. Br. J. Clin. Pharm. 85, 181–193 (2019).
Darras, B. T. et al. Risdiplam-treated infants with type 1 spinal muscular atrophy versus historical controls. N. Engl. J. Med. 385, 427–435 (2021).
Baranello, G. et al. Risdiplam in type 1 spinal muscular atrophy. N. Engl. J. Med. 384, 915–923 (2021).
Ratni, H., Scalco, R. S. & Stephan, A. H. Risdiplam, the first approved small molecule splicing modifier drug as a blueprint for future transformative medicines. ACS Med. Chem. Lett. 12, 874–877 (2021).
De Vivo, D. C. et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul. Disord. 29, 842–856 (2019).
Hua, Y. et al. Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature 478, 123–126 (2011).
Lipnick, S. L. et al. Systemic nature of spinal muscular atrophy revealed by studying insurance claims. PLoS ONE 14, e0213680 (2019).
Nery, F. C. et al. Impaired kidney structure and function in spinal muscular atrophy. Neurol. Genet. 5, e353 (2019).
Kim, J. K. et al. Muscle-specific SMN reduction reveals motor neuron-independent disease in spinal muscular atrophy models. J. Clin. Invest. 130, 1271–1287 (2020).
Mendell, J. R. et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 377, 1713–1722 (2017).
Thomsen, G. et al. Biodistribution of onasemnogene abeparvovec DNA, mRNA and SMN protein in human tissue. Nat. Med. 27, 1701–1711 (2021).
Alves, C. R. R. et al. Whole blood survival motor neuron protein levels correlate with severity of denervation in spinal muscular atrophy. Muscle Nerve 62, 351–357 (2020).
Van Alstyne, M. et al. Gain of toxic function by long-term AAV9-mediated SMN overexpression in the sensorimotor circuit. Nat. Neurosci. 24, 930–940 (2021).
Zhou, M. et al. Seamless genetic conversion of SMN2 to SMN1 via CRISPR/Cpf1 and single-stranded oligodeoxynucleotides in spinal muscular atrophy patient-specific induced pluripotent stem cells. Hum. Gene Ther. 29, 1252–1263 (2018).
Li, J. J. et al. Disruption of splicing-regulatory elements using CRISPR/Cas9 to rescue spinal muscular atrophy in human iPSCs and mice. Natl Sci. Rev. 7, 92–101 (2020).
Miccio, A., Antoniou, P., Ciura, S. & Kabashi, E. Novel genome-editing-based approaches to treat motor neuron diseases: promises and challenges. Mol. Ther. 30, 47–53 (2022).
Singh, N. N., Howell, M. D., Androphy, E. J. & Singh, R. N. How the discovery of ISS-N1 led to the first medical therapy for spinal muscular atrophy. Gene Ther. 24, 520–526 (2017).
Kosicki, M., Tomberg, K. & Bradley, A. Repair of double-strand breaks induced by CRISPR–Cas9 leads to large deletions and complex rearrangements. Nat. Biotechnol. 36, 765–771 (2018).
Leibowitz, M. L. et al. Chromothripsis as an on-target consequence of CRISPR–Cas9 genome editing. Nat. Genet. 53, 895–905 (2021).
Alanis-Lobato, G. et al. Frequent loss of heterozygosity in CRISPR–Cas9-edited early human embryos. Proc. Natl Acad. Sci. USA 118, e2004832117 (2021).
Enache, O. M. et al. Cas9 activates the p53 pathway and selects for p53-inactivating mutations. Nat. Genet. 52, 662–668 (2020).
Morgens, D. W. et al. Genome-scale measurement of off-target activity using Cas9 toxicity in high-throughput screens. Nat. Commun. 8, 15178 (2017).
Anzalone, A. V., Koblan, L. W. & Liu, D. R. Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 38, 824–844 (2020).
Gaudelli, N. M. et al. Programmable base editing of A*T to G*C in genomic DNA without DNA cleavage. Nature 551, 464–471 (2017).
Komor, A. C., Kim, Y. B., Packer, M. S., Zuris, J. A. & Liu, D. R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424 (2016).
Gaudelli, N. M. et al. Directed evolution of adenine base editors with increased activity and therapeutic application. Nat. Biotechnol. 38, 892–900 (2020).
Richter, M. F. et al. Phage-assisted evolution of an adenine base editor with improved Cas domain compatibility and activity. Nat. Biotechnol. 38, 883–891 (2020).
Walton, R. T., Christie, K. A., Whittaker, M. N. & Kleinstiver, B. P. Unconstrained genome targeting with near-PAMless engineered CRISPR–Cas9 variants. Science 368, 290–296 (2020).
Koblan, L. W. et al. Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat. Biotechnol. 36, 843–846 (2018).
Ma, H. et al. Pol III promoters to express small RNAs: delineation of transcription initiation. Mol. Ther. Nucleic Acids 3, e161 (2014).
Gao, Z., Harwig, A., Berkhout, B. & Herrera-Carrillo, E. Mutation of nucleotides around the +1 position of type 3 polymerase III promoters: the effect on transcriptional activity and start site usage. Transcription 8, 275–287 (2017).
Kim, S., Bae, T., Hwang, J. & Kim, J. S. Rescue of high-specificity Cas9 variants using sgRNAs with matched 5′ nucleotides. Genome Biol. 18, 218 (2017).
Arbab, M. et al. Determinants of base editing outcomes from target library analysis and machine learning. Cell 182, 463–480 e430 (2020).
Kim, N. et al. Deep learning models to predict the editing efficiencies and outcomes of diverse base editors. Nat. Biotechnol. https://doi.org/10.1038/s41587-023-01792-x (2023).
Miller, S. M. et al. Continuous evolution of SpCas9 variants compatible with non-G PAMs. Nat. Biotechnol. 38, 471–481 (2020).
Chatterjee, P. et al. A Cas9 with PAM recognition for adenine dinucleotides. Nat. Commun. 11, 2474 (2020).
Vakulskas, C. A. et al. A high-fidelity Cas9 mutant delivered as a ribonucleoprotein complex enables efficient gene editing in human hematopoietic stem and progenitor cells. Nat. Med. 24, 1216–1224 (2018).
Kleinstiver, B. P. et al. High-fidelity CRISPR–Cas9 nucleases with no detectable genome-wide off-target effects. Nature 529, 490–495 (2016).
Cao, X. et al. Engineering of near-PAMless adenine base editor with enhanced editing activity and reduced off-target. Mol. Ther. Nucleic Acids 28, 732–742 (2022).
Kashima, T., Rao, N. & Manley, J. L. An intronic element contributes to splicing repression in spinal muscular atrophy. Proc. Natl Acad. Sci. USA 104, 3426–3431 (2007).
Singh, N. K., Singh, N. N., Androphy, E. J. & Singh, R. N. Splicing of a critical exon of human survival motor neuron is regulated by a unique silencer element located in the last intron. Mol. Cell. Biol. 26, 1333–1346 (2006).
Cartegni, L., Hastings, M. L., Calarco, J. A., de Stanchina, E. & Krainer, A. R. Determinants of exon 7 splicing in the spinal muscular atrophy genes, SMN1 and SMN2. Am. J. Hum. Genet. 78, 63–77 (2006).
Kashima, T., Rao, N., David, C. J. & Manley, J. L. hnRNP A1 functions with specificity in repression of SMN2 exon 7 splicing. Hum. Mol. Genet. 16, 3149–3159 (2007).
Kashima, T. & Manley, J. L. A negative element in SMN2 exon 7 inhibits splicing in spinal muscular atrophy. Nat. Genet. 34, 460–463 (2003).
Lin, X. et al. Base editing-mediated splicing correction therapy for spinal muscular atrophy. Cell Res. 30, 548–550 (2020).
Kleinstiver, B. P. et al. Broadening the targeting range of Staphylococcus aureus CRISPR–Cas9 by modifying PAM recognition. Nat. Biotechnol. 33, 1293–1298 (2015).
Godena, V. K. & Ning, K. Phosphatase and tensin homologue: a therapeutic target for SMA. Signal Transduct. Target. Ther. 2, 17038 (2017).
Little, D. et al. PTEN depletion decreases disease severity and modestly prolongs survival in a mouse model of spinal muscular atrophy. Mol. Ther. 23, 270–277 (2015).
Bae, S., Park, J. & Kim, J. S. Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 30, 1473–1475 (2014).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Jiang, W., Bikard, D., Cox, D., Zhang, F. & Marraffini, L. A. RNA-guided editing of bacterial genomes using CRISPR–Cas systems. Nat. Biotechnol. 31, 233–239 (2013).
Kleinstiver, B. P. et al. Engineered CRISPR–Cas9 nucleases with altered PAM specificities. Nature 523, 481–485 (2015).
Lazzarotto, C. R. et al. CHANGE-seq reveals genetic and epigenetic effects on CRISPR–Cas9 genome-wide activity. Nat. Biotechnol. 38, 1317–1327 (2020).
Cancellieri, S. et al. Human genetic diversity alters off-target outcomes of therapeutic gene editing. Nat. Genet. 55, 34–43 (2023).
Lek, M. et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285–291 (2016).
Karczewski, K. J. et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581, 434–443 (2020).
Chen, S. et al. A genome-wide mutational constraint map quantified from variation in 76,156 human genomes. Preprint at bioRxiv https://doi.org/10.1101/2022.03.20.485034 (2022).
Koblan, L. W. et al. In vivo base editing rescues Hutchinson–Gilford progeria syndrome in mice. Nature 589, 608–614 (2021).
Levy, J. M. et al. Cytosine and adenine base editing of the brain, liver, retina, heart and skeletal muscle of mice via adeno-associated viruses. Nat. Biomed. Eng. 4, 97–110 (2020).
Hanlon, K. S. et al. Selection of an efficient AAV vector for robust CNS transgene expression. Mol. Ther. Methods Clin. Dev. 15, 320–332 (2019).
Le, T. T. et al. SMNDelta7, the major product of the centromeric survival motor neuron (SMN2) gene, extends survival in mice with spinal muscular atrophy and associates with full-length SMN. Hum. Mol. Genet. 14, 845–857 (2005).
Lutz, C. M. et al. Postsymptomatic restoration of SMN rescues the disease phenotype in a mouse model of severe spinal muscular atrophy. J. Clin. Invest. 121, 3029–3041 (2011).
Kray, K. M., McGovern, V. L., Chugh, D., Arnold, W. D. & Burghes, A. H. M. Dual SMN inducing therapies can rescue survival and motor unit function in symptomatic ∆7SMA mice. Neurobiol. Dis. 159, 105488 (2021).
Banskota, S. et al. Engineered virus-like particles for efficient in vivo delivery of therapeutic proteins. Cell 185, 250–265e216 (2022).
Thomas, N. H. & Dubowitz, V. The natural history of type I (severe) spinal muscular atrophy. Neuromuscul. Disord. 4, 497–502 (1994).
Swoboda, K. J. et al. Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Ann. Neurol. 57, 704–712 (2005).
Yeo, C. J. J. & Darras, B. T. Overturning the paradigm of spinal muscular atrophy as just a motor neuron disease. Pediatr. Neurol. 109, 12–19 (2020).
Donadon, I. et al. Rescue of spinal muscular atrophy mouse models with AAV9-Exon-specific U1 snRNA. Nucleic Acids Res. 47, 7618–7632 (2019).
Thomson, A. K. et al. Survival of motor neurone protein is required for normal postnatal development of the spleen. J. Anat. 230, 337–346 (2017).
Cabrera, A. et al. The sound of silence: transgene silencing in mammalian cell engineering. Cell Syst. 13, 950–973 (2022).
Frangoul, H. et al. CRISPR–Cas9 gene editing for sickle cell disease and beta-Thalassemia. N. Engl. J. Med. 384, 252–260 (2021).
Gillmore, J. D. et al. CRISPR–Cas9 in vivo gene editing for transthyretin amyloidosis. N. Engl. J. Med. 385, 493–502 (2021).
Gao, Y. et al. Systematic characterization of short intronic splicing-regulatory elements in SMN2 pre-mRNA. Nucleic Acids Res. 50, 731–749 (2022).
Goertsen, D. et al. AAV capsid variants with brain-wide transgene expression and decreased liver targeting after intravenous delivery in mouse and marmoset. Nat. Neurosci. 25, 106–115 (2022).
Ravindra Kumar, S. et al. Multiplexed Cre-dependent selection yields systemic AAVs for targeting distinct brain cell types. Nat. Methods 17, 541–550 (2020).
Stanton, A. C. et al. Systemic administration of novel engineered AAV capsids facilitates enhanced transgene expression in the macaque CNS. Med 4, 31–50 e38 (2023).
Rossidis, A. C. et al. In utero CRISPR-mediated therapeutic editing of metabolic genes. Nat. Med. 24, 1513–1518 (2018).
Poirier, A. et al. Risdiplam distributes and increases SMN protein in both the central nervous system and peripheral organs. Pharmacol. Res. Perspect. 6, e00447 (2018).
Zhao, X. et al. Pharmacokinetics, pharmacodynamics, and efficacy of a small-molecule SMN2 splicing modifier in mouse models of spinal muscular atrophy. Hum. Mol. Genet. 25, 1885–1899 (2016).
Passini, M. A. et al. Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Sci. Transl. Med. 3, 72ra18 (2011).
Arbab, M. et al. Base editing rescue of spinal muscular atrophy in cells and in mice. Science 380, eadg6518 (2023).
Vicencio, J. et al. Genome editing in animals with minimal PAM CRISPR–Cas9 enzymes. Nat. Commun. 13, 2601 (2022).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Kleinstiver, B. P. et al. Engineered CRISPR–Cas12a variants with increased activities and improved targeting ranges for gene, epigenetic and base editing. Nat. Biotechnol. 37, 276–282 (2019).
Rohland, N. & Reich, D. Cost-effective, high-throughput DNA sequencing libraries for multiplexed target capture. Genome Res. 22, 939–946 (2012).
Clement, K. et al. CRISPResso2 provides accurate and rapid genome editing sequence analysis. Nat. Biotechnol. 37, 224–226 (2019).
Christie, K. A. et al. Precise DNA cleavage using CRISPR–SpRYgests. Nat. Biotechnol. https://doi.org/10.1038/s41587-022-01492-y (2022).
Untergasser, A. et al. Primer3–new capabilities and interfaces. Nucleic Acids Res. 40, e115 (2012).
Beharry, A. et al. The AAV9 variant capsid AAV-F mediates widespread transgene expression in nonhuman primate spinal cord after intrathecal administration. Hum. Gene Ther. 33, 61–75 (2022).
Reilly, A. et al. Central and peripheral delivered AAV9-SMN are both efficient but target different pathomechanisms in a mouse model of spinal muscular atrophy. Gene Ther. 29, 544–554 (2022).
Acknowledgements
We acknowledge the following individuals: C. Tou (ddPCR), E. King and D. Ramos (AAV and mouse tissue extraction discussions), J. Ferreira da Silva and L. Hille (data analysis), L. Ma and N. Kim (cloning), R. Silverstein (coding and data analysis), H. Stutzman (gRNA cloning) and E. Eichelberger, R. Spellman and W. das Neves (cell culture). We acknowledge M. Mabuchi and G. B. Robb for providing purified SpRY and SpRY-HF1 protein (generated as described previously96). This work was supported by a Charles A. King Trust Postdoctoral Research Fellowship, Bank of America, N.A., Co-Trustees (C.R.R.A.); a James L. and Elisabeth C. Gamble Endowed Fund for Neuroscience Research/Mass General Neuroscience Transformative Scholar Award (C.R.R.A.); an MGH Physician/Scientist Development Award (C.R.R.A.); an MGH Executive Committee on Research Fund for Medical Discovery Fundamental Research Fellowship Award (K.A.C.); a Frederick Banting and Charles Best Canadian Institutes of Health Research Doctoral Research Award (A.R.); a St. Jude Children’s Research Hospital Collaborative Research Consortium on Novel Gene Therapies for Sickle Cell Disease (S.Q.T.); a Muscular Dystrophy Association grant 575466 (R.K.); a Muscular Dystrophy Canada grant (R.K.); a Canadian Institutes of Health Research grant PJT-156379 (R.K.); an MGH Innovation Discovery Grant (to C.R.R.A. and B.P.K.); an MGH Executive Committee on Research Howard M. Goodman Fellowship (B.P.K.); and National Institutes of Health grants R01DC017117 (C.A.M.), U01AI157189 (S.Q.T.), U01AI176471 (S.Q.T.), P01HL142494 (B.P.K.) and DP2CA281401 (B.P.K.). Some aspects of schematics in Fig. 4 and Supplementary Figs. 20 and 21 were adapted from vector art provided by Servier Medical Art by Servier, which is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0).
Author information
Authors and Affiliations
Contributions
C.R.R.A. and B.P.K. conceived of and designed the study. All authors designed, performed or supervised experiments, and/or analysed data. C.R.R.A., L.L.H., K.A.C. and R.M.D. performed cell culture, molecular and biochemical experiments. D.d.l.C and C.A.M. produced AAVs. R.Y., E.R.S., A.B., A.R. and R.K. conducted the in vivo experiments with mice and analysed data. C.R.L. and S.Q.T. designed and performed CHANGE-seq experiments. K.J.S. collected primary human fibroblasts and clinical data. C.R.R.A. and B.P.K. wrote the manuscript with contributions and/or revisions from all authors.
Corresponding authors
Ethics declarations
Competing interests
C.R.R.A., K.A.C., K.J.S. and B.P.K. are inventors on a patent application filed by Mass General Brigham (MGB) that describes genome engineering technologies to treat SMA. S.Q.T. and C.R.L are co-inventors on a patent application describing the CHANGE-seq method. S.Q.T. is a member of the scientific advisory board of Kromatid, Twelve Bio and Prime Medicine. C.A.M. has a financial interest in Sphere Gene Therapeutics, Chameleon Biosciences and Skylark Bio, companies developing gene therapy platforms. C.A.M.’s interests were reviewed and are managed by MGH and MGB in accordance with their conflict-of-interest policies. C.A.M. has a filed patent application with claims involving the AAV-F capsid. B.P.K. is an inventor on additional patents or patent applications filed by MGB that describe genome engineering technologies. B.P.K. is a consultant for EcoR1 capital and is on the scientific advisory board of Acrigen Biosciences, Life Edit Therapeutics and Prime Medicine. S.Q.T. and B.P.K. have financial interests in Prime Medicine, a company developing therapeutic CRISPR–Cas technologies for gene editing. B.P.K.‘s interests were reviewed and are managed by MGH and MGB in accordance with their conflict-of-interest policies. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary notes, figures and references.
Supplementary Table 1
CHANGE-seq data summary.
Supplementary Table 2
Off-target sequencing, and results of statistical tests.
Supplementary Table 3
gRNA target sites.
Supplementary Table 4
Plasmids.
Supplementary Table 5
Oligonucleotides and probes.
Supplementary Table 6
Base-editor data.
Supplementary Table 7
gnomAD output for SMN2.
Supplementary Table 8
Primary datasets (SI).
Source data
Figs. 1–4 and Supplementary figures
Source data for all figures and supplementary figures.
Fig. 2
Unprocessed western blots for Fig. 2e.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alves, C.R.R., Ha, L.L., Yaworski, R. et al. Optimization of base editors for the functional correction of SMN2 as a treatment for spinal muscular atrophy. Nat. Biomed. Eng 8, 118–131 (2024). https://doi.org/10.1038/s41551-023-01132-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-023-01132-z
This article is cited by
-
Crispr-Based Editing of Human Pluripotent Stem Cells for Disease Modeling
Stem Cell Reviews and Reports (2024)
-
Mutation corrections in spinal muscular atrophy
Nature Biomedical Engineering (2023)