Abstract
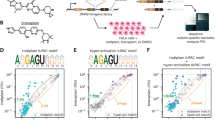
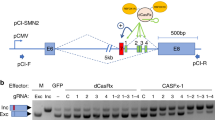
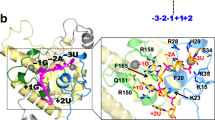
Splicing modifiers promoting SMN2 exon 7 inclusion have the potential to treat spinal muscular atrophy, the leading genetic cause of infantile death. These small molecules are SMN2 exon 7 selective and act during the early stages of spliceosome assembly. Here, we show at atomic resolution how the drug selectively promotes the recognition of the weak 5ʹ splice site of SMN2 exon 7 by U1 snRNP. The solution structure of the RNA duplex formed following 5ʹ splice site recognition in the presence of the splicing modifier revealed that the drug specifically stabilizes a bulged adenine at this exon–intron junction and converts the weak 5ʹ splice site of SMN2 exon 7 into a stronger one. The small molecule acts as a specific splicing enhancer cooperatively with the splicing regulatory network. Our investigations uncovered a novel concept for gene-specific alternative splicing correction that we coined 5ʹ splice site bulge repair.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The chemical shifts and the atomic coordinates of the RNA duplex with or without SMN-C5 have been deposited in the Biological Magnetic Resonance Bank (BMRB ID: 34311 and 34312) and in the Protein Data Bank (PDB ID: 6HMI and 6HMO). Other data and materials are available from the authors upon reasonable request.
References
Pearn, J. Classification of spinal muscular atrophies. Lancet 1, 919–922 (1980).
Paushkin, S., Gubitz, A. K., Massenet, S. & Dreyfuss, G. The SMN complex, an assemblyosome of ribonucleoproteins. Curr. Opin. Cell Biol. 14, 305–312 (2002).
Rochette, C. F., Gilbert, N. & Simard, L. R. SMN gene duplication and the emergence of the SMN2 gene occurred in distinct hominids: SMN2 is unique to Homo sapiens. Hum. Genet. 108, 255–266 (2001).
Cartegni, L. & Krainer, A. R. Disruption of an SF2/ASF-dependent exonic splicing enhancer in SMN2 causes spinal muscular atrophy in the absence of SMN1. Nat. Genet. 30, 377–384 (2002).
Kashima, T. & Manley, J. L. A negative element in SMN2 exon 7 inhibits splicing in spinal muscular atrophy. Nat. Genet. 34, 460–463 (2003).
Burghes, A. H. & Beattie, C. E. Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick? Nat. Rev. Neurosci. 10, 597–609 (2009).
Mailman, M. D. et al. Molecular analysis of spinal muscular atrophy and modification of the phenotype by SMN2. Genet. Med. 4, 20–26 (2002).
Marquis, J. et al. Spinal muscular atrophy: SMN2 pre-mRNA splicing corrected by a U7 snRNA derivative carrying a splicing enhancer sequence. Mol. Ther. 15, 1479–1486 (2007).
Hua, Y., Vickers, T. A., Baker, B. F., Bennett, C. F. & Krainer, A. R. Enhancement of SMN2 exon 7 inclusion by antisense oligonucleotides targeting the exon. PLoS Biol. 5, e73 (2007).
Rogalska, M. E. et al. Therapeutic activity of modified U1 core spliceosomal particles. Nat. Commun. 7, 11168 (2016).
Garcia-Lopez, A. et al. Targeting RNA structure in SMN2 reverses spinal muscular atrophy molecular phenotypes. Nat. Commun. 9, 2032 (2018).
Naryshkin, N. A. et al. Motor neuron disease. SMN2 splicing modifiers improve motor function and longevity in mice with spinal muscular atrophy. Science 345, 688–693 (2014).
Palacino, J. et al. SMN2 splice modulators enhance U1-pre-mRNA association and rescue SMA mice. Nat. Chem. Biol. 11, 511–517 (2015).
Hua, Y. et al. Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature 478, 123–126 (2011).
Hua, Y., Vickers, T. A., Okunola, H. L., Bennett, C. F. & Krainer, A. R. Antisense masking of an hnRNP A1/A2 intronic splicing silencer corrects SMN2 splicing in transgenic mice. Am. J. Hum. Genet. 82, 834–848 (2008).
Beusch, I., Barraud, P., Moursy, A., Clery, A. & Allain, F. H. Tandem hnRNP A1 RNA recognition motifs act in concert to repress the splicing of survival motor neuron exon 7. Elife 6, e25736 (2017).
Hache, M. et al. Intrathecal injections in children with spinal muscular atrophy: nusinersen clinical trial experience. J. Child Neurol. 31, 899–906 (2016).
Foust, K. D. et al. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat. Biotechnol. 28, 271–274 (2010).
Vigevani, L. & Valcarcel, J. A splicing magic bullet. Science 345, 624–625 (2014).
Cully, M. Neuromuscular disorders: beefing up the right splice variant to treat spinal muscular atrophy. Nat. Rev. Drug Discov. 13, 725 (2014).
Mendell, J. R. et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 377, 1713–1722 (2017).
Schneider, R. CIM Journal Club: gene therapy for spinal muscular atrophy Comment on Mendell et al. N Engl J Med 2017;377:1713-22. Clin. Invest. Med. 41, E31–E33 (2018).
Sturm, S. et al. A phase 1 healthy male volunteer single escalating dose study of the pharmacokinetics and pharmacodynamics of risdiplam (RG7916, RO7034067), a SMN2 splicing modifier. Br. J. Clin. Pharm. 85, 181–193 (2019).
Sivaramakrishnan, M. et al. Binding to SMN2 pre-mRNA-protein complex elicits specificity for small molecule splicing modifiers. Nat. Commun. 8, 1476 (2017).
Clery, A. et al. Molecular basis of purine-rich RNA recognition by the human SR-like protein Tra2-beta1. Nat. Struct. Mol. Biol. 18, 443–450 (2011).
Moursy, A., Allain, F. H. & Clery, A. Characterization of the RNA recognition mode of hnRNP G extends its role in SMN2 splicing regulation. Nucleic Acids Res. 42, 6659–6672 (2014).
Hofmann, Y. & Wirth, B. hnRNP-G promotes exon 7 inclusion of survival motor neuron (SMN) via direct interaction with Htra2-beta1. Hum. Mol. Genet. 11, 2037–2049 (2002).
Young, P. J. et al. SRp30c-dependent stimulation of survival motor neuron (SMN) exon 7 inclusion is facilitated by a direct interaction with hTra2 beta 1. Hum. Mol. Genet. 11, 577–587 (2002).
Wang, J., Schultz, P. G. & Johnson, K. A. Mechanistic studies of a small-molecule modulator of SMN2 splicing. Proc. Natl Acad. Sci. USA 115, E4604–E4612 (2018).
Ratni, H. et al. Discovery of risdiplam, a selective survival of motor Neuron-2 (SMN2) gene splicing modifier for the treatment of spinal muscular atrophy (SMA). J. Med. Chem. 61, 6501–6517 (2018).
Pinard, E. et al. Discovery of a novel class of survival motor neuron 2 splicing modifiers for the treatment of spinal muscular atrophy. J. Med. Chem. 60, 4444–4457 (2017).
Pomeranz Krummel, D. A., Oubridge, C., Leung, A. K., Li, J. & Nagai, K. Crystal structure of human spliceosomal U1 snRNP at 5.5 Å resolution. Nature 458, 475–480 (2009).
Kondo, Y., Oubridge, C., van Roon, A. M. & Nagai, K. Crystal structure of human U1 snRNP, a small nuclear ribonucleoprotein particle, reveals the mechanism of 5ʹ splice site recognition. Elife 4, e04986 (2015).
Roca, X. et al. Widespread recognition of 5ʹ splice sites by noncanonical base-pairing to U1 snRNA involving bulged nucleotides. Genes Dev. 26, 1098–1109 (2012).
Roca, X. & Krainer, A. R. Recognition of atypical 5ʹ splice sites by shifted base-pairing to U1 snRNA. Nat. Struct. Mol. Biol. 16, 176–182 (2009).
Singh, N. N., Lee, B. M. & Singh, R. N. Splicing regulation in spinal muscular atrophy by an RNA structure formed by long-distance interactions. Ann. NY Acad. Sci. 1341, 176–187 (2015).
Singh, N. N., Singh, R. N. & Androphy, E. J. Modulating role of RNA structure in alternative splicing of a critical exon in the spinal muscular atrophy genes. Nucleic Acids Res. 35, 371–389 (2007).
Rhode, B. M., Hartmuth, K., Urlaub, H. & Luhrmann, R. Analysis of site-specific protein–RNA cross-links in isolated RNP complexes, combining affinity selection and mass spectrometry. RNA 9, 1542–1551 (2003).
Stefan, M. I. & Le Novere, N. Cooperative binding. PLoS Comput. Biol. 9, e1003106 (2013).
Ehlert, F. J. Estimation of the affinities of allosteric ligands using radioligand binding and pharmacological null methods. Mol. Pharm. 33, 187–194 (1988).
Christopoulos, A. & Kenakin, T. G protein-coupled receptor allosterism and complexing. Pharm. Rev. 54, 323–374 (2002).
Jansen, J. M. et al. Inhibition of prenylated KRAS in a lipid environment. PLoS One 12, e0174706 (2017).
Hammes, G. G., Chang, Y. C. & Oas, T. G. Conformational selection or induced fit: a flux description of reaction mechanism. Proc. Natl Acad. Sci. USA 106, 13737–13741 (2009).
Krawczak, M. et al. Human gene mutation database—a biomedical information and research resource. Hum. Mutat. 15, 45–51 (2000).
Soemedi, R. et al. Pathogenic variants that alter protein code often disrupt splicing. Nat. Genet. 49, 848–855 (2017).
Disney, M. D. Short-circuiting RNA splicing. Nat. Chem. Biol. 4, 723–724 (2008).
O’Brien, K., Matlin, A. J., Lowell, A. M. & Moore, M. J. The biflavonoid isoginkgetin is a general inhibitor of pre-mRNA splicing. J. Biol. Chem. 283, 33147–33154 (2008).
Cretu, C. et al. Structural Basis of Splicing Modulation by Antitumor Macrolide Compounds. Mol. Cell 70, 265–273.e8 (2018).
Charenton, C., Wilkinson, M. E. & Nagai, K. Mechanism of 5ʹ splice site transfer for human spliceosome activation. Science 364, 362–367 (2019).
Ratni, H. et al. Specific correction of alternative survival motor neuron 2 splicing by small molecules: discovery of a potential novel medicine to treat spinal muscular atrophy. J. Med. Chem. 59, 6086–6100 (2016).
Keller, R. The Computer-Aaided Resonance Assignment Tutorial. (Cantina Verlag, 2004).
Binev, Y., Marques, M. M. & Aires-de-Sousa, J. Prediction of 1H NMR coupling constants with associative neural networks trained for chemical shifts. J. Chem. Inf. Model. 47, 2089–2097 (2007).
Guntert, P. Automated NMR structure calculation with CYANA. Methods Mol. Biol. 278, 353–378 (2004).
Case, D. A. et al. The Amber biomolecular simulation programs. J. Comput. Chem. 26, 1668–1688 (2005).
Pettersen, E. F. et al. UCSF chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Acknowledgements
We thank F. Tessaro, A. Gossert and M. Krepl for their assistance with the molecular dynamics setup, K. McCarthy and F. Metzger for useful discussions and the RNA synthesis platform of the NCCR RNA and Diseases. This work was supported by the Swiss National Science Foundation, the NCCR RNA and Diseases, cure-SMA and SMA-Europe.
Author information
Authors and Affiliations
Contributions
S.C., A.C. and F.H.-T.A. designed the research. S.C. and L.G. performed gel shift assays. S.C., S.B. and A.M. performed the splicing assays in cellular models. S.C. and S.R. performed NMR data collection. A.K. and J.H. synthesized the isotopically labeled RNA. H.R. furnished the splicing modifiers. S.C. analyzed the data and solved the structures. S.C. and F.H.-T.A. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
H.R. is an employee of F. Hoffmann-La Roche Ltd.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Table 1 and Supplementary Figs 1–10.
Supplementary Video 1
Solution structure of the apo-RNA duplex.
Supplementary Video 2
Solution structure of the RNA duplex bound to SMN-C5.
Supplementary Video 3
RNA conformational switch induced by SMN-C5—major groove view.
Supplementary Video 4
RNA conformational switch induced by SMN-C5—minor groove view.
Rights and permissions
About this article
Cite this article
Campagne, S., Boigner, S., Rüdisser, S. et al. Structural basis of a small molecule targeting RNA for a specific splicing correction. Nat Chem Biol 15, 1191–1198 (2019). https://doi.org/10.1038/s41589-019-0384-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-019-0384-5
This article is cited by
-
Small molecule approaches to targeting RNA
Nature Reviews Chemistry (2024)
-
Molecular basis of RNA-binding and autoregulation by the cancer-associated splicing factor RBM39
Nature Communications (2023)
-
Regulation of pre-mRNA splicing: roles in physiology and disease, and therapeutic prospects
Nature Reviews Genetics (2023)
-
Therapy development for spinal muscular atrophy: perspectives for muscular dystrophies and neurodegenerative disorders
Neurological Research and Practice (2022)
-
Defining molecular glues with a dual-nanobody cannabidiol sensor
Nature Communications (2022)