Abstract
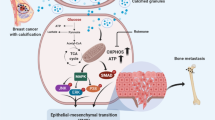
In patients with breast cancer, lower bone mineral density increases the risk of bone metastasis. Although the relationship between bone-matrix mineralization and tumour-cell phenotype in breast cancer is not well understood, mineralization-induced rigidity is thought to drive metastatic progression via increased cell-adhesion forces. Here, by using collagen-based matrices with adjustable intrafibrillar mineralization, we show that, unexpectedly, matrix mineralization dampens integrin-mediated mechanosignalling and induces a less proliferative stem-cell-like phenotype in breast cancer cells. In mice with xenografted decellularized physiological bone matrices seeded with human breast tumour cells, the presence of bone mineral reduced tumour growth and upregulated a gene-expression signature that is associated with longer metastasis-free survival in patients with breast cancer. Our findings suggest that bone-matrix changes in osteogenic niches regulate metastatic progression in breast cancer and that in vitro models of bone metastasis should integrate organic and inorganic matrix components to mimic physiological and pathologic mineralization.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. All RNA-sequencing data generated in this study are available in the NIH Gene Expression Omnibus via the accession number GSE229094. Survival analysis was conducted using publicly available datasets (Study ID: GEO2603, GSE2034). Source data for the figures are provided with this paper.
Code availability
The QuPath script used for immunochemistry analysis is available as Supplementary Information.
References
Hess, K. R. et al. Metastatic patterns in adenocarcinoma. Cancer 106, 1624–1633 (2006).
Kuchuk, I. et al. Incidence, consequences and treatment of bone metastases in breast cancer patients—experience from a single cancer centre. J. Bone Oncol. 2, 137–144 (2013).
Braun, S. et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 353, 793–802 (2005).
Guise, T. A. The vicious cycle of bone metastases. J. Musculoskelet. Neuronal Interact. 2, 570–572 (2002).
Lawson, M. A. et al. Osteoclasts control reactivation of dormant myeloma cells by remodelling the endosteal niche. Nat. Commun. 6, 8983 (2015).
Hüsemann, Y. et al. Systemic spread is an early step in breast cancer. Cancer Cell 13, 58–68 (2008).
Risson, E., Nobre, A. R., Maguer-Satta, V. & Aguirre-Ghiso, J. A. The current paradigm and challenges ahead for the dormancy of disseminated tumor cells. Nat. Cancer 1, 672–680 (2020).
Phan, T. G. & Croucher, P. I. The dormant cancer cell life cycle. Nat. Rev. Cancer 20, 398–411 (2020).
Croucher, P. I., McDonald, M. M. & Martin, T. J. Bone metastasis: the importance of the neighbourhood. Nat. Rev. Cancer 16, 373–386 (2016).
Wang, H. et al. The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell 27, 193–210 (2015).
Luo, X. et al. Stromal-initiated changes in the bone promote metastatic niche development. Cell Rep. 14, 82–92 (2016).
Yao, Z. et al. Therapy-induced senescence drives bone loss. Cancer Res. 80, 1171–1182 (2020).
Nobre, A. R. et al. Bone marrow NG2+/Nestin+ mesenchymal stem cells drive DTC dormancy via TGF-β2. Nat. Cancer 2, 327–339 (2021).
Andersen, T. L. et al. A physical mechanism for coupling bone resorption and formation in adult human bone. Am. J. Pathol. 174, 239–247 (2009).
Kraemer, B. et al. Impaired bone microenvironment: correlation between bone density and presence of disseminated tumor cells. Anticancer Res. 31, 4423–4428 (2011).
Chen, H. M., Chen, F. P., Yang, K. C. & Yuan, S. S. Association of bone metastasis with early-stage breast cancer in women with and without precancer osteoporosis according to osteoporosis therapy status. JAMA Netw. Open 2, e190429 (2019).
Blair, H. C. et al. Osteoblast differentiation and bone matrix formation in vivo and in vitro. Tissue Eng. B Rev. 23, 268–280 (2017).
Reznikov, N., Bilton, M., Lari, L., Stevens, M. M. & Kröger, R. Fractal-like hierarchical organization of bone begins at the nanoscale. Science 360, eaao2189 (2018).
Ping, H. et al. Mineralization generates megapascal contractile stresses in collagen fibrils. Science 376, 188–192 (2022).
Choi, S. et al. Intrafibrillar, bone-mimetic collagen mineralization regulates breast cancer cell adhesion and migration. Biomaterials 198, 95–106 (2019).
Chaudhuri, O., Cooper-White, J., Janmey, P. A., Mooney, D. J. & Shenoy, V. B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584, 535–546 (2020).
Page, J. M. et al. Matrix rigidity regulates the transition of tumor cells to a bone-destructive phenotype through integrin β3 and TGF-β receptor type II. Biomaterials 64, 33–44 (2015).
Turunen, M. J., Prantner, V., Jurvelin, J. S., Kröger, H. & Isaksson, H. Composition and microarchitecture of human trabecular bone change with age and differ between anatomical locations. Bone 54, 118–125 (2013).
Donnelly, E., Boskey, A. L., Baker, S. P. & van der Meulen, M. C. H. Effects of tissue age on bone tissue material composition and nanomechanical properties in the rat cortex. J. Biomed. Mater. Res. A 92, 1048–1056 (2010).
Burke, M. V., Atkins, A., Akens, M., Willett, T. L. & Whyne, C. M. Osteolytic and mixed cancer metastasis modulates collagen and mineral parameters within rat vertebral bone matrix. J. Orthop. Res. 34, 2126–2136 (2016).
Paschalis, E. P., Betts, F., DiCarlo, E., Mendelsohn, R. & Boskey, A. L. FTIR microspectroscopic analysis of normal human cortical and trabecular bone. Calcif. Tissue Int. 61, 480–486 (1997).
Mathis, K. M. et al. Bone resorption and bone metastasis risk. Med. Hypotheses 118, 36–41 (2018).
Lynch, M. E. et al. In vivo tibial compression decreases osteolysis and tumor formation in a human metastatic breast cancer model. J. Bone Miner. Res. 28, 2357–2367 (2013).
Swami, S. et al. Prevention of breast cancer skeletal metastases with parathyroid hormone. JCI Insight 2, e90874 (2017).
Farr, J. N. et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat. Med. 23, 1072–1079 (2017).
Ooi, L. L. et al. Vitamin D deficiency promotes human breast cancer growth in a murine model of bone metastasis. Cancer Res. 70, 1835–1844 (2010).
Wang, H. et al. Bone-in-culture array as a platform to model early-stage bone metastases and discover anti-metastasis therapies. Nat. Commun. 8, 15045 (2017).
Fratzl, P., Gupta, H. S., Paschalis, E. P. & Roschger, P. Structure and mechanical quality of the collagen–mineral nano-composite in bone. J. Mater. Chem. 14, 2115–2123 (2004).
Gower, L. & Elias, J. Colloid assembly and transformation (CAT): the relationship of PILP to biomineralization. J. Struct. Biol. X 6, 100059 (2022).
Chiou, A. E. et al. Fluorescent silica nanoparticles to label metastatic tumor cells in mineralized bone microenvironments. Small 17, e2001432 (2021).
Carlson, P. et al. Targeting the perivascular niche sensitizes disseminated tumour cells to chemotherapy. Nat. Cell Biol. 21, 238–250 (2019).
Olszta, M. J. et al. Bone structure and formation: a new perspective. Mater. Sci. Eng. R 58, 77–116 (2007).
Reznikov, N., Chase, H., Brumfeld, V., Shahar, R. & Weiner, S. The 3D structure of the collagen fibril network in human trabecular bone: relation to trabecular organization. Bone 71, 189–195 (2015).
Londoño-Restrepo, S. M., Jeronimo-Cruz, R., Millán-Malo, B. M., Rivera-Muñoz, E. M. & Rodriguez-García, M. E. Effect of the nano crystal size on the X-ray diffraction patterns of biogenic hydroxyapatite from human, bovine, and porcine bones. Sci. Rep. 9, 5915 (2019).
Nam, S., Lee, J., Brownfield, D. G. & Chaudhuri, O. Viscoplasticity enables mechanical remodeling of matrix by cells. Biophys. J. 111, 2296–2308 (2016).
Hall, M. S. et al. Fibrous nonlinear elasticity enables positive mechanical feedback between cells and ECMs. Proc. Natl Acad. Sci. USA 113, 14043–14048 (2016).
Masuda, T. et al. ANGPTL2 increases bone metastasis of breast cancer cells through enhancing CXCR4 signaling. Sci. Rep. 5, 9789 (2015).
Bergamaschi, A. et al. CAMK1D amplification implicated in epithelial-mesenchymal transition in basal-like breast cancer. Mol. Oncol. 2, 327–339 (2008).
Dong, X., Yang, Y., Yuan, Q., Hou, J. & Wu, G. High expression of CEMIP correlates poor prognosis and the tumor microenvironment in breast cancer as a promisingly prognostic biomarker. Front. Genet. 12, 768140 (2021).
Che, M. I. et al. β1, 4-N-acetylgalactosaminyltransferase III modulates cancer stemness through EGFR signaling pathway in colon cancer cells. Oncotarget 5, 3673–3684 (2014).
Yu, J. M. et al. TRIB3 supports breast cancer stemness by suppressing FOXO1 degradation and enhancing SOX2 transcription. Nat. Commun. 10, 5720 (2019).
Choate, J. J. & Mosher, D. F. Fibronectin concentration in plasma of patients with breast cancer, colon cancer, and acute leukemia. Cancer 51, 1142–1147 (1983).
Pathi, S. P., Lin, D. D. W., Dorvee, J. R., Estroff, L. A. & Fischbach, C. Hydroxyapatite nanoparticle-containing scaffolds for the study of breast cancer bone metastasis. Biomaterials 32, 5112–5122 (2011).
Bruning, P. F. et al. Bone mineral density after adjuvant chemotherapy for premenopausal breast cancer. Br. J. Cancer 61, 308–310 (1990).
Balestrini, J. L., Chaudhry, S., Sarrazy, V., Koehler, A. & Hinz, B. The mechanical memory of lung myofibroblasts. Integr. Biol. 4, 410–421 (2012).
Li, C. X. et al. MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells. Nat. Mater. 16, 379–389 (2016).
He, F. et al. Hydroxyapatite mineral enhances malignant potential in a tissue-engineered model of ductal carcinoma in situ (DCIS). Biomaterials 224, 119489 (2019).
Chiou, A. E. et al. Breast cancer-secreted factors perturb murine bone growth in regions prone to metastasis. Sci. Adv. 7, eabf2283 (2021).
Zhang, W. et al. The bone microenvironment invigorates metastatic seeds for further dissemination. Cell 184, 2471–2486.e20 (2021).
Thiagarajan, P. S. et al. Development of a fluorescent reporter system to delineate cancer stem cells in triple-negative breast cancer. Stem Cells 33, 2114–2125 (2015).
Ginestier, C. et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1, 555–567 (2007).
Raha, D. et al. The cancer stem cell marker aldehyde dehydrogenase is required to maintain a drug-tolerant tumor cell subpopulation. Cancer Res. 74, 3579–3590 (2014).
Pan, G. et al. A negative feedback loop of transcription factors that controls stem cell pluripotency and self-renewal. FASEB J. 20, 1730–1732 (2006).
Krishnakumar, R. et al. FOXD3 regulates pluripotent stem cell potential by simultaneously initiating and repressing enhancer activity. Cell Stem Cell 18, 104–117 (2016).
Chu, T. L. et al. FoxD3 deficiency promotes breast cancer progression by induction of epithelial–mesenchymal transition. Biochem. Biophys. Res. Commun. 446, 580–584 (2014).
Li, W. et al. Unraveling the roles of CD44/CD24 and ALDH1 as cancer stem cell markers in tumorigenesis and metastasis. Sci. Rep. 7, 13856 (2017).
Paszek, M. J. et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 (2005).
Seo, B. R. et al. Collagen microarchitecture mechanically controls myofibroblast differentiation. Proc. Natl Acad. Sci. USA 117, 11387–11398 (2020).
Barney, L. E. et al. Tumor cell-organized fibronectin maintenance of a dormant breast cancer population. Sci. Adv. 6, eaaz4157 (2020).
Reznikov, N. et al. Biological stenciling of mineralization in the skeleton: local enzymatic removal of inhibitors in the extracellular matrix. Bone 138, 115447 (2020).
Boys, A. J. et al. Top-down fabrication of spatially controlled mineral-gradient scaffolds for interfacial tissue engineering. ACS Biomater. Sci. Eng. https://doi.org/10.1021/acsbiomaterials.9b00176 (2019).
Zhou, H., Boys, A. J., Harrod, J. B., Bonassar, L. J. & Estroff, L. A. Mineral distribution spatially patterns bone marrow stromal cell behavior on monolithic bone scaffolds. Acta Biomater. 112, 274–285 (2020).
Fornetti, J., Welm, A. L. & Stewart, S. A. Understanding the bone in cancer metastasis. J. Bone Miner. Res. 33, 2099–2113 (2018).
Crapo, P. M., Gilbert, T. W. & Badylak, S. F. An overview of tissue and whole organ decellularization processes. Biomaterials 32, 3233–3243 (2011).
Gao, Y. et al. Metastasis organotropism: redefining the congenial soil. Dev. Cell 49, 375–391 (2019).
Cox, T. R. The matrix in cancer. Nat. Rev. Cancer 21, 217–238 (2021).
Reuten, R. et al. Basement membrane stiffness determines metastases formation. Nat. Mater. 20, 892–903 (2021).
Nam, S., Hu, K. H., Butte, M. J. & Chaudhuri, O. Strain-enhanced stress relaxation impacts nonlinear elasticity in collagen gels. Proc. Natl Acad. Sci. USA 113, 5492–5497 (2016).
Aguirre-Ghiso, J. A., Liu, D., Mignatti, A., Kovalski, K. & Ossowski, L. Urokinase receptor and fibronectin regulate the ERK(MAPK) to p38(MAPK) activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Mol. Biol. Cell 12, 863–879 (2001).
Margadant, C. & Sonnenberg, A. Integrin-TGF-Β crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 11, 97–105 (2010).
Ruppender, N. et al. Cellular adhesion promotes prostate cancer cells escape from dormancy. PLoS ONE 10, e0130565 (2015).
Bragado, P. et al. TGF-β2 dictates disseminated tumour cell fate in target organs through TGF-β-RIII and p38α/β signalling. Nat. Cell Biol. 15, 1351–1361 (2013).
Sosa, M. S. et al. NR2F1 controls tumour cell dormancy via SOX9- and RARβ-driven quiescence programmes. Nat. Commun. 6, 6170 (2015).
Marturano-Kruik, A. et al. Human bone perivascular niche-on-a-chip for studying metastatic colonization. Proc. Natl Acad. Sci. USA 115, 1256–1261 (2018).
Thrivikraman, G. et al. Rapid fabrication of vascularized and innervated cell-laden bone models with biomimetic intrafibrillar collagen mineralization. Nat. Commun. 10, 3520 (2019).
Singh, D. K., Patel, V. G., Oh, W. K. & Aguirre-Ghiso, J. A. Prostate cancer dormancy and reactivation in bone marrow. J. Clin. Med. 10, 2648 (2021).
Boskey, A. L. & Coleman, R. Critical reviews in oral biology and medicine: aging and bone. J. Dent. Res. 89, 1333–1348 (2010).
Azarin, S. M. et al. In vivo capture and label-free detection of early metastatic cells. Nat. Commun. 6, 8094 (2015).
Sadtler, K. et al. Divergent immune responses to synthetic and biological scaffolds. Biomaterials 192, 405–415 (2019).
Coleman, R. et al. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet 386, 1353–1361 (2015).
Coleman, R. E. et al. Breast-cancer adjuvant therapy with zoledronic acid. N. Engl. J. Med. 365, 1396–1405 (2011).
Pelham, R. J. & Wang, Y. L. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl Acad. Sci. USA 94, 13661 (1997).
Przybyla, L., Lakins, J. N., Sunyer, R., Trepat, X. & Weaver, V. M. Monitoring developmental force distributions in reconstituted embryonic epithelia. Methods 94, 101–113 (2016).
Han, S. J., Oak, Y., Groisman, A. & Danuser, G. Traction microscopy to identify force modulation in subresolution adhesions. Nat. Methods 12, 653–656 (2015).
Dobin, A. & Gingeras, T. R. Mapping RNA-seq reads with STAR. Curr. Protoc. Bioinformatics 51, 11.14.1–11.14.19 (2015).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Grüneboom, A. et al. A network of trans-cortical capillaries as mainstay for blood circulation in long bones. Nat .Metab. 1, 236–250 (2019).
Bankhead, P. et al. QuPath: open source software for digital pathology image analysis. Sci. Rep. 7, 16878 (2017).
Acknowledgements
We thank all members of the Fischbach lab for valuable discussions of this research; the Wiesner group (Cornell University) for providing fluorescent silica nanoparticles; J. Kuo (Cornell University) for help with illustrations; L. O’Keeffe for help with preparation of bone scaffolds; the Cornell Animal Health Diagnostic Core for paraffin embedding and sectioning; J. Massague (Memorial Sloan Kettering Cancer Center) for providing bone tropic BoM1-2287 and BoM-1833; and the Cornell Center for Animal Resources and Education (CARE) staff for animal care. Financial support was provided by the Human Frontier Science Program (RGP0016/2017); the National Cancer Institute through the Center on the Physics of Cancer Metabolism (1U54CA210184); NIH F31 (F31CA228448) to A.E.C.; fellowship support by the Stem Cell Program of Cornell University to N.D.S.; and NSF GRFP (DGE-1650441) to M.A.W. and A.A.S. This work used the Cornell Center for Materials Research (CCMR), which is supported through the NSF MRSEC program (DMR-1719875); the Cornell NanoScale Science & Technology Facility (CNF), a member of the NSF-supported National Nanotechnology Coordinated Infrastructure (NNCI-2025233); and the Cornell University Biotechnology Resource Center (BRC) facilities, including a Zeiss LSM 710 confocal microscope (NIH 1S10RR025502), Zeiss LSM880 confocal/multiphoton microscope (NYSTEM (C029155) and NIH (S10OD018516)), light-sheet microscope (NIH S10OD023466), ZEISS/Xradia Versa 520 X-ray microscope (NIH S10OD012287), IVIS Spectrum (NIH S10OD025049) and Genomics Facility (RRID:SCR_021727).
Author information
Authors and Affiliations
Contributions
S.C., M.A.W., L.A.E. and C.F. designed the project. S.C. and M.A.W. conducted most of the experiments. A.A.S. performed and analysed FACS and RNA-seq data. N.D.S performed and analysed the hMSC experiments. A.E.C. performed the animal study for light-sheet microscopy. J.E.D. performed FACS. A.V. and O.E. analysed RNA-seq data with the SSGSEA method. S.C.L. analysed IHC images. Z.C, A.A.S. and M.P. conducted and analysed TFM. S.C., M.A.W., L.A.E. and C.F. analysed the data and wrote the paper. All authors discussed the results and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
O.E. was supported by Janssen, J&J, Astra-Zeneca, Volastra and Eli Lilly research grants. He is scientific advisor and equity holder in Freenome, Owkin, Volastra Therapeutics and One Three Biotech, and a paid scientific advisor to Champions Oncology and Pionyr Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Julie A. Rhoades, Hai Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
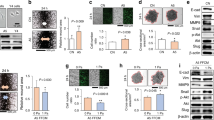
Extended Data Fig. 1 Mineral-to-matrix ratio of collagen and mineralized collagen.
Mineral to matrix ratio of mineralized collagen as calculated by dividing the phosphate peak area (907–1183 cm−1) of FT-IR spectra by the amide I peak area (1580–1727 cm−1) (n = 3; ****p < 0.0001). Data are mean ± SD. Data were calculated using two-tailed unpaired t-test.
Extended Data Fig. 2 Storage and loss moduli of collagen and mineralized collagen.
a, b, Representative time sweeps (a) and quantification of storage (Gˊ) and loss (G˝) moduli (n = 3; **p = 0.0083; ##p = 0.0035) (b). Data are mean ± SD. Data in b were calculated using two-tailed unpaired t-test.
Extended Data Fig. 3 Heatmap of differentially expressed genes (DEGs).
DEGs were defined as the genes with FDR-adjusted p-values < 0.05 and log2FC > 1. Relative expression levels of all DEGs (left) or top 50 genes (right) is split into collagen and mineralized collagen conditions.
Extended Data Fig. 4 Top-10 enrichment plot from gene-set enrichment analysis (GSEA).
a, GSEA indicates that biological processes associated with transcription and cell migration were overrepresented by collagen mineralization. Red labeled gene sets are associated with actin remodeling and migration curated from the Molecular Signatures Data Base (MSigDB), while blue labeled gene sets have been directly linked to stemness. The top 3 significant transcription factors (FOXJ2, MED25, ZNF746) do not have clearly defined functions beyond complexing with RNA polymerase II but FOXD3 was also enriched albeit to a less significant extent (FDR q-value = 0.062). Dashed red line designates FDR q-value cutoff of 0.05. b, Enrichment plot for FOXD3 target genes as determined by GSEA. Normalized Enrichment Score (NES) = 1.68, nominal p-value = 0.009, FDR q-value = 0.062. c, Enrichment plot for the Wu Cell Migration gene signature from the MSigDB as determined by GSEA. Normalized Enrichment Score (NES) = 1.60, nominal p-value < 0.001, FDR q-value = 0.157. While cell migration was not a focus of our study, this gene set includes several genes directly related to cytoskeletal remodeling (for example NEDD9, KRT19, S100A4, and FN1).
Extended Data Fig. 5 Pre-adsorbed fibronectin does not affect the growth of MDA-MB231.
MDA-MB231 cell growth on the different substrates for 7 d. Substrates were incubated with various concentrations of fibronectin (FN) before cell seeding (n = 4). Data are mean ± SD. Data were calculated using two-way ANOVA with Tukey’s multiple comparisons.
Extended Data Fig. 6 Matrix mineralization correlates with decreased tumour-cell growth in the presence of bone-resident cells.
a, Representative confocal micrographs and corresponding quantification of co-cultured osteogenically differentiated hMSCs and labeled MDA-MB231 cells (n = 3; **p = 0.0061). Scale bar = 200 µm. b, Representative bright field micrographs of Alizarin Red S stained hMSCs cultured with control or osteogenic media and in the presence or absence of tumor-conditioned media (TCM). Scale bar, 500 μm and 100 μm (inset). c, Representative confocal micrographs of hMSCs stained for fibronectin. Scale bar = 200 µm. Data are mean ± SD. Data in a were calculated using two-tailed Mann Whitney U test.
Extended Data Fig. 7 Characterization of mineralized collagen at different time points of dissolution.
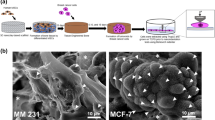
a, FT-IR spectra of mineralized collagen following hydroxyapatite dissolution for up to 6 days. Non-mineralized collagen served as control. b,c, FT-IR analysis of mineral to matrix ratio (n = 3; ****p < 0.0001) (b) and crystallinity index (CI) of matrices after different periods of mineral dissolution (n = 3; ****p < 0.0001) (c). CI was calculated by drawing a baseline between 450 cm−1 and 750 cm−1 (red dashed line) and measuring the heights of phosphate peaks at 567 cm−1 and 603 cm−1 and the height of the lowest point between them relative to the baseline. d, Representative SEM and BSE images visualizing differences in mineral content and collagen fibril diameter after different periods of mineral dissolution. Pseudocolor was generated from BSE images by converting grey-scale pixel intensity to a linear 256-bit color scale. Fibril diameter was measured from SEM images. Scale bar = 2 µm. Data are mean ± SD. Data in b and c were calculated using one-way ANOVA with Dunnett’s multiple comparisons.
Extended Data Fig. 8 E-cadherin expression of MCF7 is reduced by mineralized collagen.
Representative confocal micrographs and corresponding quantification of E-cadherin after 7 d of culture of MCF7 cells on the different substrates (n = 3; **p = 0.0012; ****p < 0.0001). Scale bar = 20 µm. Data are mean ± SD. Data were calculated using one-way ANOVA with Tukey’s multiple comparisons.
Extended Data Fig. 9 Total traction forces of different breast-cancer cell lines.
Cells were precultured on tissue culture polystyrene (PS), collagen, and mineralized collagen. Cells were precultured on the different substrates for 7 days prior to reseeding on PA gels for traction force microscopy measurements (MDA-MB231 (PS: 44 cells examined from 3 gels; Col and MN-col: at least 61 cells examined from 6 gels); MCF7 (at least 31cells examined from 5 gels); BoM-1833 (PS: 21 cells examined from 2 gels; Col and MN-col: at least 33 cells examined from 3 gels)). *p = 0.0459; **p = 0.0076; ***p = 0.0001; #p = 0.0193. Data are mean ± SD. Data were calculated using Kruskal Wallis test with Dunn’s multiple comparisons.
Extended Data Fig. 10 Survival analysis of patients with breast cancer.
a, Overall survival of patients with breast cancer by stratification based on mineral-induced gene expression using different breast cancer subtypes in METABRIC database. b, Overall bone metastasis-free survival of patients with breast cancer scoring high or low for expression of the mineral-induced gene signature (Supplementary Table 2).
Supplementary information
Supplementary Information
Supplementary Figures and Tables.
Data
Source data and statistics for Supplementary Fig. 1.
Data
Unprocessed western blot of Supplementary Fig. 2.
Data
Source data of Supplementary Fig. 4.
Code
Script for QuPath to analyse tissue sections in Fig. 6d.
Source data
Source Data Figs. 1–6 and Extended Data Figs. 1–10
Source data and statistics.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Choi, S., Whitman, M.A., Shimpi, A.A. et al. Bone-matrix mineralization dampens integrin-mediated mechanosignalling and metastatic progression in breast cancer. Nat. Biomed. Eng 7, 1455–1472 (2023). https://doi.org/10.1038/s41551-023-01077-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-023-01077-3
This article is cited by
-
Potential interplay between tumor size and vitamin D receptor (VDR) polymorphisms in breast cancer prognosis: a prospective cohort study
Cancer Causes & Control (2024)
-
Bone mineral slows down breast cancer cells
Nature Biomedical Engineering (2023)