Abstract
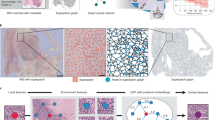
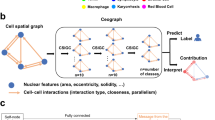
Multiplexed immunofluorescence imaging allows the multidimensional molecular profiling of cellular environments at subcellular resolution. However, identifying and characterizing disease-relevant microenvironments from these rich datasets is challenging. Here we show that a graph neural network that leverages spatial protein profiles in tissue specimens to model tumour microenvironments as local subgraphs captures distinctive cellular interactions associated with differential clinical outcomes. We applied this spatial cellular-graph strategy to specimens of human head-and-neck and colorectal cancers assayed with 40-plex immunofluorescence imaging to identify spatial motifs associated with cancer recurrence and with patient survival after treatment. The graph deep learning model was substantially more accurate in predicting patient outcomes than deep learning approaches that model spatial data on the basis of the local composition of cell types, and it generated insights into the effect of the spatial compartmentalization of tumour cells and granulocytes on patient prognosis. Local graphs may also aid in the analysis of disease-relevant motifs in histology samples characterized via spatial transcriptomics and other -omics techniques.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data supporting the results in this study involve patient data and are available from the corresponding author on reasonable request.
Code availability
Commercial software (Akoya Biosciences, Enable Medicine) was used to preprocess images and to classify cells using methods based on published algorithms. Deepcell (https://github.com/vanvalenlab/deepcell-tf) was used to segment cells from multiplexed immunofluorescence images. The codes used for the construction of spatial cellular graphs and for the following analyses are available at https://gitlab.com/enable-medicine-public/space-gm.
References
Luca, B. A. et al. Atlas of clinically distinct cell states and ecosystems across human solid tumors. Cell 184, 5482–5496 (2021).
Wu, S. Z. et al. A single-cell and spatially resolved atlas of human breast cancers. Nat. Genet. 53, 1334–1347 (2021).
Bejarano, L., Jordāo, M. J. C. & Joyce, J. A. Therapeutic targeting of the tumor microenvironment. Cancer Discov. 11, 933–959 (2021).
Lomakin, A. et al. Spatial genomics maps the structure, character and evolution of cancer clones. Preprint at bioRxiv https://doi.org/10.1101/2021.04.16.439912 (2021).
Rodriques, S. G. et al. Slide-seq: a scalable technology for measuring genome-wide expression at high spatial resolution. Science 363, 1463–1467 (2019).
Wang, X. et al. Three-dimensional intact-tissue sequencing of single-cell transcriptional states. Science 361, eaat5691 (2018).
Moffitt, J. R. et al. High-throughput single-cell gene-expression profiling with multiplexed error-robust fluorescence in situ hybridization. Proc. Natl Acad. Sci. USA 113, 11046–11051 (2016).
Goltsev, Y. et al. Deep profiling of mouse splenic architecture with CODEX multiplexed imaging. Cell 174, 968–981 (2018).
Angelo, M. et al. Multiplexed ion beam imaging of human breast tumors. Nat. Med. 20, 436–442 (2014).
Lin, J.-R., Fallahi-Sichani, M., Chen, J.-Y. & Sorger, P. K. Cyclic immunofluorescence (CycIF), a highly multiplexed method for single-cell imaging. Curr. Protoc. Chem. Biol. 8, 251–264 (2016).
Ali, H. R. et al. Imaging mass cytometry and multiplatform genomics define the phenogenomic landscape of breast cancer. Nat. Cancer 1, 163–175 (2020).
Schürch, C. M. et al. Coordinated cellular neighborhoods orchestrate antitumoral immunity at the colorectal cancer invasive front. Cell 182, 1341–1359 (2020); erratum 183, 838 (2020).
Bhate, S. S., Barlow, G. L., Schürch, C. M. & Nolan, G. P. Tissue schematics map the specialization of immune tissue motifs and their appropriation by tumors. Cell Syst. 13, 109–130 (2022).
Zhou, Y. et al. Cgc-net: cell graph convolutional network for grading of colorectal cancer histology images. In Proc. IEEE/CVF International Conference on Computer Vision Workshops 388–398 (IEEE, 2019).
Lu, W., Graham, S., Bilal, M., Rajpoot, N. & Minhas, F. Capturing cellular topology in multi-gigapixel pathology images. In Proc. IEEE/CVF Conference on Computer Vision and Pattern Recognition Workshops 260–261 (IEEE, 2020).
Anand, D., Gadiya, S. & Sethi, A. Histographs: graphs in histopathology. In Medical Imaging 2020: Digital Pathology Vol. 11320 (eds Tomaszewski, J. E. & Ward, A. D.) 150–155 (SPIE, 2020).
Gilmer, J., Schoenholz, S. S., Riley, P. F., Vinyals, O. & Dahl, G. E. Neural message passing for quantum chemistry. In Proc. 34th International Conference on Machine Learning Vol. 70 (eds Precup, D. & Teh, Y. W.) 1263–1272 (JMLR.org, 2017).
Wu, Z. et al. A comprehensive survey on graph neural networks. IEEE Trans. Neural Netw. Learn. Syst. 32, 4–24 (2021).
Brbić, M. et al. Annotation of spatially resolved single-cell data with STELLAR. Preprint at bioRxiv https://doi.org/10.1101/2021.11.24.469947 (2021).
Innocenti, C. et al. An unsupervised graph embeddings approach to multiplex immunofluorescence image exploration. Preprint at bioRxiv https://doi.org/10.1101/2021.06.09.447654 (2021).
Fischer, D. S., Schaar, A. C. & Theis, F. J. Learning cell communication from spatial graphs of cells. Preprint at bioRxiv https://doi.org/10.1101/2021.07.11.451750 (2021).
Kim, J. et al. Unsupervised discovery of tissue architecture in multiplexed imaging. Preprint at bioRxiv https://doi.org/10.1101/2022.03.15.484534 (2022).
Xu, K., Hu, W., Leskovec, J. & Jegelka, S. How powerful are graph neural networks? In Proc.7th International Conference on Learning Representations (OpenReview, 2019); https://openreview.net/forum?id=ryGs6iA5Km
Argiris, A., Karamouzis, M. V., Raben, D. & Ferris, R. L. Head and neck cancer. Lancet 371, 1695–1709 (2008).
Dalerba, P. et al. CDX2 as a prognostic biomarker in stage II and stage III colon cancer. N. Engl. J. Med. 374, 211–222 (2016).
Uppaluri, R. et al. Neoadjuvant and adjuvant pembrolizumab in resectable locally advanced, human papillomavirus–unrelated head and neck cancer: a multicenter, phase II trial. Clin. Cancer Res. 26, 5140–5152 (2020).
McInnes, L., Healy, J. & Melville, J. UMAP: uniform manifold approximation and projection for dimension reduction. Preprint at https://doi.org/10.48550/arXiv.1802.03426 (2018).
Blise, K. E., Sivagnanam, S., Banik, G. L., Coussens, L. M. & Goecks, J. Single-cell spatial architectures associated with clinical outcome in head and neck squamous cell carcinoma. NPJ Precis. Oncol. 6, 10 (2022).
Jackson, H. W. et al. The single-cell pathology landscape of breast cancer. Nature 578, 615–620 (2020).
Trellakis, S. et al. Polymorphonuclear granulocytes in human head and neck cancer: enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. Int. J. Cancer 129, 2183–2193 (2011).
Lonardi, S. et al. Tumor-associated neutrophils (TANs) in human carcinoma-draining lymph nodes: a novel TAN compartment. Clin. Transl. Immunol. 10, e1252 (2021).
Coffelt, S. B., Wellenstein, M. D. & de Visser, K. E. Neutrophils in cancer: neutral no more. Nat. Rev. Cancer 16, 431–446 (2016).
Shojaei, F. et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 450, 825–831 (2007).
Di Mitri, D. et al. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature 515, 134–137 (2014).
Coffelt, S. B. et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522, 345–348 (2015).
Wculek, S. K. & Malanchi, I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 528, 413–417 (2015).
Regev, A. et al. The Human Cell Atlas. eLife 6, e27041 (2017).
HuBMAP Consortium. The human body at cellular resolution: the NIH Human Biomolecular Atlas Program. Nature 574, 187–192 (2019).
Greenwald, N. F. et al. Whole-cell segmentation of tissue images with human-level performance using large-scale data annotation and deep learning. Nat. Biotechnol. 40, 555–565 (2021).
Hickey, J. W., Tan, Y., Nolan, G. P. & Goltsev, Y. Strategies for accurate cell type identification in CODEX multiplexed imaging data. Front. Immunol. 12, 727626 (2021).
Blondel, V. D., Guillaume, J.-L., Lambiotte, R. & Lefebvre, E. Fast unfolding of communities in large networks. J. Stat. Mech. Theory Exp. 2008, P10008 (2008).
Kvamme, H., Borgan, Ø. & Scheel, I. Time-to-event prediction with neural networks and Cox regression. J. Mach. Learn. Res. 20, 1–30 (2019).
Kingma, D. P. & Ba, J. Adam: a method for stochastic optimization. In Proc. 3rd International Conference on Learning Representations (ICLR, 2015); https://www.iclr.cc/archive/www/2015.html
Pedregosa, F. et al. Scikit-learn: machine learning in Python. J. Mach. Learn. Res. 12, 2825–2830 (2011).
Paszke, A. et al. PyTorch: An imperative style, high-performance deep learning library. In Proc. 33rd International Conference on Neural Information Processing Systems 8026–8037 (Curran Associates, 2019).
Fey, M. & Lenssen, J. E. Fast graph representation learning with PyTorch geometric. Preprint at https://doi.org/10.48550/arXiv.1903.02428 (2019).
Kipf, T. N., & Welling, M. Semi-supervised classification with graph convolutional networks. In Proc. 5th International Conference on Learning Representations (OpenReview, 2017); https://openreview.net/forum?id=SJU4ayYgl
Veličković, P. et al. Graph attention networks. In Proc. 6th International Conference on Learning Representations (OpenReview, 2018); https://openreview.net/forum?id=rJXMpikCZ
Hamilton, W., Ying, Z., & Leskovec, J. Inductive representation learning on large graphs. In Proc. 31st International Conference on Neural Information Processing Systems 1025–1035 (Curran Associates, 2017).
Li, Y., Tarlow, D., Brockschmidt, M. & Zemel, R. Gated graph sequence neural networks. In Proc. 4th International Conference on Learning Representations (OpenReview, 2016); https://openreview.net/forum?id=HSgW989Kp-q
Vinyals, O., Bengio, S. & Kudlur, M. Order matters: sequence to sequence for sets. In Proc. 4th International Conference on Learning Representations (ICLR, 2016); https://www.iclr.cc/archive/www/2016.html
Acknowledgements
J.Z. discloses support for the research described in this study from NSF CAREER (grant no. 1942926). K.S. discloses support for the research described in this study from a Knight-Hennessy Fellowship.
Author information
Authors and Affiliations
Contributions
Z.W. and A.E.T. designed the model and computational experiments in consultation with E.W. and K.S. Z.W. and A.E.T. analysed the data. Z.W. and A.E.T. wrote the manuscript with input from all authors. G.W.C., P.D.D., A.M.E., R.U. and U.D. provided samples for the experiments. H.J.K., H.B.D. and R.P. performed the experiments and data preprocessing. J.Z. and A.T.M. were responsible for the overall direction and planning of the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Tae Hyun Hwang, Jonathan Nowak and Jianyu Rao for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Comparison of composition-based clusters and microenvironment clusters.
A. and B. We generate composition-based clusters with cell type compositions of 3-hop subgraphs (microenvironments) following the same procedure. Note that the average predictions of microenvironment clusters are much more polarized. C. The composition cluster highly enriched with granulocytes has neutral average predictions/labels, it could be further dissected into multiple sub-clusters that belong to different microenvironment clusters. We see a pair of granulocyte/tumor microenvironments that have opposite outcome labels, see Results for further discussion. D. Similarly, the composition cluster enriched with vessel/lymph vessel cells could be dissected into multiple sub-clusters, from which we notice two microenvironment clusters that are both enriched with lymph vessel cells but have different composition and outcome predictions, see Supplementary Note 7 for further discussion. E. Comparison of the two microenvironments enriched in lymph vessel cells: the left column shows a microenvironment with more lymphocytes and has overall positive outcomes; the right column shows a contrasting group with more tumor cells and much worse outcome predictions. Observation of tumor cells in close vicinity of lymph vessels indicates potential lymphovascular invasion and will lead to worse prognosis, which aligns with model predictions.
Supplementary information
Supplementary Information
Supplementary Notes 1–7, Tables 1–7 and Figs.1–10.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, Z., Trevino, A.E., Wu, E. et al. Graph deep learning for the characterization of tumour microenvironments from spatial protein profiles in tissue specimens. Nat. Biomed. Eng 6, 1435–1448 (2022). https://doi.org/10.1038/s41551-022-00951-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-022-00951-w
This article is cited by
-
Unsupervised and supervised discovery of tissue cellular neighborhoods from cell phenotypes
Nature Methods (2024)
-
Integrating single-cell multi-omics and prior biological knowledge for a functional characterization of the immune system
Nature Immunology (2024)
-
Multimodal bioimaging across disciplines and scales: challenges, opportunities and breaking down barriers
npj Imaging (2024)
-
CellCharter reveals spatial cell niches associated with tissue remodeling and cell plasticity
Nature Genetics (2024)
-
The covariance environment defines cellular niches for spatial inference
Nature Biotechnology (2024)