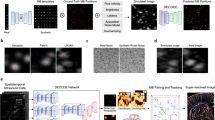
Abstract
Ultrafast ultrasound localization microscopy can be used to detect the subwavelength acoustic scattering of intravenously injected microbubbles to obtain haemodynamic maps of the vasculature of animals and humans. The quality of the haemodynamic maps depends on signal-to-noise ratios and on the algorithms used for the localization of the microbubbles and the rendering of their trajectories. Here we report the results of benchmarking of the performance of seven microbubble-localization algorithms. We used metrics for localization errors, localization success rates, processing times and a measure of the reprojection of the localization of the microbubbles on the original beamformed grid. We combined eleven metrics into an overall score and tested the algorithms in three simulated microcirculation datasets, and in angiography datasets of the brain of a live rat after craniotomy, an excised rat kidney and a mammary tumour in a live mouse. The algorithms, metrics and datasets, which we have made openly available at https://github.com/AChavignon/PALA and https://doi.org/10.5281/zenodo.4343435, will facilitate the identification or generation of optimal microbubble-localization algorithms for specific applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. All data generated in this study, including source data and the data used to make the figures, are available from Zenodo at https://doi.org/10.5281/zenodo.4343435.
Code availability
All codes used for data acquisition and for data analysis are available on GitHub at https://github.com/AChavignon/PALA.
References
Tanter, M. & Fink, M. Ultrafast imaging in biomedical ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 61, 102–119 (2014).
Macé, E. et al. Functional ultrasound imaging of the brain. Nat. Methods 8, 662–664 (2011).
Macé, E. et al. High sensitivity brain angiography using ultrafast Doppler. IEEE Int. Ultrason. Symp. https://doi.org/10.1109/ultsym.2010.5935810 (2010).
Demene, C. et al. Spatiotemporal clutter filtering of ultrafast ultrasound data highly increases Doppler and fUltrasound sensitivity. IEEE Trans. Med. Imaging 34, 2271–2285 (2015).
Frinking, P., Segers, T., Luan, Y. & Tranquart, F. Three decades of ultrasound contrast agents: a review of the past, present and future improvements. Ultrasound Med. Biol. 46, 892–908 (2020).
Couture, O., Besson, B., Montaldo, G., Fink, M. & Tanter, M. Microbubble ultrasound super-localisation imaging (MUSLI). IEEE Int. Ultrason. Symp. https://doi.org/10.1109/ULTSYM.2011.6293576 (2011).
Couture, O., Tanter, M. & Fink, M. Method and device for sound imaging. Patent 889 Cooperation Treaty (PCT)/FR2011/052810 (2011).
Couture, O., Hingot, V., Heiles, B., Muleki-Seya, P. & Tanter, M. Ultrasound localisation microscopy and super-resolution: a state of the art. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 65, 1304–1320 (2018).
Errico, C. et al. Ultrafast ultrasound localisation microscopy for deep super-resolution vascular imaging. Nature 527, 499–502 (2015).
Christensen-Jeffries, K., Browning, R. J., Tang, M.-X., Dunsby, C. & Eckersley, R. J. In vivo acoustic super-resolution and super-resolved velocity mapping using microbubbles. IEEE Trans. Med. Imaging 34, 433–440 (2015).
Heiles, B. et al. Volumetric ultrasound localization microscopy of the whole brain microvasculature. Preprint at bioRxiv https://doi.org/10.1101/2021.09.17.460797 (2021).
Viessmann, O. M., Eckersley, R. J., Christensen-Jeffries, K., Tang, M. X. & Dunsby, C. Acoustic super-resolution with ultrasound and microbubbles. Phys. Med. Biol. 58, 6447–6458 (2013).
Lin, F. et al. 3-D ultrasound localisation microscopy for identifying microvascular morphology features of tumour angiogenesis at a resolution beyond the diffraction limit of conventional ultrasound. Theranostics 7, 196–204 (2017).
OˈReilly, M. A. & Hynynen, K. A super-resolution ultrasound method for brain vascular mapping: super-resolution ultrasound method for brain vascular mapping. Med. Phys. 40, 110701 (2013).
Song, P. et al. Improved super-resolution ultrasound microvessel imaging with spatiotemporal nonlocal means filtering and bipartite graph-based microbubble tracking. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 65, 149–167 (2018).
Brown, J. et al. Investigation of microbubble detection methods for super-resolution imaging of microvasculature. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 66, 676–691 (2019).
Christensen-Jeffries, K. et al. Microbubble axial localisation errors in ultrasound super-resolution imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 64, 1644–1654 (2017).
Kanoulas, E. et al. Super-resolution contrast-enhanced ultrasound methodology for the identification of in vivo vascular dynamics in 2D. Invest. Radiol. 54, 500–516 (2019).
Song, P., Manduca, A., Trzasko, J. D., Daigle, R. E. & Chen, S. On the effects of spatial sampling quantisation in super-resolution ultrasound microvessel imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 65, 2264–2276 (2018).
Hingot, V. et al. Microvascular flow dictates the compromise between spatial resolution and acquisition time in ultrasound localisation microscopy. Sci. Rep. 9, 2456 (2019).
Kuhn, H. W. The Hungarian method for the assignment problem. Nav. Res. Logist. Q. 2, 83–97 (1955).
Zhang, G. et al. Acoustic wave sparsely activated localisation microscopy (AWSALM): super-resolution ultrasound imaging using acoustic activation and deactivation of nanodroplets. Appl. Phys. Lett. 113, 014101 (2018).
Lowerison, M. R., Huang, C., Lucien, F., Chen, S. & Song, P. Ultrasound localisation microscopy of renal tumor xenografts in chicken embryo is correlated to hypoxia. Sci. Rep. 10, 2478 (2020).
Forsberg, F., Leeman, S. & Jensen, J. A. Assessment of hybrid speckle reduction algorithms. Phys. Med. Biol. 36, 1539–1549 (1991).
Ledoux, L. A. F., Brands, P. J. & Hoeks, A. P. G. Reduction of the clutter component in doppler ultrasound signals based on singular value decomposition: a simulation study. Ultrason. Imaging 19, 1–18 (1997).
Desailly, Y. et al. Contrast enhanced ultrasound by real-time spatiotemporal filtering of ultrafast images. Phys. Med. Biol. 62, 31–42 (2017).
Acknowledgements
The project was funded by the European Research Council under the European Union Horizon H2020 programme/ERC Consolidator grant agreement no. 772786-ResolveStroke. The project was also supported by Agence Nationale de la Recherche (ANR), within the project ANR Predic and the Plan Cancer UICT. We thank the laboratories Institut Langevin and PhysMed Paris for technical support and particularly L. Rahal for helping with data acquisition; C. Orset (INSERM UMR-S U1237, Physiopathology and Imaging of Neurological Disorders, GIP Cyceron, Institut Blood and Brain @ Caen-Normandie (BB@C), Caen, France) for the preparation of the animals and the perfusion of the contrast agent, and the biomedical imaging platform CYCERON (UMS 3408 Unicaen/CNRS, Caen, France), particularly H. Skeif and V. Beaudouin, for the micro-CT of brains; R. Hlushchuk for all his valuable advice for µAngiofil perfusion; D. Maresca for useful discussion, and our collaborators at Plateforme d’Imagerie du Vivant, Institut Cochin (U1016, INSERM Paris, France); and F. Lager, G. Renault and A. Nicolas-Boluda for helping with the mouse tumour model and the in vivo experiments.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception of the project. E.T. and P.L. were the main collaborators on the kidney and tumour experiments. B.H. and V.H. laid out the general framework and algorithm for ULM, with contributions from all authors. B.H. wrote the original codes for the localization algorithms, with significant improvements introduced by A.C. A.C. wrote the simulation framework and implemented the metrics. V.H., A.C., E.T. and P.L. acquired in vivo data. B.H. wrote the manuscript, with input from all authors. O.C. directed the work of B.H., A.C. and V.H. as thesis advisor or postdoctoral supervisor.
Corresponding author
Ethics declarations
Competing interests
O.C. is a co-inventor of an ultrasound super-resolution patent ((PCT)/FR2011/052810). All other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks James Greenleaf and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, figures and tables.
Supplementary Data
Dynamic table for calculating the global score for the performance assessment of microbubble-localization algorithms.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Heiles, B., Chavignon, A., Hingot, V. et al. Performance benchmarking of microbubble-localization algorithms for ultrasound localization microscopy. Nat. Biomed. Eng 6, 605–616 (2022). https://doi.org/10.1038/s41551-021-00824-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-021-00824-8
This article is cited by
-
An all-ultrasound cranial imaging method to establish the relationship between cranial FUS incidence angle and transcranial attenuation in non-human primates in 3D
Scientific Reports (2024)
-
Transthoracic ultrasound localization microscopy of myocardial vasculature in patients
Nature Biomedical Engineering (2024)
-
An Automated Heart Shunt Recognition Pipeline Using Deep Neural Networks
Journal of Imaging Informatics in Medicine (2024)
-
Evaluation of tumor microvasculature with 3D ultrasound localization microscopy based on 2D matrix array
European Radiology (2024)
-
Wireless agents for brain recording and stimulation modalities
Bioelectronic Medicine (2023)