Abstract
In breast cancer, genetic heterogeneity, the lack of actionable targets and immune evasion all contribute to the limited clinical response rates to immune checkpoint blockade therapy. Here, we report a high-throughput screen based on the functional interaction of mouse- or patient-derived breast tumour organoids and tumour-specific cytotoxic T cells for the identification of epigenetic inhibitors that promote antigen presentation and potentiate T-cell-mediated cytotoxicity. We show that the epigenetic inhibitors GSK-LSD1, CUDC-101 and BML-210, identified by the screen, display antitumour activities in orthotopic mammary tumours in mice, that they upregulate antigen presentation mediated by the major histocompatibility complex class I on breast tumour cells and that treatment with BML-210 substantially sensitized breast tumours to the inhibitor of the checkpoint programmed death-1. Standardized measurements of tumour-cell killing activity facilitated by tumour-organoid–T-cell screens may help with the identification of candidate immunotherapeutics for a range of cancers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are too large to be publicly shared, yet they are available for research purposes from the corresponding authors on reasonable request. The RNA sequence data are available from the GEO database under accession number GSE182954. Source data are provided with this paper.
Change history
30 August 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41551-023-01096-0
References
Gatti-Mays, M. E. et al. If we build it they will come: targeting the immune response to breast cancer. NPJ Breast Cancer 5, 37 (2019).
Dijkstra, K. K. et al. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell 174, 1586–1598 (2018).
Sachs, N. et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell 172, 373–386 (2018).
Stevanovic, S. et al. Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science 356, 200–205 (2017).
Schmid, P. et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 379, 2108–2121 (2018).
Zacharakis, N. et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat. Med. 24, 724–730 (2018).
Kim, I. S. et al. Immuno-subtyping of breast cancer reveals distinct myeloid cell profiles and immunotherapy resistance mechanisms. Nat. Cell Biol. 21, 1113–1126 (2019).
Villanueva, L., Alvarez-Errico, D. & Esteller, M. The contribution of epigenetics to cancer immunotherapy. Trends Immunol. 41, 676–691 (2020).
Romero, D. HDAC inhibitors tested in phase III trial. Nat. Rev. Clin. Oncol. 16, 465 (2019).
Sulaiman, A. et al. Co-inhibition of mTORC1, HDAC and ESR1α retards the growth of triple-negative breast cancer and suppresses cancer stem cells. Cell Death Dis. 9, 815 (2018).
Dravis, C. et al. Epigenetic and transcriptomic profiling of mammary gland development and tumor models disclose regulators of cell state plasticity. Cancer Cell 34, 466–482 (2018).
D’Abreo, N. & Adams, S. Immune-checkpoint inhibition for metastatic triple-negative breast cancer: safety first? Nat. Rev. Clin. Oncol. 16, 399–400 (2019).
Wein, L., Luen, S. J., Savas, P., Salgado, R. & Loi, S. Checkpoint blockade in the treatment of breast cancer: current status and future directions. Br. J. Cancer 119, 4–11 (2018).
Feder-Mengus, C., Ghosh, S., Reschner, A., Martin, I. & Spagnoli, G. C. New dimensions in tumor immunology: what does 3D culture reveal? Trends Mol. Med. 14, 333–340 (2008).
Friedrich, J., Seidel, C., Ebner, R. & Kunz-Schughart, L. A. Spheroid-based drug screen: considerations and practical approach. Nat. Protoc. 4, 309–324 (2009).
Junttila, M. R. & de Sauvage, F. J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 501, 346–354 (2013).
Palucka, A. K. & Coussens, L. M. The basis of oncoimmunology. Cell 164, 1233–1247 (2016).
Neal, J. T. et al. Organoid modeling of the tumor immune microenvironment. Cell 175, 1972–1988 (2018).
Grassi, L. et al. Organoids as a new model for improving regenerative medicine and cancer personalized therapy in renal diseases. Cell Death Dis. 10, 201 (2019).
Crespo, M. et al. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat. Med. 23, 878–884 (2017).
Drost, J. et al. Organoid culture systems for prostate epithelial and cancer tissue. Nat. Protoc. 11, 347–358 (2016).
Lee, S. H. et al. Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell 173, 515–528 (2018).
Kim, M. et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat. Commun. 10, 3991 (2019).
Broutier, L. et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 23, 1424–1435 (2017).
Minton, K. Studying tumour-specific T cell responses in 3D. Nat. Rev. Immunol. 18, 602–603 (2018).
de Souza, N. A model for tumor-immune interaction. Nat. Methods 15, 762 (2018).
Cattaneo, C. M. et al. Tumor organoid-T-cell coculture systems. Nat. Protoc. 15, 15–39 (2020).
Cafri, G. et al. Memory T cells targeting oncogenic mutations detected in peripheral blood of epithelial cancer patients. Nat. Commun. 10, 449 (2019).
Clarke, S. R. et al. Characterization of the ovalbumin-specific TCR transgenic line OT-I: MHC elements for positive and negative selection. Immunol. Cell Biol. 78, 110–117 (2000).
Rapoport, A. P. et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat. Med. 21, 914–921 (2015).
Wu, R. et al. Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma current status and future outlook. Cancer J. 18, 160–175 (2012).
Banh, R. S. et al. PTP1B controls non-mitochondrial oxygen consumption by regulating RNF213 to promote tumour survival during hypoxia. Nat. Cell Biol. 18, 803–813 (2016).
Gogna, R., Madan, E., Kuppusamy, P. & Pati, U. Re-oxygenation causes hypoxic tumor regression through restoration of p53 wild-type conformation and post-translational modifications. Cell Death Dis. 3, e286 (2012).
Quail, D. F. & Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437 (2013).
Joyce, J. A. & Pollard, J. W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 9, 239–252 (2009).
Ivashkiv, L. B. IFNγ: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 18, 545–558 (2018).
Motyka, B. et al. Mannose 6-phosphate/insulin-like growth factor II receptor is a death receptor for granzyme B during cytotoxic T cell-induced apoptosis. Cell 103, 491–500 (2000).
Croft, M. The role of TNF superfamily members in T-cell function and diseases. Nat. Rev. Immunol. 9, 271–285 (2009).
Sheng, W. et al. LSD1 ablation stimulates anti-tumor immunity and enables checkpoint blockade. Cell 174, 549–563 (2018).
Blum, J. S., Wearsch, P. A. & Cresswell, P. Pathways of antigen processing. Annu Rev. Immunol. 31, 443–473 (2013).
Leone, P. et al. MHC class I antigen processing and presenting machinery: organization, function, and defects in tumor cells. J. Natl Cancer Inst. 105, 1172–1187 (2013).
Ostrand-Rosenberg, S. & Fenselau, C. Myeloid-derived suppressor cells: immune-suppressive cells that impair antitumor immunity and are sculpted by their environment. J. Immunol. 200, 422–431 (2018).
Sacchi, A. et al. Myeloid-derived suppressor cells specifically suppress IFN-γ production and antitumor cytotoxic activity of Vδ2 T cells. Front. Immunol. 9, 1271 (2018).
Gray, M. J. et al. Phosphatidylserine-targeting antibodies augment the anti-tumorigenic activity of anti-PD-1 therapy by enhancing immune activation and downregulating pro-oncogenic factors induced by T-cell checkpoint inhibition in murine triple-negative breast cancers. Breast Cancer Res. 18, 50 (2016).
Gray, M., Gong, J., Nguyen, V., Hutchins, J. & Freimark, B. Targeting of phosphatidylserine by monoclonal antibodies augments the activity of immune checkpoint inhibitor PD-1/PD-L1 therapy in murine breast tumors. Cancer Res. 76, abstr. P4-04-03 (2016).
Ceccacci, E. & Minucci, S. Inhibition of histone deacetylases in cancer therapy: lessons from leukaemia. Br. J. Cancer 114, 605–611 (2016).
Munster, P. N. et al. A phase II study of the histone deacetylase inhibitor vorinostat combined with tamoxifen for the treatment of patients with hormone therapy-resistant breast cancer. Br. J. Cancer 104, 1828–1835 (2011).
Terranova-Barberio, M. et al. HDAC inhibition potentiates immunotherapy in triple negative breast cancer. Oncotarget 8, 114156–114172 (2017).
Phan, N. et al. A simple high-throughput approach identifies actionable drug sensitivities in patient-derived tumor organoids. Commun. Biol. 2, 78 (2019).
Bradley, C. A. Gastrointestinal cancer: organoids predict clinical responses. Nat. Rev. Gastroenterol. Hepatol. 15, 189 (2018).
Clevers, H. Modeling development and disease with organoids. Cell 165, 1586–1597 (2016).
Kondo, J. & Inoue, M. Application of cancer organoid model for drug screening and personalized therapy. Cells 8, 470 (2019).
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Fatehullah, A., Tan, S. H. & Barker, N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 18, 246–254 (2016).
Appay, V., Douek, D. C. & Price, D. A. CD8+ T cell efficacy in vaccination and disease. Nat. Med. 14, 623–628 (2008).
Qiu, G. Z. et al. Reprogramming of the tumor in the hypoxic niche: the emerging concept and associated therapeutic strategies. Trends Pharmacol. Sci. 38, 669–686 (2017).
Lai, C. J. et al. CUDC-101, a multitargeted inhibitor of histone deacetylase, epidermal growth factor receptor, and human epidermal growth factor receptor 2, exerts potent anticancer activity. Cancer Res. 70, 3647–3656 (2010).
Jayathilaka, N. et al. Inhibition of the function of class IIa HDACs by blocking their interaction with MEF2. Nucleic Acids Res. 40, 5378–5388 (2012).
Banik, D., Moufarrij, S. & Villagra, A. Immunoepigenetics combination therapies: an overview of the role of HDACs in cancer immunotherapy. Int. J. Mol. Sci. 20, 2241 (2019).
Xu, H. et al. Organoid technology and applications in cancer research. J. Hematol. Oncol. 11, 116 (2018).
Yang, W. et al. Potentiating the antitumour response of CD8+ T cells by modulating cholesterol metabolism. Nature 531, 651–655 (2016).
Li, Y. et al. Heterozygous deletion of chromosome 17p renders prostate cancer vulnerable to inhibition of RNA polymerase II. Nat. Commun. 9, 4394 (2018).
Liu, Y. H. et al. TP53 loss creates therapeutic vulnerability in colorectal cancer. Nature 520, 697–U286 (2015).
Van der Jeught, K. et al. ST2 as checkpoint target for colorectal cancer immunotherapy. JCI Insight 5, e136073 (2020).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
McCarthy, D. J., Chen, Y. & Smyth, G. K. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 40, 4288–4297 (2012).
Dennis, G.Jr. et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 4, R60 (2003).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849 (2016).
Acknowledgements
We thank the staff at the Laboratory Animal Resource Center of Indiana University for their technical support in animal studies; the staff at the Indiana Center for Biological Microscopy, the Flow Cytometry Resource Facility (FCRF) and the Center for Medical Genomics of Indiana University for use of instruments and technical assistance; and the staff at the Indiana University Simon Comprehensive Cancer Center (IUSCCC) for providing human breast tissue samples. This work was supported in part by US National Institutes of Health grants R01CA203737 (to X.L.), R01CA206366 (to X.L. and X.H.), R01CA243023 (to X.L. and X.H.) and R01CA222251 (to X.L.), and by the Indiana University Strategic Research Initiative fund (to X.L.) and the Vera Bradley Foundation for Breast Cancer Research (to X.L. and X.Z.).
Author information
Authors and Affiliations
Contributions
Z.Z., X.Z. and X.L. designed experiments in the study. K.V.d.J., Y.F., T.Y., Y.L. and L.Z. provided technical support and conducted animal studies and immunological analyses. Y.Y. and H.E. provided technical assistance in molecular studies. Z.A., X.W. and F.G. provided technical support for tumour organoid studies. M.L.C. coordinated breast cancer tissue procurement. G.J., B.P.S., X.H. and F.G. discussed results and provided valuable advice for the project. S.L. and J.W. conducted bioinformatics and statistical analyses. Z.Z., X.Z. and X.L. wrote and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
X.L. and X.Z. are the inventors on the US Provisional Patent Application (Serial No. 63/129,762) submitted by The Trustees of Indiana University for the organoid-based screen method and identified drugs in this study.
Additional information
Peer review information Nature Biomedical Engineering thanks Paola Scaffidi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
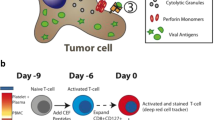
Extended Data Fig. 1 Functional evaluation of the compounds screened from the 2D and tumour-organoid systems.
a, Pie chart of the positive compounds screened from the 2D and tumour organoid systems. b, Effect of the above compounds (a) on T cell infiltration in tumour organoids. The CD8+ T cells (from OT-I mouse) were co-cultured with the compound-treated GFP+Luc+OVA+ EO771 tumour organoids for 48 h. The tumour organoids with T cells infiltrated or attached were dissociated to single cells and stained with APC/Cy7-conjugated anti-mouse CD8 and SYTOX Blue reagent for 15 min. The T cell proportions from the tumour organoids were analyzed by flow cytometry. Data from 3 biologically parallel experiments were analyzed using One-way ANOVA and presented as mean ± SD. ***, p < 0.001; ****, p < 0.0001. c, Representative flow cytometry data showing the CD8+ T cell proportion in the GFP+Luc+OVA+ EO771 tumour organoids. A total of 20,000 events per cell sample were collected for data analysis. d, Representative optical images of CD8+ T cells co-cultured with the GFP+Luc+OVA+ EO771 tumour organoids. e-g, Gross dissected mammary tumour images (e), tumour growth (f) and weight (g) of the EO771 tumours from the tumour-bearing C57BL/6 mice treated with vehicle control, PFI-1 (20 mg kg-1), or Bromosporine (20 mg kg-1). Tumours were harvested at day 28 post injection. For statistical analysis of data, one-way ANOVA test was used in (f,g). Data are presented as mean ± SD. ns, no significance.
Extended Data Fig. 2 Validation of antitumour activity of the three drug candidates in human tumour organoids.
a, Schematic illustration of the drug-validation test using NY-ESO-1+ MDA-MB-468 organoids co-cultured with NY-ESO-1-specific CD8+ T cells. Human breast cancer-associated fibroblasts (CAFs) were used with MDA-MB-468 cells to generate tumour organoids. b, Optical images showing the co-culture of the CD8+ T cells and MDA-MB-468 tumour organoids treated with control or drug candidates.
Extended Data Fig. 3 Antitumour activity of drug candidates in mouse breast tumour models.
a, Drug-treatment scheme of mouse breast tumour models. b, Gross dissected mammary tumour images of the EO771 tumours from the tumour-bearing C57BL/6 mice treated with vehicle control, BML-210 (20 mg kg-1), or CUDC-101 (20 mg kg-1). c, IHC staining images of Ki67+ and cleaved Caspase 3+ cells in the tumour tissues. d, Gross dissected mammary tumour images of the EO771 tumours from the tumour-bearing nude mice treated with vehicle control, BML-210 (20 mg kg-1), or CUDC-101 (20 mg kg-1). Tumours from nude mice were harvested at day 18 post injection. e, Gross mammary tumour images of the EO771 tumours from the tumour-bearing C57BL/6 mice treated with isotype control, CUDC-101 (20 mg kg-1), BML-210 (20 mg kg-1), anti-CD8 (10 mg kg-1) + CUDC-101 (20 mg kg-1), anti-CD8 (10 mg kg-1) + BML-210 (20 mg kg-1), anti-CD4 (10 mg kg-1) + CUDC-101 (20 mg kg-1) or anti-CD4 (10 mg kg-1) + BML-210 (20 mg kg-1). Tumours from tumour-bearing C57BL/6 mice were harvested at day 28 post injection.
Extended Data Fig. 4 Antitumour activity of BML-210 and CUDC-101 in mouse breast tumour models with CD4+ or CD8+ T-cell depletion.
a, Drug-treatment scheme of mouse breast tumour models. b, Flow cytometry gating strategy for analysis of CD4+ and CD8+ T cells from EO771 tumours in C57BL/6 mice. c, Typical graphs showing the proportion of CD4+ and CD8+ T cells in total T cells (CD3+) from EO771 tumours treated with vehicle control or indicated compound, and with CD4 or CD8 depletion. d,e, Proportions of CD8+ (d) and CD4+ (e) T cells in total T cells from EO771 tumours with CD4 or CD8 depletion. f,g, Weight of the EO771 tumours from the tumour-bearing C57BL/6 mice treated with control, CUDC-101 (20 mg kg-1), BML-210 (20 mg kg-1), anti-CD8 (10 mg kg-1) + CUDC-101 (20 mg kg-1), anti-CD8 (10 mg kg-1) + BML-210 (20 mg kg-1), anti-CD4 (10 mg kg-1) + CUDC-101 (20 mg kg-1) or anti-CD4 (10 mg kg-1) + BML-210 (20 mg kg-1). Tumours from tumour-bearing C57BL/6 mice were harvested at day 28 post injection. For statistical analysis of data, two-way ANOVA test was used in (d,e). One-way ANOVA test was used in (f,g). Data are presented as mean ± SD. ****, p < 0.0001; ns, no significance.
Extended Data Fig. 5 Antitumour activity of GSK-LSD1 in mouse breast tumour models.
a, Drug-treatment scheme of mouse breast tumour models. b-d, Gross dissected mammary tumour images (b), tumour growth (c) and weight (d) of the EO771 tumours from the tumour-bearing C57BL/6 mice treated with vehicle control, GSK-LSD1 (20 mg kg-1). e, Proportions of total T, CD4+ T, and CD8+ T cells in total immune (CD45+) cells in the EO771 tumours treated with control, GSK-LSD1. f, Percentage of active cells in total CD8+ T cells, indicated by GZMB+, IFNγ+, TNFα+ in flow cytometry analysis. g, IHC staining images of Ki67+ and cleaved Caspase 3+ cells in the tumour tissues. h,i, Quantitative results for (g). For statistical analysis of data, Two-sided Student’s t-test was used in (c,d,h,i) and two-way ANOVA test was used in (e,f). Data are presented as mean ± SD. ***, p < 0.001; ****, p < 0.0001; ns, no significance.
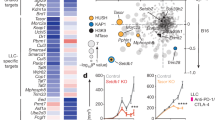
Extended Data Fig. 6 Drug candidates promotes the expression of genes in the antigen presentation of breast tumour cells.
a, Gene-set enrichment plot of the antigen processing and presentation pathway in EO771 cells treated with BML-210 in comparison with the cells treated with vehicle control. b, Validation of up-regulated genes from Fig. 6c in human MDA-MB-468 cells treated with vehicle control or BML-210 (1.0 μM) by quantitative RT-PCR. c, Levels of HLA-A,B,C on the NY-ESO-1+ MDA-MB-468 cells treated with control or BML-201, determined by MFI in flow cytometry analysis. d, H-2k expression levels in the OVA+ EO771 cells treated with CUDC-101 or GSK-LSD1 were determined by qRT-PCR. e, HLA gene expression levels in NY-ESO-1+ MDA-MB-468 cells treated with CUDC-101 or GSK-LSD1 were determined by qRT-PCR. f, B2M expression levels in the OVA+ EO771 cells treated with CUDC-101 or GSK-LSD1 were determined by qRT-PCR. g,h, The effect of drug treatment on the H-2Kb and HLA-A2 antigen presentation on OVA+ EO771 cells and NY-ESO-1+ MDA-MB-468 cells, respectively. Data (b,d-f) from 3 biologically parallel experiments were analyzed using Two-way ANOVA. Data (c,g,h) from 3 biologically parallel experiments were analyzed using One-way ANOVA. ***, p < 0.001; ****, p < 0.0001; ns, no significance.
Extended Data Fig. 7 BML-210 promotes the expression of genes in the antigen presentation of breast tumour cells.
a,b, Confocal images showing H-2Kb and HLA-A2 on OVA+ EO771 cells (a) and NY-ESO-1+ MDA-MB-468 cells (b), respectively. The immunofluorescence images were analyzed by ImageJ. c, Quantitative analysis of confocal images in (a) for assessing H-2Kb antigen presentation. d, Quantitative analysis of confocal images in (b) for assessing HLA-A2 antigen presentation. e, Western blot showing B2M protein expression levels in OVA+ EO771 cells treated with BML-210 drug with 0, 0.1, 1 μM for 48 h. f, Western blot showing B2M knockdown in OVA+ EO771 cells. One-way ANOVA test was conducted for statistical analysis in (c,d). Data are presented as mean ± SD. ****, p < 0.0001.
Supplementary information
Source data
Source Data Fig. 2
Source data for tumour burden (panel e).
Source Data Fig. 5
Source data for tumour burden (panels a, g, h and i).
Source Data Fig. 7
Source data for tumour burden (panel e).
Source Data Extended Data Fig. 1
Source data for tumour burden (panel f).
Source Data Extended Data Fig. 5
Source data for tumour burden (panel c).
Source Data Extended Data Fig. 7
Uncropped western blots (panels e and f).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Z., Van der Jeught, K., Fang, Y. et al. An organoid-based screen for epigenetic inhibitors that stimulate antigen presentation and potentiate T-cell-mediated cytotoxicity. Nat Biomed Eng 5, 1320–1335 (2021). https://doi.org/10.1038/s41551-021-00805-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-021-00805-x
This article is cited by
-
Patient-derived organoids in human cancer: a platform for fundamental research and precision medicine
Molecular Biomedicine (2024)
-
Drug repurposing for cancer therapy
Signal Transduction and Targeted Therapy (2024)
-
Harnessing 3D in vitro systems to model immune responses to solid tumours: a step towards improving and creating personalized immunotherapies
Nature Reviews Immunology (2024)
-
Breast cancer organoids and their applications for precision cancer immunotherapy
World Journal of Surgical Oncology (2023)
-
Newly developed 3D in vitro models to study tumor–immune interaction
Journal of Experimental & Clinical Cancer Research (2023)