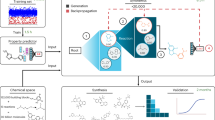
Abstract
The emergence of drug-resistant bacteria calls for the discovery of new antibiotics. Yet, for decades, traditional discovery strategies have not yielded new classes of antimicrobial. Here, by mining the human proteome via an algorithm that relies on the sequence length, net charge, average hydrophobicity and other physicochemical properties of antimicrobial peptides, we report the identification of 2,603 encrypted peptide antibiotics, many of which are encoded in proteins with biological function unrelated to the immune system. We show that the encrypted peptides kill pathogenic bacteria by targeting their membrane, modulate gut and skin commensals, do not readily select for bacterial resistance, and possess anti-infective activity in skin abscess and thigh infection mouse models. We also show, in vitro and in the two mouse models of infection, that encrypted antibiotic peptides from the same biogeographical area display synergistic antimicrobial activity. Our algorithmic strategy allows for the rapid mining of proteomic data and opens up new routes for the discovery of candidate antibiotics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The list of the identified encrypted peptides, and source data for the normalized abundance of genes encoding different protein classes, for amino acid frequency in encrypted peptides compared with known AMPs, for expression levels of proteins containing encrypted peptides, and for antimicrobial activity (in vitro and in vivo), as well as data on synergistic interactions, evolution of resistance and mechanism of action, are provided with this paper. Source data are provided with this paper.
Code availability
The custom Python code for scanning the human proteome to detect candidate encrypted peptides is available as Supplementary Information.
Change history
01 December 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41551-022-00967-2
References
2019 Antimicrobial Resistant Threats Report (Centers for Disease Control and Prevention, 2019).
World Health Organization. Antimicrobial resistance: Global Health Report on Surveillance. Bull. World Health Organ. https://doi.org/10.1007/s13312-014-0374-3 (2014).
Lepore, C., Silver, L., Theuretzbacher, U., Thomas, J. & Visi, D. The small-molecule antibiotics pipeline: 2014–2018. Nat. Rev. Drug Discov. https://doi.org/10.1038/d41573-019-00130-8 (2019).
de la Fuente-Nunez, C., Torres, M. D., Mojica, F. J. & Lu, T. K. Next-generation precision antimicrobials: towards personalized treatment of infectious diseases. Curr. Opin. Microbiol. 37, 95–102 (2017).
Torres, P. H. M., Sodero, A. C. R., Jofily, P. & Silva-Jr, F. P. Key topics in molecular docking for drug design. Int. J. Mol. Sci. 20, 4574 (2019).
Torres, M. D. T., Cao, J., Franco, O. L., Lu, T. K. & de la Fuente-Nunez, C. Synthetic biology and computer-based frameworks for antimicrobial peptide discovery. ACS Nano 15, 2143–2164 (2021).
Torres, M. D. T. & de la Fuente-Nunez, C. Toward computer-made artificial antibiotics. Curr. Opin. Microbiol. 51, 30–38 (2019).
Porto, W. F. et al. In silico optimization of a guava antimicrobial peptide enables combinatorial exploration for peptide design. Nat. Commun. 9, 1490 (2018).
Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 415, 389–395 (2002).
Pane, K. et al. Antimicrobial potency of cationic antimicrobial peptides can be predicted from their amino acid composition: application to the detection of “cryptic” antimicrobial peptides. J. Theor. Biol. 419, 254–265 (2017).
Mi, H., Muruganujan, A., Ebert, D., Huang, X. & Thomas, P. D. PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 47, D419–D426 (2019).
Pirtskhalava, M. et al. DBAASP v.2: an enhanced database of structure and antimicrobial/cytotoxic activity of natural and synthetic peptides. Nucleic Acids Res. 44, D1104–D1112 (2016).
Kang, X. et al. DRAMP 2.0, an updated data repository of antimicrobial peptides. Sci. Data 6, 148 (2019).
Cullen, T. W. et al. Antimicrobial peptide resistance mediates resilience of prominent gut commensals during inflammation. Science 347, 170–175 (2015).
Almeida, A. et al. A new genomic blueprint of the human gut microbiota. Nature 568, 499–504 (2019).
Kim, Y.-H., O’Neill, H. M. & Whitehead, J. P. Carboxypeptidase X-1 (CPX-1) is a secreted collagen-binding glycoprotein. Biochem. Biophys. Res. Commun. 468, 894–899 (2015).
Kim, Y. et al. Identification of carboxypeptidase X (CPX)‐1 as a positive regulator of adipogenesis. FASEB J. 30, 2528–2540 (2016).
Lei, Y., Xin, X., Morgan, D., Pintar, J. E. & Fricker, L. D. Identification of mouse CPX-1, a novel member of the metallocarboxypeptidase gene family with highest similarity to CPX-2. DNA Cell Biol. 18, 175–185 (1999).
Cani, P. D. & de Vos, W. M. Next-generation beneficial microbes: the case of Akkermansia muciniphila. Front. Microbiol. 8, 1765 (2017).
Brenner, A. V. et al. Common single nucleotide polymorphisms in genes related to immune function and risk of papillary thyroid cancer. PLoS ONE 8, e57243 (2013).
Bork, P. & Beckmann, G. The CUB domain. J. Mol. Biol. 231, 539–545 (1993).
Nordahl, E. A. et al. Activation of the complement system generates antibacterial peptides. Proc. Natl Acad. Sci. USA 101, 16879–16884 (2004).
Durack, J. & Lynch, S. V. The gut microbiome: relationships with disease and opportunities for therapy. J. Exp. Med. 216, 20–40 (2019).
Shreiner, A. B., Kao, J. Y. & Young, V. B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 31, 69–75 (2015).
Uberoi, A. et al. Commensal microbiota regulates skin barrier function and repair via signaling through the aryl hydrocarbon receptor. Cell Host Microbe 29, 1235–1248.e8 (2021).
Grice, E. The skin microbiome: potential for novel diagnostic and therapeutic approaches to cutaneous disease. Semin. Cutan. Med. Surg. 33, 98–103 (2014).
Sanford, J. A. & Gallo, R. L. Functions of the skin microbiota in health and disease. Semin. Immunol. 25, 370–377 (2013).
Robinson, S. D. et al. A comprehensive portrait of the venom of the giant red bull ant, Myrmecia gulosa, reveals a hyperdiverse hymenopteran toxin gene family. Sci. Adv. 4, eaau4640 (2018).
Torres, M. D. T. et al. Structure-function-guided exploration of the antimicrobial peptide polybia-CP identifies activity determinants and generates synthetic therapeutic candidates. Commun. Biol. 1, 221 (2018).
Magana, M. et al. The value of antimicrobial peptides in the age of resistance. Lancet Infect. Dis. https://doi.org/10.1016/S1473-3099(20)30327-3 (2020).
Breidenstein, E. B. M., de la Fuente-Núñez, C. & Hancock, R. E. W. Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol. 19, 419–426 (2011).
Pachori, P., Gothalwal, R. & Gandhi, P. Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit; a critical review. Genes Dis. 6, 109–119 (2019).
Tyers, M. & Wright, G. D. Drug combinations: a strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 17, 141–155 (2019).
Reffuveille, F., de la Fuente-Núñez, C., Mansour, S. & Hancock, R. E. W. A broad-spectrum antibiofilm peptide enhances antibiotic action against bacterial biofilms. Antimicrob. Agents Chemother. 58, 5363–5371 (2014).
Merg, F. et al. Big dynorphin as a putative endogenous ligand for the kappa-opioid receptor. J. Neurochem. 97, 292–301 (2006).
Wang, Z. et al. Elabela-apelin receptor signaling pathway is functional in mammalian systems. Sci. Rep. 5, 8170 (2015).
Cheah, S.-E. et al. Polymyxin resistance in Acinetobacter baumannii: genetic mutations and transcriptomic changes in response to clinically relevant dosage regimens. Sci. Rep. 6, 26233 (2016).
Samaras, P. et al. ProteomicsDB: a multi-omics and multi-organism resource for life science research. Nucleic Acids Res. 48, D1153–D1163 (2019).
Fleming, A. Penicillin. Nobel lecture. Nobel Foundation https://www.nobelprize.org/uploads/2018/06/fleming-lecture.pdf (1945).
Fensterseifer, I. C. M. et al. Selective antibacterial activity of the cationic peptide PaDBS1R6 against Gram-negative bacteria. Biochim. Biophys. Acta Biomembr. 1861, 1375–1387 (2019).
Cardoso, M. H. et al. A computationally designed peptide derived from Escherichia coli as a potential drug template for antibacterial and antibiofilm therapies. ACS Infect. Dis. 4, 1727–1736 (2018).
Cândido, E. S. et al. Short cationic peptide derived from archaea with dual antibacterial properties and anti-infective potential. ACS Infect. Dis. 5, 1081–1086 (2019).
Pane, K. et al. Identification of novel cryptic multifunctional antimicrobial peptides from the human stomach enabled by a computational–experimental platform. ACS Synth. Biol. 7, 2105–2115 (2018).
Ling, L. L. et al. A new antibiotic kills pathogens without detectable resistance. Nature 517, 455–459 (2015).
Gudmundsson, S. & Erlendsdóttir, H. in Handbook of Animal Models of Infection (eds Zak, O. & Sande M. A.) 137–144 (Elsevier, 1999).
Sberro, H. et al. Large-scale analyses of human microbiomes reveal thousands of small, novel genes. Cell 178, 1245–1259.e14 (2019).
Donia, M. S. & Fischbach, M. A. Small molecules from the human microbiota. Science 349, 1254766 (2015).
Sugimoto, Y. et al. A metagenomic strategy for harnessing the chemical repertoire of the human microbiome. Science 366, eaax9176 (2019).
Culp, E. J. et al. Hidden antibiotics in actinomycetes can be identified by inactivation of gene clusters for common antibiotics. Nat. Biotechnol. 37, 1149–1154 (2019).
The UniProt Consortium. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 47, D506–D515 (2019).
Acknowledgements
C.d.l.F.-N. holds a Presidential Professorship at the University of Pennsylvania, is a recipient of the Langer Prize by the AIChE Foundation and acknowledges funding from the Institute for Diabetes, Obesity, and Metabolism, the Penn Mental Health AIDS Research Center of the University of Pennsylvania, the Nemirovsky Prize, and the Dean’s Innovation Fund from the Perelman School of Medicine at the University of Pennsylvania. Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R35GM138201 and the Defense Threat Reduction Agency (DTRA; HDTRA11810041 and HDTRA1-21-1-0014). The authors also acknowledge resource and expertise contributions from Simon Knight and Elizabeth Grice at the University of Pennsylvania, and support by the Burroughs Wellcome Fund PATH Award (Principal investigator (PI), Elizabeth Grice), the Linda Pechenik Montague Investigator Award (Perelman School of Medicine; PI, Grice), and the Penn Skin Biology and Diseases Resource-based Center (NIH/NIAMS P30AR069589; PI, Grice). L.F. was supported by the Dermatology Research Training Grant (NIH/NIAMS T32AR007465; PIs, Grice and Margolis). Mark Goulian kindly donated E. coli AIC221 and AIC222. All figures were prepared using the Biorender drawing toolkit. The authors thank E. Broset and all members of the de la Fuente Lab for insightful discussions.
Author information
Authors and Affiliations
Contributions
M.D.T.T. and C.d.l.F.N. designed the experiments. M.D.T.T. performed all biological activity experiments, mechanism of action assays and animal models. M.C.R.M., O.C. and E.N. performed algorithm development and database searches. L.F. isolated the skin commensals described in ref. 25 and used in this study. M.D.T.T. and C.d.l.F.N. wrote the first draft of the manuscript. M.C.R.M., O.C. and E.N. revised the manuscript. C.d.l.F.N. supervised and administered the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Biomedical Engineering thanks Mohan Babu, Kim Lewis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Expression levels of proteins containing encrypted peptides.
a, Schematic of the biogeographic region within the human body where proteins containing encrypted peptides are located. Expression levels are displayed in a gradient; organs in blue indicate high expression levels and organs in red, low expression levels. b, Normalized expression level values expressed in log10 intensity based absolute quantification (iBAQ), a commonly used metric for protein abundance47.
Extended Data Fig. 2 Synergy between encrypted peptides found within the same area of the human body.
a, Experimental layout of the 96-well plates used for two-way synergy experiments using pairs of encrypted peptides. The following encrypted peptides were used: apelin-36, apelin receptor early endogenous 1, natriuretic peptide, big dynorphin, FIBa-GVV27, vWF-PQR19, SRFP1-KKI32, SRFP1-FAL48, INTb-FTR26, SCUB1-SKE25, SCUB3-KHK26, and SCUB3-MLP22. Briefly, two-fold dilutions ranging from 0 to 50 μmol L-1 of the peptide solutions were plated in 96-well plates and 106 bacterial cells in NB were added to each well to reach a final volume of 200 μL. b, The FIC value, which indicates the degree of synergy between two antimicrobial agents against a target microorganism (in this case, P. aeruginosa PAO1) was calculated based on the MICs of the peptides used alone and in combination. FIC index values ≤0.5 indicate synergy; additive effects are captured by 0.5≤FIC≤1; 1≤FIC≤4 indicates indifference; and FIC index ≥4 represents antagonism. Assays were performed in three independent replicates.
Extended Data Fig. 3 Membrane permeabilization and depolarization assays for several bacterial strains.
a, Outer membrane permeabilization experiments showed that encrypted peptides (SCUB1-SKE25, SCUB3-KHK26 and natriuretic peptide) permeabilized the outer membranes of P. aeruginosa PAO1, B. fragilis ATCC25285, and S. epidermidis as much as they permeabilized the A. baumannii ATCC19606 outer membrane (Fig. 3e). b, Cytoplasmic membrane depolarization assays performed against P. aeruginosa PAO1 and the gut commensal B. fragilis ATCC25285. As shown for A. baumannii ATCC19606 (Fig. 3d), the encrypted peptides did not depolarize the cytoplasmic membrane. Data in a and b are the mean ± s.d. Assays were performed in three independent replicates.
Extended Data Fig. 4 Anti-infective activity and synergistic interactions of encrypted peptides against P. aeruginosa PAO1 in a neutropenic thigh infection mouse model.
a, SCUB1-SKE25 (25 μmol L-1; 77.9 μg mL-1) and SCUB3-MLP22 (25 μmol L-1; 66.9 μg mL-1) showed inhibitory activity against P. aeruginosa PAO1, especially when used in combination at their MIC (obtained from in vitro synergy experiments; 3.12 and 6.25 μmol L-1, respectively). b, Mouse weight was monitored throughout the duration of the neutropenic thigh infection model (8 days) and under all conditions tested to rule out potential toxic effects mediated by the encrypted peptides. The statistical significance in a was determined using one-way ANOVA, ***p < 0.001, ****p < 0.0001, features on the violin plots represent median and upper and lower quartiles. The data in b are the mean ± s.d. Eight mice were used per group.
Extended Data Fig. 5 Anti-infective activity of encrypted peptides in a neutropenic thigh infection mouse model.
Treatment with 4-fold MIC of SCUB1-SKE25 (100 μmol L-1; 311.6 μg mL-1) and SCUB3-MLP22 (100 μmol L-1; 267.6 μg mL-1) alone and in combination (at 12.5 and 25 μmol L-1, respectively). Both monotherapy and combination therapy displayed similar antimicrobial activity against A. baumannii ATCC19606 to treatment groups using the MIC of each peptide. Polymyxin B and levofloxacin were used as controls, the former of which completely cleared the infection. The statistical significance was determined using one-way ANOVA, ****p < 0.0001, features on the violin plots represent median and upper and lower quartiles. Four mice were used per group.
Supplementary information
Supplementary Information
Supplementary tables and figures.
Supplementary Dataset 1
List of encrypted peptides found in the human proteome according to our physicochemical features-based scoring function.
Supplementary Dataet 2
Source data for the Supplementary figures.
Supplementary Code
Python code for scanning the human proteome to detect candidate encrypted peptides.
Source data
Source data for Fig. 1
Source data.
Source data for Fig. 2
Source data.
Source data for Fig. 3
Source data.
Source data for Fig. 4
Source data.
Source data for Extended Data Fig. 1
Source data.
Source data for Extended Data Fig. 2
Source data.
Source data for Extended Data Fig. 3
Source data.
Source data for Extended Data Fig. 4
Source data.
Source data for Extended Data Fig. 5
Source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Torres, M.D.T., Melo, M.C.R., Flowers, L. et al. Mining for encrypted peptide antibiotics in the human proteome. Nat Biomed Eng 6, 67–75 (2022). https://doi.org/10.1038/s41551-021-00801-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-021-00801-1
This article is cited by
-
Promiscuous, persistent and problematic: insights into current enterococcal genomics to guide therapeutic strategy
BMC Microbiology (2024)
-
Machine learning for antimicrobial peptide identification and design
Nature Reviews Bioengineering (2024)
-
Antimicrobial peptides designed by computational analysis of proteomes
Antonie van Leeuwenhoek (2024)
-
Evaluation of antibiotic use and analysis of ciprofloxacin and gentamicin residue in fish samples from farms in Lagos, Nigeria
Environmental Monitoring and Assessment (2024)
-
Mining for antimicrobial peptides in sequence space
Nature Biomedical Engineering (2023)