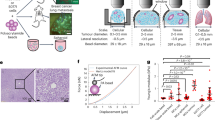
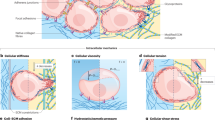
Abstract
Solid stress and tissue stiffness affect tumour growth, invasion, metastasis and treatment. Unlike stiffness, which can be precisely mapped in tumours, the measurement of solid stresses is challenging. Here, we show that 2D spatial maps of the solid stress and the resulting elastic energy in excised or in situ tumours with arbitrary shapes and a wide range of sizes can be obtained via three distinct and quantitative techniques that rely on the measurement of tissue displacement after disruption of the confining structures. Application of these methods in models of primary tumours and metastasis revealed that (i) solid stress depends on both cancer cells and their microenvironments, (ii) solid stress increases with tumour size and (iii) mechanical confinement by the surrounding tissue substantially contributes to intratumoral solid stress. Further study of the genesis and consequences of solid stress, facilitated by the engineering principles presented here, may lead to new discoveries and therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jain, R. K., Martin, J. D. & Stylianopoulos, T . The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng. 16, 321–346 (2014).
Chaudhuri, O. et al. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 13, 970–978 (2014).
Mouw, J. K. et al. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat. Med. 20, 360–367 (2014).
Samuel, M. S. et al. Actomyosin-mediated cellular tension drives increased tissue stiffness and β-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell 19, 776–791 (2011).
Wirtz, D., Konstantopoulos, K. & Searson, P. C. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat. Rev. Cancer 11, 512–522 (2011).
Goetz, J. G. et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell 146, 148–163 (2011).
Tung, J. C. et al. Tumor mechanics and metabolic dysfunction. Free Radic. Biol. Med. 79, 269–280 (2015).
Helmlinger, G., Netti, P. A., Lichtenbeld, H. C., Melder, R. J. & Jain, R. K. Solid stress inhibits the growth of multicellular tumor spheroids. Nat. Biotechnol. 15, 778–783 (1997).
Stylianopoulos, T. et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc. Natl Acad. Sci. USA 109, 15101–15108 (2012).
Boucher, Y. & Jain, R. K. Microvascular pressure is the principal driving force for interstitial hypertension in solid tumors: implications for vascular collapse. Cancer Res. 52, 5110–5114 (1992).
Griffon-Etienne, G., Boucher, Y., Brekken, C., Suit, H. D. & Jain, R. K. Taxane-induced apoptosis decompresses blood vessels and lowers interstitial fluid pressure in solid tumors: clinical implications. Cancer Res. 59, 3776–3782 (1999).
Padera, T. P. et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science 296, 1883–1886 (2002).
Padera, T. P. et al. Pathology: cancer cells compress intratumour vessels. Nature 427, 695 (2004).
Chauhan, V. P. et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 4, 2516 (2013).
Jain, R. K. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell 26, 605–622 (2014).
Fernández-Sánchez, M. E. et al. Mechanical induction of the tumorigenic β-catenin pathway by tumour growth pressure. Nature 523, 92–95 (2015).
Tse, J. M. et al. Mechanical compression drives cancer cells toward invasive phenotype. Proc. Natl Acad. Sci. USA 109, 911–916 (2012).
Provenzano, P. P. et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21, 418–429 (2012).
Chauhan, V. P. et al. Compression of pancreatic tumor blood vessels by hyaluronan is caused by solid stress and not interstitial fluid pressure. Cancer Cell 26, 14–15 (2014).
US National Library of Medicine. Proton w/FOLFIRINOX-Losartan for pancreatic cancer. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT01821729 (2013).
US National Library of Medicine. PEGPH20 plus nab-paclitaxel plus Gemcitabine compared with nab-paclitaxel plus Gemcitabine in subjects with stage IV untreated pancreatic cancer (HALO-109-202). ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT01839487 (2013).
Chuong, C. & Fung, Y. in Frontiers in Biomechanics (eds Schmid-Schönbein, G. W. et al. ) Ch. 9 (Springer, 1986).
Taber, L. A. & Humphrey, J. D . Stress-modulated growth, residual stress, and vascular heterogeneity. J. Biomech. Eng. 123, 528–535 (2001).
Campas, O. et al. Quantifying cell-generated mechanical forces within living embryonic tissues. Nat. Methods 11, 183–189 (2014).
Grashoff, C. et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263–266 (2010).
Timoshenko, S. & Goodier, J. N. Theory of Elasticity (McGraw-Hill, 1951).
Plodinec, M. et al. The nanomechanical signature of breast cancer. Nat. Nanotech. 7, 757–765 (2012).
Lopez, J., Kang, I., You, W., McDonald, D. & Weaver, V. In situ force mapping of mammary gland transformation. Integr. Biol. (Camb). 3, 910–921 (2011).
Vakoc, B. J. et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat. Med. 15, 1219–1223 (2009).
Simon, D., Horgan, C. & Humphrey, J. Mechanical restrictions on biological responses by adherent cells within collagen gels. J. Mech. Behav. Biomed. Mat. 14, 216–226 (2012).
Orimo, A. et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121, 335–348 (2005).
Stylianopoulos, T. et al. Coevolution of solid stress and interstitial fluid pressure in tumors during progression: implications for vascular collapse. Cancer Res. 73, 3833–3841 (2013).
Voutouri, C., Mpekris, F., Papageorgis, P., Odysseos, A. D. & Stylianopoulos, T. Role of constitutive behavior and tumor-host mechanical interactions in the state of stress and growth of solid tumors. PLoS ONE 9, e104717 (2014).
Fukumura, D., Incio, J., Shankaraiah, R. & Jain, R. K. Obesity and cancer: an angiogenic and inflammatory link. Microcirculation 23, 191–206 (2016).
Incio, J. et al. Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov. 6, 852–869 (2016).
Van den Eynden, G. G. et al. The histological growth pattern of colorectal cancer liver metastases has prognostic value. Clin. Exp. Metastasis 29, 541–549 (2012).
Eefsen, R. et al. Growth pattern of colorectal liver metastasis as a marker of recurrence risk. Clin. Exp. Metastasis 32, 369–381 (2015).
Conklin, M. W. et al. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am. J. Pathol. 178, 1221–1232 (2011).
Good, D. W. et al. Elasticity as a biomarker for prostate cancer: a systematic review. BJU. Int. 113, 523–534 (2014).
Choi, W. J. et al. Predicting prognostic factors of breast cancer using shear wave elastography. Ultrasound Med. Biol. 40, 269–274 (2014).
Milosevic, M. F. et al. High tumor interstitial fluid pressure identifies cervical cancer patients with improved survival from radiotherapy plus cisplatin versus radiotherapy alone. Int. J. Cancer 135, 1692–1699 (2014).
Roh, H. et al. Interstitial hypertension in carcinoma of uterine cervix in patients: possible correlation with tumor oxygenation and radiation response. Cancer Res. 51, 6695–6698 (1991).
Fyles, A. et al. Long-term performance of interstial fluid pressure and hypoxia as prognostic factors in cervix cancer. Radiother. Oncol. 80, 132–137 (2006).
Discher, D. E., Janmey, P. & Wang, Y. L. Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 (2005).
Nia, H. T., Han, L., Li, Y., Ortiz, C. & Grodzinsky, A. Poroelasticity of cartilage at the nanoscale. Biophys. J. 101, 2304–2313 (2011).
Grodzinsky, A. J. Fields, Forces, and Flows in Biological Systems Ch. 4 (Garland Science, 2011).
Hutter, J. L. & Bechhoefer, J. Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 64, 1868–1873 (1993).
Kiviranta, P. et al. Collagen network primarily controls Poisson's ratio of bovine articular cartilage in compression. J. Orthop. Res. 24, 690–699 (2006).
Buschmann, M. D. et al. Stimulation of aggrecan synthesis in cartilage explants by cyclic loading is localized to regions of high interstitial fluid flow. Arch. Biochem. Biophys. 366, 1–7 (1999).
Netti, P. A., Berk, D. A., Swartz, M. A., Grodzinsky, A. J. & Jain, R. K. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 60, 2497–2503 (2000).
Morimoto-Tomita, M., Ohashi, Y., Matsubara, A., Tsuiji, M. & Irimura, T. Mouse colon carcinoma cells established for high incidence of experimental hepatic metastasis exhibit accelerated and anchorage-independent growth. Clin. Exp. Metastasis 22, 513–521 (2005).
Yuan, F. et al. Vascular permeability and microcirculation of gliomas and mammary carcinomas transplanted in rat and mouse cranial windows. Cancer Res. 54, 4564–4568 (1994).
Nia, H. T. et al. Dataset for solid stress and elastic energy as measures of tumour mechanopathology. figsharehttp://dx.doi.org/10.6084/m9.figshare.3796092 (2016).
Acknowledgements
We thank S. Roberge, C. Smith, J. Kahn and M. Duquette for technical assistance. We also thank P. Huang, N. Bardeesy and T. Irimura for providing MMTV-M3C, AK4.4 and SL4 cells, respectively. This work was supported in part by funding from the National Cancer Institute (P01-CA080124), an NCI Outstanding Investigator Award (R35-CA197743) and a Department of Defense Breast Cancer Research Innovator award (W81XWH-10-1-0016) to R.K.J., a DP2 OD008780 to T.P.P., a R01 grant (HL128168) to L.L.M., T.P.P. and J.W.B., a Susan G. Komen Foundation Fellowship (PDF14301739) to G.S., a National Institutes of Health award (F31HL126449) to M.D., and an UNCF-Merck Science Initiative Postdoctoral Fellowship, Burroughs Wellcome Fund Postdoctoral Enrichment Program Award and a NCI grant (F32CA183465) to D.J.
Author information
Authors and Affiliations
Contributions
H.T.N. and R.K.J. designed the study; H.T.N., H.L., G.S., M.D., D.J., N.R., J.I., K.J. performed the research; H.T.N., H.L., G.S., M.D., D.J., N.R., J.I., V.P.C., K.J., J.D.M., V.A., T.P.P., D.F., Y.B., F.J.H., A.J.G., J.W.B., L.L.M. and R.K.J. analysed the data; H.T.N., M.D., G.S., V.P.C., L.L.M. and R.K.J. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
R.K.J. received consultant fees from Ophthotech, SPARC, SynDevRx and XTuit. R.K.J. owns equity in Enlight, Ophthotech, SynDevRx and XTuit, and serves on the Board of Directors of XTuit and the Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, the Tekla Healthcare Opportunities Fund and the Tekla World Healthcare Fund. No reagents or funding from these companies were used in these studies.
Supplementary information
Supplementary information
Supplementary Notes, Figures and Tables (PDF 1138 kb)
MATLAB scripts
Custom MATLAB code (ZIP 4 kb)
Rights and permissions
About this article
Cite this article
Nia, H., Liu, H., Seano, G. et al. Solid stress and elastic energy as measures of tumour mechanopathology. Nat Biomed Eng 1, 0004 (2017). https://doi.org/10.1038/s41551-016-0004
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-016-0004
This article is cited by
-
Linking cell mechanical memory and cancer metastasis
Nature Reviews Cancer (2024)
-
The impact of tumor microenvironment: unraveling the role of physical cues in breast cancer progression
Cancer and Metastasis Reviews (2024)
-
3D bioprinted tumor model: a prompt and convenient platform for overcoming immunotherapy resistance by recapitulating the tumor microenvironment
Cellular Oncology (2024)
-
Measuring and modelling tumour heterogeneity across scales
Nature Reviews Bioengineering (2023)
-
Cell–extracellular matrix mechanotransduction in 3D
Nature Reviews Molecular Cell Biology (2023)