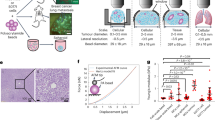
Abstract
Solid stress, distinct from both tissue stiffness and fluid pressure, is a mechanical stress that is often elevated in both murine and human tumors. The importance of solid stress in tumor biology has been recognized in initial studies: solid stress promotes tumor progression and lowers the efficacy of anticancer therapies by compressing blood vessels and contributing to hypoxia. However, robust, reproducible, and objective methods that go beyond demonstration and bulk measurements have not yet been established. We have developed three new techniques to rigorously measure and map solid stress in both human and murine tumors that are able to account for heterogeneity in the tumor microenvironment. We describe here these methods and their independent advantages: 2D spatial mapping of solid stress (planar-cut method), sensitive estimation of solid stress in small tumors (slicing method), and in situ solid-stress quantification (needle-biopsy method). Furthermore, the preservation of tissue morphology and structure allows for subsequent histological analyses in matched tumor sections, facilitating quantitative correlations between solid stress and markers of interest. The three procedures each require ∼2 h of experimental time per tumor. The required skill sets include basic experience in tumor resection and/or biopsy (in mice or humans), as well as in intravital imaging (e.g., ultrasonography).
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Helmlinger, G., Netti, P.A., Lichtenbeld, H.C., Melder, R.J. & Jain, R.K. Solid stress inhibits the growth of multicellular tumor spheroids. Nat. Biotechnol. 15, 778–783 (1997).
Padera, T.P. et al. Pathology: cancer cells compress intratumour vessels. Nature 427, 695 (2004).
Stylianopoulos, T. et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc. Natl. Acad. Sci. USA 109, 15101–15108 (2012).
Chauhan, V.P. et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 4, 2516 (2013).
Diop-Frimpong, B., Chauhan, V.P., Krane, S., Boucher, Y. & Jain, R.K. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc. Natl. Acad. Sci. USA 108, 2909–2914 (2011).
ClinicalTrials.gov: Proton w/FOLFIRINOX-Losartan for Pancreatic Cancer; identifier NCT01821729. Available from: https://clinicaltrials.gov/ct2/show/NCT01821729.
Murphy, J.E. et al. TGF-B1 inhibition with losartan in combination with FOLFIRINOX (F-NOX) in locally advanced pancreatic cancer (LAPC): preliminary feasibility and R0 resection rates from a prospective phase II study. J. Clin. Oncol. 35, suppl. 4S; abstr. 386 (2017).
Liu, H. et al. Use of angiotensin system inhibitors is associated with immune activation and longer survival in non-metastatic pancreatic ductal adenocarcinoma. Clin. Cancer Res. 23, 5959 (2017).
Nia, H.T. et al. Solid stress and elastic energy as measures of tumour mechanopathology. Nat. Biomed. Eng. 1, 0004 (2016).
Campas, O. et al. Quantifying cell-generated mechanical forces within living embryonic tissues. Nat. Methods 11, 183–189 (2014).
Choi, W.J. et al. Predicting prognostic factors of breast cancer using shear wave elastography. Ultrasound Med. Biol. 40, 269–274 (2014).
Conklin, M.W. et al. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am. J. Pathol. 178, 1221–1232 (2011).
Lu, P., Weaver, V.M. & Werb, Z. The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 196, 395–406 (2012).
ClinicalTrials.gov: PEGPH20 Plus Nab-Paclitaxel Plus Gemcitabine Compared With Nab-Paclitaxel Plus Gemcitabine in Subjects With Stage IV Untreated Pancreatic Cancer (HALO-109-202). Available from: https://clinicaltrials.gov/ct2/show/NCT01839487.
Scarcelli, G. et al. Noncontact three-dimensional mapping of intracellular hydromechanical properties by Brillouin microscopy. Nat. Methods 12, 1132–1134 (2015).
Hajjarian, Z. et al. Laser speckle rheology for evaluating the viscoelastic properties of hydrogel scaffolds. Sci. Rep. 6, 37949 (2016).
Kiviranta, P. et al. Collagen network primarily controls Poisson′s ratio of bovine articular cartilage in compression. J. Orthop. Res. 24, 690–699 (2006).
Buschmann, M.D. et al. Stimulation of aggrecan synthesis in cartilage explants by cyclic loading is localized to regions of high interstitial fluid flow. Arch. Biochem. Biophys. 366, 1–7 (1999).
Nia, H.T., Han, L., Li, Y., Ortiz, C. & Grodzinsky A . Poroelasticity of cartilage at the nanoscale. Biophys. J. 101, 2304–2313 (2011).
Netti, P.A., Berk, D.A., Swartz, M.A., Grodzinsky, A.J. & Jain, R.K. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 60, 2497–2503 (2000).
Vakoc, B.J. et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat. Med. 15, 1219–1223 (2009).
Grodzinsky, A.J. Fields, Forces, and Flows in Biological Systems Chapter 4 139–173 (Garland Science, 2011).
Jain, R.K., Martin, J.D. & Stylianopoulos, T. The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng. 16, 321 (2014).
Roose, T., Netti, P.A., Munn, L.L., Boucher, Y. & Jain, R.K. Solid stress generated by spheroid growth estimated using a linear poroelasticity model. Microvasc. Res. 66, 204–212 (2003).
Xue, S.-L., Li, B., Feng, X.-Q. & Gao, H. Biochemomechanical poroelastic theory of avascular tumor growth. J. Mech. Phys. Solids 94, 409–432 (2016).
Buschmann, M.D. et al. Stimulation of aggrecan synthesis in cartilage explants by cyclic loading is localized to regions of high interstitial fluid flow 1. Arch. Biochem. Biophys. 366, 1–7 (1999).
Meijer, E.F. et al. Murine chronic lymph node window for longitudinal intravital lymph node imaging. Nat. Protoc. 12, 1513–1520 (2017).
Jeong, H.-S. et al. Investigation of the lack of angiogenesis in the formation of lymph node metastases. J. Natl. Cancer Inst. 107 djv155 (2015).
Incio, J. et al. Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov. 6, 852–69 (2016).
Rahbari, N.N. et al. Anti-VEGF therapy induces ECM remodeling and mechanical barriers to therapy in colorectal cancer liver metastases. Sci. Transl. Med. 8, 360ra135 (2016).
Peterson, T.E. et al. Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc. Natl. Acad. Sci. USA 113, 4470–4475 (2016).
Acknowledgements
We thank S. Roberge for technical assistance. We thank N. Bardeesy and T. Irimura for providing AK4.4 and SL4 cells, respectively. This work was supported in part by the National Cancer Institute (P01-CA080124, R35-CA197743, and R01-CA208205 to R.K.J.); a fellowship from the National Cancer Institute (F32-CA216944-01 to H.T.N.); a fellowship from the Susan G. Komen Foundation (PDF14201739 to G.S.); and a fellowship from the National Heart, Lung, and Blood Institute (F31HL126449 to M.D.).
Author information
Authors and Affiliations
Contributions
H.T.N., M.D., and R.K.J. designed the study; H.T.N. and M.D. were responsible for acquisition of the data. H.T.N., M.D., G.S., P.H., L.L.M., and R.K.J. contributed to analysis and interpretation of the data. H.T.N., M.D., G.S., P.H., L.L.M., and R.K.J. were involved in drafting of the article and revising it for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
R.K.J. has received consultant fees from Enlight, Merck, Ophthotech, Pfizer, SPARC, and SynDevRx; owns equity in Enlight, Ophthotech, SynDevRx, and XTuit; and serves on the Board of Directors of XTuit and the Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund, and Tekla World Healthcare Fund. No funding or reagents from these companies were used in this study. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Nia, H., Datta, M., Seano, G. et al. Quantifying solid stress and elastic energy from excised or in situ tumors. Nat Protoc 13, 1091–1105 (2018). https://doi.org/10.1038/nprot.2018.020
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2018.020
This article is cited by
-
Crystal ribcage: a platform for probing real-time lung function at cellular resolution
Nature Methods (2023)
-
Intravital measurements of solid stresses in tumours reveal length-scale and microenvironmentally dependent force transmission
Nature Biomedical Engineering (2023)
-
Minimal Morphoelastic Models of Solid Tumour Spheroids: A Tutorial
Bulletin of Mathematical Biology (2023)
-
Magnetic resonance elastography from fundamental soft-tissue mechanics to diagnostic imaging
Nature Reviews Physics (2022)
-
Combining losartan with radiotherapy increases tumor control and inhibits lung metastases from a HER2/neu-positive orthotopic breast cancer model
Radiation Oncology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.