Abstract
Salinity is one of the most severe abiotic stresses that adversely affect plant growth and agricultural productivity. The plant Na+/H+ antiporter Salt Overly Sensitive 1 (SOS1) located in the plasma membrane extrudes excess Na+ out of cells in response to salt stress and confers salt tolerance. However, the molecular mechanism underlying SOS1 activation remains largely elusive. Here we elucidate two cryo-electron microscopy structures of rice (Oryza sativa) SOS1, a full-length protein in an auto-inhibited state and a truncated version in an active state. The SOS1 forms a dimeric architecture, with an NhaA-folded transmembrane domain portion in the membrane and an elongated cytosolic portion of multiple regulatory domains in the cytoplasm. The structural comparison shows that SOS1 adopts an elevator transport mechanism accompanied by a conformational transition of the highly conserved Pro148 in the unwound transmembrane helix 5 (TM5), switching from an occluded conformation in the auto-inhibited state to a conducting conformation in the active state. These findings allow us to propose an inhibition–release mechanism for SOS1 activation and elucidate how SOS1 controls Na+ homeostasis in response to salt stress.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analysed in this paper are presented in the main text, figures, extended data figures and supplementary videos or are available from the corresponding author upon request. The cryo-EM maps of the OsSOS1 full-length (OsSOS1FL) and truncated versions (OsSOS1976) have been deposited in the Electron Microscopy Data Bank with accession codes EMD-35775 and EMD-35950, respectively, and their structural models have been deposited in the PDB with accession codes 8IWO and 8J2M, respectively (Extended Data Table 1). Source data are provided with this paper.
References
Munns, R. & Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681 (2008).
Yang, Y. & Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 60, 796–804 (2018).
Yang, Y. & Guo, Y. Elucidating the molecular mechanisms mediating plant salt–stress responses. New Phytol. 217, 523–539 (2018).
Zhu, J. K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 53, 247–273 (2002).
Zhu, J. K. Abiotic stress signaling and responses in plants. Cell 167, 313–324 (2016).
Gong, Z. et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 63, 635–674 (2020).
Xie, Q., Zhou, Y. & Jiang, X. Structure, function, and regulation of the plasma membrane Na(+)/H(+) antiporter salt overly sensitive 1 in plants. Front. Plant Sci. 13, 866265 (2022).
Wu, S. J., Ding, L. & Zhu, J. K. SOS1, a genetic locus essential for salt tolerance and potassium acquisition. Plant Cell 8, 617–627 (1996).
Shi, H., Quintero, F. J., Pardo, J. M. & Zhu, J. K. The putative plasma membrane Na(+)/H(+) antiporter SOS1 controls long-distance Na(+) transport in plants. Plant Cell 14, 465–477 (2002).
Shi, H., Ishitani, M., Kim, C. & Zhu, J. K. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc. Natl Acad. Sci. USA 97, 6896–6901 (2000).
Guo, Y., Halfter, U., Ishitani, M. & Zhu, J. K. Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance. Plant Cell 13, 1383–1400 (2001).
Liu, J., Ishitani, M., Halfter, U., Kim, C. S. & Zhu, J. K. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc. Natl Acad. Sci. USA 97, 3730–3734 (2000).
Ishitani, M. et al. SOS3 function in plant salt tolerance requires N-myristoylation and calcium binding. Plant Cell 12, 1667–1678 (2000).
Liu, J. & Zhu, J. K. A calcium sensor homolog required for plant salt tolerance. Science 280, 1943–1945 (1998).
Qiu, Q. S., Guo, Y., Dietrich, M. A., Schumaker, K. S. & Zhu, J. K. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc. Natl Acad. Sci. USA 99, 8436–8441 (2002).
Shi, H., Lee, B. H., Wu, S. J. & Zhu, J. K. Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nat. Biotechnol. 21, 81–85 (2003).
Yue, Y., Zhang, M., Zhang, J., Duan, L. & Li, Z. SOS1 gene overexpression increased salt tolerance in transgenic tobacco by maintaining a higher K(+)/Na(+) ratio. J. Plant Physiol. 169, 255–261 (2012).
Xu, F.-C. et al. The Na+/H+ antiporter GbSOS1 interacts with SIP5 and regulates salt tolerance in Gossypium barbadense. Plant Sci. 330, 111658 (2023).
Brett, C. L., Donowitz, M. & Rao, R. Evolutionary origins of eukaryotic sodium/proton exchangers. Am. J. Physiol. Cell Physiol. 288, C223–C239 (2005).
Chanroj, S. et al. Conserved and diversified gene families of monovalent cation/H(+) antiporters from algae to flowering plants. Front. Plant Sci. 3, 25 (2012).
Isayenkov, S. V., Dabravolski, S. A., Pan, T. & Shabala, S. Phylogenetic diversity and physiological roles of plant monovalent cation/H(+) antiporters. Front. Plant Sci. 11, 573564 (2020).
Masrati, G. et al. Broad phylogenetic analysis of cation/proton antiporters reveals transport determinants. Nat. Commun. 9, 4205 (2018).
Nunez-Ramirez, R. et al. Structural insights on the plant salt-overly-sensitive 1 (SOS1) Na(+)/H(+) antiporter. J. Mol. Biol. 424, 283–294 (2012).
Guo, Y. et al. Transgenic evaluation of activated mutant alleles of SOS2 reveals a critical requirement for its kinase activity and C-terminal regulatory domain for salt tolerance in Arabidopsis thaliana. Plant Cell 16, 435–449 (2004).
Martinez-Atienza, J. et al. Conservation of the salt overly sensitive pathway in rice. Plant Physiol. 143, 1001–1012 (2007).
Hunte, C. et al. Structure of a Na+/H+ antiporter and insights into mechanism of action and regulation by pH. Nature 435, 1197–1202 (2005).
Wohlert, D., Kuhlbrandt, W. & Yildiz, O. Structure and substrate ion binding in the sodium/proton antiporter PaNhaP. Elife 3, e03579 (2014).
Dong, Y. et al. Structure and mechanism of the human NHE1–CHP1 complex. Nat. Commun. 12, 3474 (2021).
Flynn, G. E., Black, K. D., Islas, L. D., Sankaran, B. & Zagotta, W. N. Structure and rearrangements in the carboxy-terminal region of SpIH channels. Structure 15, 671–682 (2007).
Quintero, F. J. et al. Activation of the plasma membrane Na/H antiporter salt-overly-sensitive 1 (SOS1) by phosphorylation of an auto-inhibitory C-terminal domain. Proc. Natl Acad. Sci. USA 108, 2611–2616 (2011).
Lee, C. et al. A two-domain elevator mechanism for sodium/proton antiport. Nature 501, 573–577 (2013).
Paulino, C., Wohlert, D., Kapotova, E., Yildiz, O. & Kuhlbrandt, W. Structure and transport mechanism of the sodium/proton antiporter MjNhaP1. Elife 3, e03583 (2014).
Winkelmann, I. et al. Crystal structure of the Na(+)/H(+) antiporter NhaA at active pH reveals the mechanistic basis for pH sensing. Nat. Commun. 13, 6383 (2022).
Dong, Y. et al. Structural basis of autoinhibition of the human NHE3–CHP1 complex. Sci. Adv. 8, eabn3925 (2022).
Winklemann, I. et al. Structure and elevator mechanism of the mammalian sodium/proton exchanger NHE9. EMBO J. 39, e105908 (2020).
Matsuoka, R. et al. Structure, mechanism and lipid-mediated remodeling of the mammalian Na(+)/H(+) exchanger NHA2. Nat. Struct. Mol. Biol. 29, 108–120 (2022).
Ache, P. et al. GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K(+)-selective, K(+)-sensing ion channel. FEBS Lett. 486, 93–98 (2000).
Xue, J., Han, Y., Zeng, W., Wang, Y. & Jiang, Y. Structural mechanisms of gating and selectivity of human rod CNGA1 channel. Neuron 109, 1302–1313.e4 (2021).
Lu, Y. et al. Structural basis for the activity regulation of a potassium channel AKT1 from Arabidopsis. Nat. Commun. 13, 5682 (2022).
Zagotta, W. N. et al. Structural basis for modulation and agonist specificity of HCN pacemaker channels. Nature 425, 200–205 (2003).
Lee, C. H. & MacKinnon, R. Voltage sensor movements during hyperpolarization in the HCN channel. Cell 179, 1582–1589.e7 (2019).
Wang, X. H. et al. Structural basis for activity of TRIC counter-ion channels in calcium release. Proc. Natl Acad. Sci. USA 116, 4238–4243 (2019).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Wu, C., Huang, X., Cheng, J., Zhu, D. & Zhang, X. High-quality, high-throughput cryo-electron microscopy data collection via beam tilt and astigmatism-free beam-image shift. J. Struct. Biol. 208, 107396 (2019).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife, https://doi.org/10.7554/eLife.42166 (2018).
asarnow/pyem: UCSF pyem v0.5. Zenodo. (2019); https://github.com/asarnow/pyem
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D 75, 861–877 (2019).
Barad, B. A. et al. EMRinger: side chain-directed model and map validation for 3D cryo-electron microscopy. Nat. Methods 12, 943–946 (2015).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Acknowledgements
This project is financially supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA24020305 to Y.C.) and the National Key Research and Development Program of China (2020YFA0509903 to Y.C. and 2021YFA1300702 to M.S.).
Author information
Authors and Affiliations
Contributions
X.Z. performed protein purification, cryo-EM data collection, yeast experiments and data analysis; L.T. and X. Han performed cryo-EM data collection, structural determination and structural analysis; C.Z. performed model building, structural analysis and sequence analysis; J.N., X. Han, Q.L., X. Huang, L.Q. and M.W. performed experiments; F.Y., M.S., R.X., Y.W., Y.G. and Q.X. analysed data; Y.C. initiated the project, planned and analysed experiments, supervised the research and wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Jose M. Pardo, Huazhong Shi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
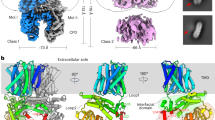
Extended Data Fig. 1 Structure-based sequence alignments for the representative SOS1s from both dicots and monocots.
The cryo-EM structure of OsSOS1 is used to restrict sequence gaps to inter-helical segments, with the superior coils and arrows defining extents of the secondary elements. The sequences include representative members from dicots and monocots: Oryza sativa Japonica Group (XP_015619351.1), Zea mays (XP_008645741.2), Sorghum bicolor (XP_002443674.1), Aegilops tauschii (XP_020190641.1), Brachypodium distachyon (XP_003576505.1), Dendrobium officinale (XP_020699162.1), Asparagus officinalis (XP_020256864.1), Musa acuminata subsp. malaccensis (XP_018686090.1), Phoenix dactylifera (XP_008798100.1), Amborella trichopoda (XP_006849492.1), Arabidopsis thaliana (NP_178307.2), Brassica rapa (XP_009114217.1), Eutrema salsugineum (XP_006395824.1), Beta vulgaris subsp. vulgaris (XP_010680103.2), Glycine max (NP_001244939.1), Daucus carota (XP_017226136.1), Cucumis melo (XP_008466844.1), Vitis vinifera (NP_001268140.1), Juglans regia (XP_018828660.1), Eucalyptus grandis (XP_010066529.2). The highly conserved inhibitory motifs (motif-1 and motif-2) are shown in boxes. Some critical residues are highlighted as following: 1. The putative phosphorylation residues are highlighted in cyan (S1113 and S1135 in OsSOS1). 2. The mutated residues are highlighted in yellow.
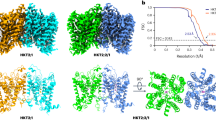
Extended Data Fig. 2 Structural determination of the OsSOS1FL.
(a) The workflow for image processing of the OsSOS1FL. 2D classification of the OsSOS1FL particles from the cryo-EM sample are shown. (b) Fourier shell correlation (FSC) curve suggests an overall resolution at 3.1 Å, as estimated using the 0.143 cut-off criterion (Left). The FSC curves for cross-validation between the model and the unmasked (Black) or masked (Pink) cryo-EM maps of OsSOS1 (Right). (c) Representative cryo-EM density map for the TM segments of TMD, helical elements of HD, CNBD, CTD and auto-inhibitory motif segments.
Extended Data Fig. 3 Structural determination of the OsSOS1976.
(a) The workflow for image processing of the OsSOS1976. 2D classification ns of the OsSOS1976 particles from the cryo-EM sample are shown. (b) Fourier shell correlation (FSC) curve suggests an overall resolution at 3.4 Å, as estimated using the 0.143 cut-off criterion (Left). The FSC curves for cross-validation between the model and the unmasked (Black) or masked (Pink) cryo-EM maps of OsSOS1976 (Right). (c) Representative cryo-EM density map for the TM segments of the TMD.
Extended Data Fig. 4 Domain organization of OsSOS1 and Salt tolerance assay.
(a) Ribbons drawing of the OsSOS1 protomer is shown, orientated as in Fig. 1a. (b) Salt tolerance assay of the OsSOS1 and its C-terminal truncated mutants in the Na+-extrusion defective yeast cells (S. cerevisiae AXT3K).
Extended Data Fig. 5 Structural analysis of the TMD of the OsSOS1.
(a) Superimposition of the TMD (OsSOS1, as colored in Fig. 2a) with archaeal Na+/H+ exchanger NhaP from Pyrococcus abyssii (pink), with an r.m.s.d. of 2.6 Å/386 superimposed Ca and 20% sequence identity. (b) Superimposition of the TMD (OsSOS1, as colored in Fig. 2a) with human Na+/H+ exchanger HsNHE1 from (light purple), with an r. m. s. d. of 2.9 Å/389 superimposed Ca and 26% sequence identity. (c) Cavities within the TMD. Zoom view are shown, as indicated. Two endogenously bound lipid molecules at the dimeric interface are shown as sticks. (d) A conserved inter-helices salt bridge (R341 vs E171) and a kink (~18°) at the middle of TM4 in the TMD.
Extended Data Fig. 6 Structural analysis of the HD of the OsSOS1.
(a) Ribbons drawing and domain topology of the OsSOS1 HD, with one protomer in green and the other in grey. (b) Electrostatic potential at helical domain surface. Electronegative and electropositive potential are colored in degrees of red and blue saturation, respectively. One protomer is drawn as ribbon diagram, and colored in green. Highly conserved charged residues at the dimeric HD interface and ion-conducting pathway are shown in stick. Two conserved glycine-mediate kinks, H6 (~35°) and H7 (~34°), are indicated.
Extended Data Fig. 7 Structure-based sequence alignment of CNBDs.
The structures for both CNBDs (OsSOS1 and HCN) have been superimposed and used to restrict sequence gaps to inter-secondary structural elements. Superior coils and arrows define extents of the secondary structural elements in OsSOS1 CNBD (top) and HCN CNBD (bottom). Two loss-of-function mutations in the b-roll structure of the CNBD, including G777D (sos1-8) and G784E (sos1-9), led to impaired transport function in Arabidopsis are highlighted in magenta. The residues critical for cAMP binding in the HCN CNBD are highlighted in cyan.
Extended Data Fig. 8 Structural analysis of the domain interfaces.
(a) The TMD-HD domain interface. The dimeric TMD is shown in ribbon drawing, with one protomer in light blue and the other one in grey. The dimeric HD is shown as surface, colored with electrostatic potential. Electronegative and electropositive potential are shown in degrees of red and blue saturation, respectively. (b) Domain organization of TMD (light bule) and HD (salmon), with a dihedral angle of ~26°. (c) The HD-CNBD domain interface. The dimeric HD is shown in ribbon drawing, with one protomer in green and the other one in grey. The CNBD-CTD is shown as surface, colored with electrostatic potential. Electronegative and electropositive potential are shown in degrees of red and blue saturation, respectively.
Extended Data Fig. 9 Comparison of the truncated OsSOS1976 with the auto-inhibited OsSOS1FL.
(a, b) The elution profile of the truncated SOS1976 (magenta) and the full-length OsSOS1FL (sky-blue) on size-exclusion column Superose 6 (Increase 10/300 GL). The truncated SOS1976 elutes at 13.8 ml, whereas the full-length OsSOS1FL elutes at 14.3 ml. The high-molecular weight aggregates elute at avoid volume are indicated with *. The eluted fraction were analyzed by SDS-PAGE. (c) The superimposition of the auto-inhibited OsSOS1FL structure and the truncated SOS1976 structure, based on their dimeric TMD portions (with an r.m.s.d. of 2.38 Å/848 superimposed Ca), reveals distinctive structural differences occurred in the cytoplasmic regions. The structure of the truncated SOS1976 is represented as a density map, with the TMD portion depicted in purple and the unmodeled cytoplasmic portion in gray. The cytosolic portion of the OsSOS1FL is represented in cartoon format (light blue). This comparison highlights a substantial conformational change upon SOS1 activation. The masks of the detergent boundary are illustrated in white.
Supplementary information
Supplementary Data 1
Validation report for the full-length SOS1FL (auto-inhibited state).
Supplementary Data 2
Validation report for the truncated SOS1976 (constitutively active state).
Source data
Source Data Fig. 1
Unprocessed size-exclusion profile and SDS–PAGE gels for ED-9a/b.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Xy., Tang, Lh., Nie, Jw. et al. Structure and activation mechanism of the rice Salt Overly Sensitive 1 (SOS1) Na+/H+ antiporter. Nat. Plants 9, 1924–1936 (2023). https://doi.org/10.1038/s41477-023-01551-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-023-01551-5
This article is cited by
-
Salt Stress Tolerance and Calcium Signalling Components: Where We Stand and How Far We can Go?
Journal of Plant Growth Regulation (2024)