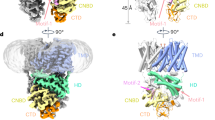
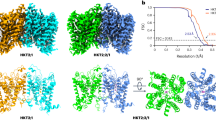
Abstract
The plasma membrane Na+/H+ exchanger Salt Overly Sensitive 1 (SOS1) is crucial for plant salt tolerance. Unlike typical sodium/proton exchangers, SOS1 contains a large cytoplasmic domain (CPD) that regulates Na+/H+ exchange activity. However, the underlying modulation mechanism remains unclear. Here we report the structures of SOS1 from Arabidopsis thaliana in two conformations, primarily differing in CPD flexibility. The CPD comprises an interfacial domain, a cyclic nucleotide-binding domain-like domain (CNBD-like domain) and an autoinhibition domain. Through yeast cell-based Na+ tolerance test, we reveal the regulatory role of the interfacial domain and the activation role of the CNBD-like domain. The CPD forms a negatively charged cavity that is connected to the ion binding site. The transport of Na+ may be coupled with the conformational change of CPD. These findings provide structural and functional insight into SOS1 activity regulation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The 3D cryo-EM density maps have been deposited to the Electron Microscopy Data Bank (EMDB) under the accession codes EMD-36077 (SOS1 with stable CPD, Class 1) and EMD-36076 (SOS1 with flexible CPD, Class 2). The atomic coordinates for the corresponding model have been deposited to the Protein Data Bank (PDB) under the accession codes 8JD9 (https://doi.org/10.2210/pdb8JD9/pdb) (SOS1 with stable CPD, Class 1) and 8JDA (https://doi.org/10.2210/pdb8JDA/pdb) (SOS1 with flexible CPD, Class 2). Source data are provided with this paper.
References
Yang, Y. & Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 60, 796–804 (2018).
Yang, Y. & Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 217, 523–539 (2018).
Zhu, J. K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 53, 247–273 (2002).
Wu, S. J., Ding, L. & Zhu, J. K. SOS1, a genetic locus essential for salt tolerance and potassium acquisition. Plant Cell 8, 617–627 (1996).
Liu, J. & Zhu, J. K. An Arabidopsis mutant that requires increased calcium for potassium nutrition and salt tolerance. Proc. Natl Acad. Sci. USA 94, 14960–14964 (1997).
Zhu, J. K., Liu, J. & Xiong, L. Genetic analysis of salt tolerance in Arabidopsis. Evidence for a critical role of potassium nutrition. Plant Cell 10, 1181–1191 (1998).
Shi, H., Ishitani, M., Kim, C. & Zhu, J. K. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc. Natl Acad. Sci. USA 97, 6896–6901 (2000).
Qiu, Q. S., Barkla, B. J., Vera-Estrella, R., Zhu, J. K. & Schumaker, K. S. Na+/H+ exchange activity in the plasma membrane of Arabidopsis. Plant Physiol. 132, 1041–1052 (2003).
Shi, H., Lee, B. H., Wu, S. J. & Zhu, J. K. Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nat. Biotechnol. 21, 81–85 (2003).
Shi, H., Quintero, F. J., Pardo, J. M. & Zhu, J. K. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+. transport in plants. Plant Cell 14, 465–477 (2002).
Qiu, Q. S., Guo, Y., Dietrich, M. A., Schumaker, K. S. & Zhu, J. K. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc. Natl Acad. Sci. USA 99, 8436–8441 (2002).
Quintero, F. J., Ohta, M., Shi, H., Zhu, J. K. & Pardo, J. M. Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. Proc. Natl Acad. Sci. USA 99, 9061–9066 (2002).
Liu, J., Ishitani, M., Halfter, U., Kim, C. S. & Zhu, J. K. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc. Natl Acad. Sci. USA 97, 3730–3734 (2000).
Halfter, U., Ishitani, M. & Zhu, J. K. The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc. Natl Acad. Sci. USA 97, 3735–3740 (2000).
Quintero, F. J. et al. Activation of the plasma membrane Na/H antiporter Salt-Overly-Sensitive 1 (SOS1) by phosphorylation of an auto-inhibitory C-terminal domain. Proc. Natl Acad. Sci. USA 108, 2611–2616 (2011).
Gong, D., Guo, Y., Schumaker, K. S. & Zhu, J. K. The SOS3 family of calcium sensors and SOS2 family of protein kinases in Arabidopsis. Plant Physiol. 134, 919–926 (2004).
Hunte, C. et al. Structure of a Na+/H+ antiporter and insights into mechanism of action and regulation by pH. Nature 435, 1197–1202 (2005).
Winkelmann, I. et al. Crystal structure of the Na+/H+ antiporter NhaA at active pH reveals the mechanistic basis for pH sensing. Nat. Commun. 13, 6383 (2022).
Dong, Y. et al. Structure and mechanism of the human NHE1-CHP1 complex. Nat. Commun. 12, 3474 (2021).
Winklemann, I. et al. Structure and elevator mechanism of the mammalian sodium/proton exchanger NHE9. EMBO J. 39, e105908 (2020).
Rodriguez-Rosales, M. P. et al. Plant NHX cation/proton antiporters. Plant Signal. Behav. 4, 265–276 (2009).
Sanchez-Barrena, M. J., Martinez-Ripoll, M., Zhu, J. K. & Albert, A. The structure of the Arabidopsis thaliana SOS3: molecular mechanism of sensing calcium for salt stress response. J. Mol. Biol. 345, 1253–1264 (2005).
Sanchez-Barrena, M. J. et al. The structure of the C-terminal domain of the protein kinase AtSOS2 bound to the calcium sensor AtSOS3. Mol. Cell 26, 427–435 (2007).
Nunez-Ramirez, R. et al. Structural insights on the plant salt-overly-sensitive 1 (SOS1) Na(+)/H(+) antiporter. J. Mol. Biol. 424, 283–294 (2012).
Dutta, D., Esmaili, M., Overduin, M. & Fliegel, L. Expression and detergent free purification and reconstitution of the plant plasma membrane Na(+)/H(+) antiporter SOS1 overexpressed in Pichia pastoris. Biochim. Biophys. Acta Biomembr. 1862, 183111 (2020).
Punjani, A. & Fleet, D. J. 3D variability analysis: resolving continuous flexibility and discrete heterogeneity from single particle cryo-EM. J. Struct. Biol. 213, 107702 (2021).
Paulino, C., Wohlert, D., Kapotova, E., Yildiz, O. & Kuhlbrandt, W. Structure and transport mechanism of the sodium/proton antiporter MjNhaP1. Elife 3, e03583 (2014).
Wohlert, D., Kuhlbrandt, W. & Yildiz, O. Structure and substrate ion binding in the sodium/proton antiporter PaNhaP. Elife 3, e03579 (2014).
Dong, Y. et al. Structural basis of autoinhibition of the human NHE3-CHP1 complex. Sci. Adv. 8, eabn3925 (2022).
The PyMOL Molecular Graphics System, Version 2.3.0 (Schrödinger, LLC, 2019).
Katiyar-Agarwal, S. et al. The plasma membrane Na+/H+ antiporter SOS1 interacts with RCD1 and functions in oxidative stress tolerance in Arabidopsis. Proc. Natl Acad. Sci. USA 103, 18816–18821 (2006).
Chai, H. et al. The plasma-membrane polyamine transporter PUT3 is regulated by the Na+/H+ antiporter SOS1 and protein kinase SOS2. New Phytol. 226, 785–797 (2020).
Cha, J. Y. et al. The Na+/H+ antiporter SALT OVERLY SENSITIVE 1 regulates salt compensation of circadian rhythms by stabilizing GIGANTEA in Arabidopsis. Proc. Natl Acad. Sci. USA 119, e2207275119 (2022).
Rodenas, R. et al. Insights into the mechanisms of transport and regulation of the Arabidopsis high-affinity K+ transporter HAK51. Plant Physiol. 185, 1860–1874 (2021).
Lu, Y. et al. Structural basis for the activity regulation of a potassium channel AKT1 from Arabidopsis. Nat. Commun. 13, 5682 (2022).
Fu, H. et al. SALT OVERLY SENSITIVE 1 is inhibited by clade D protein phosphatase 2C D6 and D7 in Arabidopsis thaliana. Plant Cell 35, 279–297 (2023).
Xu, H. et al. Functional characterization of a wheat plasma membrane Na+/H+ antiporter in yeast. Arch. Biochem. Biophys. 473, 8–15 (2008).
Martinez-Atienza, J. et al. Conservation of the salt overly sensitive pathway in rice. Plant Physiol. 143, 1001–1012 (2007).
Olias, R. et al. The plasma membrane Na+/H+ antiporter SOS1 is essential for salt tolerance in tomato and affects the partitioning of Na+ between plant organs. Plant Cell Environ. 32, 904–916 (2009).
Zhang, M. et al. A putative plasma membrane Na(+)/H(+) antiporter GmSOS1 is critical for salt stress tolerance in Glycine max. Front. Plant Sci. 13, 870695 (2022).
Zhou, X. et al. The classical SOS pathway confers natural variation of salt tolerance in maize. New Phytol. 236, 479–494 (2022).
Quan, R. et al. SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell 19, 1415–1431 (2007).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Grant, T. & Grigorieff, N. Measuring the optimal exposure for single particle cryo-EM using a 2.6 A reconstruction of rotavirus VP6. Elife 4, e06980 (2015).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Li, Z. et al. Uni-Fold: an open-source platform for developing protein folding models beyond AlphaFold. Preprint at bioRxiv https://doi.org/10.1101/2022.08.04.502811 (2022).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D 58, 1948–1954 (2002).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2020).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Guo, Y., Halfter, U., Ishitani, M. & Zhu, J. K. Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance. Plant Cell 13, 1383–1400 (2001).
Rodriguez-Navarro, A. & Ramos, J. Dual system for potassium transport in Saccharomyces cerevisiae. J. Bacteriol. 159, 940–945 (1984).
Fiser, A. & Sali, A. Modeller: generation and refinement of homology-based protein structure models. Methods Enzymol. 374, 461–491 (2003).
Stansfeld, P. J. et al. MemProtMD: automated insertion of membrane protein structures into explicit lipid membranes. Structure 23, 1350–1361 (2015).
Stansfeld, P. J. & Sansom, M. S. From coarse grained to atomistic: a serial multiscale approach to membrane protein simulations. J. Chem. Theory Comput. 7, 1157–1166 (2011).
Berendsen, H. J. C., Postma, J. P. M., Gunsteren, W. F. V., DiNola, A. & Haak, J. R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690 (1984).
Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 81, 511–519 (1984).
Hoover, W. G. Canonical dynamics: equilibrium phase-space distributions. Phys. Rev. A Gen. Phys. 31, 1695–1697 (1985).
Parrinello, M. & Rahman, A. Polymorphic transitions in single crystals: a new molecular dynamics method. J. Appl. Phys. 52, 7182–7190 (1981).
Hess, B., Bekker, H., Berendsen, H. J. C. & Fraaije, J. G. E. M. LINCS: a linear constraint solver for molecular simulations. J. Comput. Chem. 18, 1463–1472 (1997).
Essmann, U. et al. A smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577–8593 (1995).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1, 19–25 (2015).
Best, R. B. et al. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone phi, psi and side-chain chi(1) and chi(2) dihedral angles. J. Chem. Theory Comput. 8, 3257–3273 (2012).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Acknowledgements
The cryo-EM data for SOS1 were collected at Shuimu BioSciences. We thank Z. Li of the Proteinomics Facility at China Agricultural University and X. Meng of the Proteinomics Facility at Tsinghua University for protein MS analysis. This work was supported by the National Key R&D Program of China (2022YFA1303400), the National Natural Science Foundation of China (32171188 to G.Y.), the Young Elite Scientists Sponsorship Program of the China Association for Science and Technology (to G.Y.) and the Chinese Universities Scientific Fund (2020RC008, 2020TC177, 2021RC012, 2022RC017, 2022TC144, 2023RC012 to G.Y.).
Author information
Authors and Affiliations
Contributions
G.Y. initiated and supervised the project. Y.Z., J.Z., Y.J. and H. Wu prepared the samples. X.N. prepared and analysed the phenotype of the sos1 mutant plants. Y.Z., X.N. and J.Z. performed the yeast growth experiment to reveal Na+ transport. Y.J., X.X. and Y.Z. collected the EM data. P.F. and G.Y. analysed the EM data and calculated the EM map. P.F. and G.Y. built and refined the atomic model. Q.W. and H. Wen performed and analysed the molecular dynamics simulation. G.Y. and Y.G. designed and analysed the biochemical experiments. All authors discussed the results. G.Y. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Jose M. Pardo, Huazhong Shi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Alleles with sos1 mutants are sensitive to salt stress.
a, Seven mutants reduce the salt tolerance of Arabidopsis7. The location of sos mutants in protein SOS1 and amino acid(s) change are listed. Asterisk represents the translation stop. The location of these mutants is individually labelled. b, Salt sensitivity analysis of gl1 (WT), sos1-1, sos1-3, sos1-6, sos1-8, sos1-9, sos1-11 and sos1-12. Six-day-old seedlings grown on half-strength Murashige and Skoog (1/2 MS) medium were transferred to 1/2 MS medium or 1/2 MS medium containing 50 mM NaCl. Photographs were taken after 6-day treatment. c, Primary root length and fresh weight analysis of seedlings in b. Error bars represent the mean ± SD, n = 15 (three seedlings as a group were weighed together, and the average of each group was used for analysis), two-sided Student’s t test, *p < 0.05, ****p < 0.0001. (Exact P values of primary root length are 0.5512 for gl1, 3E-15 for sos1-11, the others are <1E-15; Exact P values of fresh weight are 0.3318, 6.03E-13, 1.19E-11, 2.38E-11, 7.62E-13, 2.65E-13, 2.33E-8, 5.82E-9 correspondingly). d, Net Na+ flux analysis of gl1 (WT), sos1-1, sos1-3, sos1-6, sos1-8, sos1-9, sos1-11 and sos1-12 by NMT. Seven-day-old seedlings grown on 1/2 MS medium were transferred to 1/2 MS medium with 50 mM NaCl for 12-h treatment, then the net Na+ flux was tested at the meristem zone. The flux data were shown every 12 s over 6 min. Based on the methods mentioned above, the positive net Na+ fluxes indicate the net Na+ efflux. Error bars represent the mean ± SD, n = 8. e, Calculated net Na+ fluxes in c Error bars represent the mean ± SD, n = 8. Different letters represent significant differences at p < 0.01 (one-way ANOVA). Exact P values are listed in Source Data Extended Data Fig. 1.
Extended Data Fig. 2 Protein purification of Arabidopsis thaliana SOS1.
A representative gel-filtration chromatography of SOS1. The peak fractions were visualized on SDS–PAGE by Coomassie staining. kDa, kilodaltons. MW, molecular weight marker. We have purified the protein three times and got similar results.
Extended Data Fig. 3 Cryo-EM data processing and analysis for SOS1 2class1.
a, A flowchart of the EM data processing by CryoSPARC46. 9060 micrographs were selected after CTF estimation and motion correction from 9098 movie stacks. b, Representative results of 2D classification of Class1 after particle picking and extraction. The red arrow indicates the stable CPD of Class1. c, The overall resolution of the reconstruction for SOS1 class1 is estimated to be 2.87 Å. d, Angular distribution of the particles used for reconstruction of SOS1. e, Local resolution estimation in Å of the SOS1 class1. f, FSC curves of the refined model versus the overall 2.87 Å map that it was refined against (black); of the models refined against the first half maps versus the same maps (purple); and of the models refined against the first half maps versus the second half maps (green). The small difference between the red and green curves indicates that the refinement of the atomic coordinates did not suffer from overfitting.
Extended Data Fig. 4 Cryo-EM data processing and analysis for SOS1 class2.
a, A flowchart of the EM data processing by CryoSPARC46. Similar to class1, class2 particles were separated from the same whole 1,035,234 particles. b, Representative results of 2D classification of Class2 in which no stable CPD seen. The red arrow indicates the flexible CPD of Class2. c, The overall resolution of the reconstruction for SOS1 class2 is estimated to be 3.67 Å. d, Angular distribution of the particles used for reconstruction of SOS1. e, Local resolution estimation in Å of the SOS1 class1. Although the resolution is inferior to class1, it is enough to build the distinct TMD. f, FSC curves of the refined model versus the overall 3.67 Å map that it was refined against (black); of the models refined against the first half maps versus the same maps (purple); and of the models refined against the first half maps versus the second half maps (green). The small difference between the red and green curves indicates that the refinement of the atomic coordinates did not suffer from overfitting.
Extended Data Fig. 6 Comparison of Class1 and Class2.
a, Comparison of cryo-EM maps of SOS1 Class1 and Class2 colored with grey and pink respectively. In the low-pass filtered maps, the relative downward motion of its CPD can be observed in the average densities. The arrows indicated the moving directions. b, Structural alignment of the TMD of Class1 and Class2. Compared to Class1, the shift of TM13 in Class2 triggers a slight movement of the TMD away from the symmetry axis of the dimer.
Extended Data Fig. 7 Dimeric assembly of SOS1 and cryo-EM analysis of the SOS1Δ998.
a, Co-purified phospholipids are located between the dimer domains and stabilize the dimer through hydrophobic effects. The contour level of the lipid density is 5 σ. b-c, Views from extracellular or intracellular side of SOS1. d, Structure alignment of SOS1 TMD with human NHE1 and Equus caballus NHE919,20. Superimposition of the dimeric TMD of SOS1 and NHE1 or NHE9 reveal an RMSD of 2.691 Å (over 534 Cα atoms) or 3.526 Å (over 570 Cα atoms), respectively. e, The two IFDs intertwine with each other. The nine helices are labeled as α1-α9. f, The result of 2D classification and the 3D reconstruction reveals highly flexible CPD and TMD of SOS1Δ998.
Extended Data Fig. 8 The Na+ coordination and putative conserved binding site in AtSOS1.
a, All-atom molecular dynamics simulations of SOS1 in the presence of high K+ concentration and varied Na+ concentrations. The ensemble of Na+ ions are shown as orange spheres while K+ ions are presented in palecyan. In the presence of high K+ concentration, both K+ and Na+ ions could move into the large cavity lined with negatively charged residues from CPD, reflecting the electrostatic feature of the CPD. b, Sequence alignment of SOS1 and other Na+/H+ exchangers revealed a conserved aspartic acid across different species. The equivalent residue in SOS1 is Asp201. Yeast growth on the medium with 25 mM NaCl reveals that the mutation Asp201Asn abolished Na+/H+ exchange compared to the wild-type SOS1 in the presence a reported constitutively active version of SOS2 (SOS2T168D/Δ308)53.
Extended Data Fig. 9 Structural mapping of mutants on SOS1 reveals molecular mechanism of functional regulation.
a, The locations of seven mutants in the structure and potential structural changes of SOS1. b, Closed-up view of sos1-3, sos1-8, sos1-9 and sos1-12. c, Sequence alignment of CNBD (-like) domains across different species reveals the conservation of Gly784.
Supplementary information
Supplementary Video 1
Movie of the 3D variability analysis of Class 1, illustrating the conformational dynamics occurring in the TMD and CPD regions of Class 1. It appears that the movement of the CPD potentially influences the conformation of the TMD.
Supplementary Video 2
Movie of the 3D variability analysis of Class 2, highlighting the dynamic behaviour of the TMD in Class 2, showing relative rotations between the core domain and the dimer domain. These observed changes may be associated with the high flexibility exhibited by the CPD of Class 2.
Source data
Source Data Extended Data Fig. 1
Statistical source data for Extended Data Fig. 1c,e.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Zhou, J., Ni, X. et al. Structural basis for the activity regulation of Salt Overly Sensitive 1 in Arabidopsis salt tolerance. Nat. Plants 9, 1915–1923 (2023). https://doi.org/10.1038/s41477-023-01550-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-023-01550-6