Abstract
The nitrate (NO3−) electroreduction into ammonia (NH3) represents a promising approach for sustainable NH3 synthesis. However, the variation of adsorption configurations renders great difficulties in the simultaneous optimization of binding energy for the intermediates. Though the extensively reported Cu-based electrocatalysts benefit NO3− adsorption, one of the key issues lies in the accumulation of nitrite (NO2−) due to its weak adsorption, resulting in the rapid deactivation of catalysts and sluggish kinetics of subsequent hydrogenation steps. Here we report a tandem electrocatalyst by combining Cu single atoms catalysts with adjacent Co3O4 nanosheets to boost the electroreduction of NO3− to NH3. The obtained tandem catalyst exhibits a yield rate for NH3 of 114.0 mg\({}_{{{{{{\rm{NH}}}}}}_3}\) h−1 cm−2, which exceeds the previous values for the reported Cu-based catalysts. Mechanism investigations unveil that the combination of Co3O4 regulates the adsorption configuration of NO2− and strengthens the binding with NO2−, thus accelerating the electroreduction of NO3− to NH3.
Similar content being viewed by others
Introduction
As one of the nitrogen-containing species, nitrate (NO3−) widely exists in industrial and agricultural wastewater with a high concentration, mainly caused by the emission of low-level nuclear waste and intensive usage of fertilizers1,2,3. Excessive NO3− has significantly threatened ecological balance, inducing acid rain and photochemical smog4. Additionally, NO3− in human body is easily converted into toxic nitrite (NO2−), leading to serious health issues5. Among the methods for removing NO3−, electroreduction process using renewable electricity is regarded as an appealing technology under mild conditions6,7,8,9. The controllable products including nontoxic nitrogen (N2) and valuable ammonia (NH3) could be obtained after NO3− electroreduction10,11,12. Since NH3 is a fundamental chemical compound and a promising green hydrogen carrier, NO3− electroreduction into NH3 instead of N2 is more desirable. Taken together, it is highly imperative to achieve efficient electroreduction of NO3− into NH3 from the perspective of environmental protection and sustainable NH3 synthesis.
In view of the multiple nitrogen-containing intermediates (e.g. *NO3, *NO2, and *NO) involved in the NO3− electroreduction, an optimal catalyst should satisfy the simultaneously optimized adsorption of intermediates. The moderate binding energy of intermediates serves as one of the key factors for efficient NO3− electroreduction into NH313,14. Classically, given that the coordination of N atom in NO3− is saturated by three O atoms, *NO3 tends to bond with active sites through O atoms. Whereas, *NO2 is preferentially adsorbed on active sites through N and O atoms. As for *NO, N atom in *NO is inclined to connect with active sites. The variation of adsorption configurations renders great difficulties in the simultaneous optimization of binding energy for the intermediates. A typical instance is Cu-based electrocatalysts which have been reported extensively for NO3− electroreduction14,15,16,17,18,19,20. Though Cu-based electrocatalysts benefit NO3− adsorption, one of the key issues lies in the accumulation of NO2−, resulting in the rapid deactivation of catalysts and sluggish kinetics of the subsequent hydrogenation steps for NH3 production15,17. However, it remains a grand challenge to design an efficient catalyst to satisfy the simultaneously optimized adsorption of intermediates with different configurations.
Herein, we report a tandem electrocatalyst by combining Cu single atoms anchored on N-doped carbon with adjacent Co3O4 nanosheets (denoted as Co3O4/Cu1-N-C) to boost the electroreduction of NO3− to NH3. The obtained Co3O4/Cu1-N-C catalyst exhibits a remarkable yield rate for NH3 of 114.0 mg\({}_{{{{{{\rm{NH}}}}}}_3}\) h−1 cm−2, which exceeds the previous values for all of the reported Cu-based catalysts. Mechanism investigations unveil that the combination of Co3O4 regulates the adsorption configuration of NO2− and strengthens the binding with NO2−, thus accelerating the electroreduction of NO3− to NH3.
Results
Catalyst synthesis and characterizations
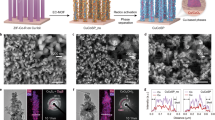
Co3O4/Cu1-N-C catalyst was synthesized by adding sodium borohydride to the mixture containing Cu single-atom catalysts and cobalt nitrate. Cu single atoms dispersed on N-doped carbon (denoted as Cu1-N-C) were prepared via pyrolyzing Cu-doped ZIF-8 at 900 °C under Ar atmosphere (Supplementary Figs. 1 and 2). Figure 1a shows the high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) image of Co3O4/Cu1-N-C. As displayed in the high resolution TEM (HRTEM) image and the corresponding selected area electron diffraction pattern (SAED), Co3O4 nanosheets were successfully deposited on the surface of Cu1-N-C (Supplementary Fig. 3). Figure 1b shows the aberration-corrected HAADF-STEM image of Co3O4/Cu1-N-C. The lattice fringes with an interplanar spacing of 0.201 nm were ascribed to the (400) facet of Co3O4. Besides, abundant Cu single atoms were observed around Co3O4 nanosheets. Based on energy-dispersive X-ray spectroscopy (EDS) elemental mapping, Co, Cu, and N elements were uniformly distributed throughout the whole structure (Fig. 1c). The uniform distribution of Cu sites and Co3O4 species constituted the adjacent catalytic centers. The metal content of Cu and Co in Co3O4/Cu1-N-C were determined to be 0.60 wt% and 4.70 wt%, respectively, by inductively coupled plasma-optical emission spectroscopy analysis (ICP-OES). For comparison, Co3O4 nanosheets dispersed on N-doped carbon (denoted as Co3O4/N-C) were prepared with a similar synthetic procedure of Co3O4/Cu1-N-C except for the addition of Cu precursor (Supplementary Fig. 4). Figure 1d shows the Raman spectra for Co3O4/Cu1-N-C, Cu1-N-C, and Co3O4/N-C. All of the Raman spectra displayed two peaks located at 1356 and 1591 cm−1, assigned to the D band and G band of graphite carbon, respectively21. The similar intensity ratios of D band to G band (ID/IG) for the three samples indicated that the carbon support possessed similar degree of structural disorder (Supplementary Fig. 5). Compared with Cu1-N-C, three distinguishable peaks located at 482, 527, and 689 cm−1 were observed for both Co3O4/Cu1-N-C and Co3O4/N-C, corresponding to Eg, F2g, and A1g vibration modes of Co3O4 crystals, respectively22. The structure of graphite carbon supports was further confirmed by X-ray diffraction patterns (Supplementary Fig. 6). Figure 1e shows the Cu K-edge X-ray absorption near edge structure (XANES) spectra of Co3O4/Cu1-N-C and Cu1-N-C. Obviously, the energy absorption edge profiles for both Co3O4/Cu1-N-C and Cu1-N-C were located between those of CuO and Cu2O, elucidating that the valence state of Cu species in the two catalysts were between +1 to +2. As shown in Fig. 1f, a dominant peak at 1.93 Å was observed in the extended X-ray absorption fine structure (EXAFS) spectra of Cu K-edge for Co3O4/Cu1-N-C and Cu1-N-C, which were attributed to the Cu-N bond. The absence of Cu-Cu bond in the two catalysts further confirmed the atomic dispersion of Cu species. Besides, the EXAFS fitting results indicate that the coordination numbers of Cu-N shell in both Co3O4/Cu1-N-C and Cu1-N-C were approximately 4.0 (Supplementary Fig. 7 and Table 1). After the deposition of Co3O4 nanosheets, the coordination structure of Cu single atoms (CuN4) in Co3O4/Cu1-N-C was unchanged. Besides, the wavelet transformed EXAFS (WT-EXAFS) spectra of Co3O4/Cu1-N-C and Cu1-N-C also confirmed the Cu-N bonding in the two catalysts (Supplementary Fig. 8). For the Co K-edge XANES spectra, the edge energy for both Co3O4/Cu1-N-C and Co3O4/N-C were similar to that for Co3O4 reference (Fig. 1g). Figure 1h shows that the Co-O coordination in Co3O4/Cu1-N-C and Co3O4/N-C were approximate to that in Co3O4, certifying the similar coordination structure of Co3O4 species in the two catalysts (Supplementary Fig. 9 and Table 2). Figure 1i shows the Co 2p X-ray photoelectron spectroscopy (XPS) spectra. Specifically, the peaks at 798.0, 782.7, 796.1, and 780.8 eV in Co3O4/Cu1-N-C and Co3O4/N-C corresponded to Co2+ 2p1/2, Co2+ 2p3/2, Co3+ 2p1/2, and Co3+ 2p3/2, respectively23. The indiscernible shift of Co 2p peaks demonstrated that Cu1-N-C as the support did not significantly affect the valence state of Co.
a HAADF-STEM image, b aberration-corrected HAADF-STEM image, and c EDS elemental mappings of Co3O4/Cu1-N-C. d Raman spectra of Co3O4/Cu1-N-C, Cu1-N-C, and Co3O4/N-C. e Cu K-edge XANES spectra and f EXAFS spectra for Co3O4/Cu1-N-C, Cu1-N-C, Cu foil, Cu2O, and CuO. g Co K-edge XANES spectra and h EXAFS spectra for Co3O4/Cu1-N-C, Co3O4/N-C, Co foil, CoO, and Co3O4. i Co 2p XPS spectra for Co3O4/Cu1-N-C and Co3O4/N-C.
Catalytic performance toward NO3 − electroreduction
The catalytic performance of the catalysts was investigated in a three-electrode H-type cell toward NO3− electroreduction (Supplementary Fig. 10). The concentration of NH3 product was quantified by the indophenol blue method (Supplementary Fig. 11). To preliminarily explore the process of tandem catalysis, we conducted the linear sweep voltammetry (LSV) curves of Co3O4/Cu1-N-C, Cu1-N-C, and Co3O4/N-C with 1 M NO3−/NO2−, respectively. As shown in Fig. 2a, the current density of Cu1-N-C in the presence of NO3− was higher than that of Co3O4/N-C, suggesting that Cu1-N-C possessed higher activity toward NO3− electroreduction. Whereas, Co3O4/N-C exhibited a larger current density relative to Cu1-N-C in NO2− (Fig. 2b). The superior activity of Co3O4 species toward NO2− electroreduction was further demonstrated by the higher Faradaic efficiency (FE) and yield rate for NH3 of Co3O4/N-C in NO2− electroreduction relative to Cu1-N-C (Supplementary Fig. 12). Considering that NO2− is one of the vital intermediates, the combination of Cu1-N-C and Co3O4 would couple the separate functions of Cu sites and Co3O4 species for the sequential reduction of NO3− and NO2−. As expected, Co3O4/Cu1-N-C displayed the highest current density among the three catalysts in the electrolyte of NO3−. Besides, the tremendous discrepancy of the LSV curves of Co3O4/Cu1-N-C in 1 M KOH with/without NO3− also implied the superior activity of Co3O4/Cu1-N-C toward NO3− electroreduction (Supplementary Fig. 13).
LSV curves of Cu1-N-C, Co3O4/N-C, and Co3O4/Cu1-N-C with (a) 1 M NO3− and (b) 1 M NO2−. c jNH3, d normalized jNH3 based on Cdl, e FE for NH3, and f yield rate for NH3 of Cu1-N-C, Co3O4/N-C, and Co3O4/Cu1-N-C at different applied potentials with 1 M NO3−. g Yield rate for NH3 of Co3O4/Cu1-N-C at −1.0 V vs RHE under 20 rounds of successive reactions. The solution resistance was determined to be 4.4 ± 0.2 ohm in the electrolytes by potentiostatic electrochemical impedance spectroscopy.
Figure 2c provides the partial current density for NH3 (jNH3) of Co3O4/Cu1-N-C, Cu1-N-C, and Co3O4/N-C at various applied potentials toward NO3− electroreduction. The jNH3 of Co3O4/Cu1-N-C exceeded those of Cu1-N-C and Co3O4/N-C. Especially, at −1.0 V vs reversible hydrogen electrode (RHE), the jNH3 of Co3O4/Cu1-N-C reached −1437.5 mA cm−2, which was 2.2 times and 3.6 times as high as that of Cu1-N-C and Co3O4/N-C, respectively. Moreover, the normalized jNH3 based on double-layer capacitance (Cdl) for Co3O4/Cu1-N-C was the largest among the three catalysts, indicating the highest intrinsic activity for Co3O4/Cu1-N-C toward NO3− electroreduction (Fig. 2d and Supplementary Fig. 14). In addition, the FE for NH3 of Co3O4/Cu1-N-C was higher with respect to the other two counterparts at all applied potentials (Fig. 2e). Especially, Co3O4/Cu1-N-C achieved the maximum FE for NH3 of 97.7% at −0.8 V vs RHE. Furthermore, at −1.0 V vs RHE, the yield rate of NH3 for Co3O4/Cu1-N-C reached up to 114.0 mg\({}_{{{{{{\rm{NH}}}}}}_3}\) h−1 cm−2, which exceeded all of the reported value for Cu-based catalysts14,16,18,19,24,25,26,27,28,29,30 (Fig. 2f and Supplementary Table 3). The yield rate of NH4+ in the electrolyte after the electroreduction process was also determined by 1H nuclear magnetic resonance (NMR) analysis, which was approximated to the results detected via the indophenol blue method (Supplementary Fig. 15 and Table 4). Other liquid and gaseous products including NO2−, NH2OH, NO, NO2, N2O, H2, and N2 for Co3O4/Cu1-N-C were also measured (Supplementary Figs. 16–18). NH3 were the only main product after NO3−/NO2− electroreduction (Supplementary Table 5). Besides, the FE for NH3 of Co3O4/Cu1-N-C with NO3− concentrations ranging from 10 mM to 500 mM all exceeded 91.2%, indicating a wide tolerance range for the concentration of NO3− (Supplementary Fig. 19). The durability of Co3O4/Cu1-N-C was examined by 20 rounds of successive reactions. The negligible decay of the yield rate demonstrated the satisfactory durability of Co3O4/Cu1-N-C (Fig. 2g). The Raman and XAFS measurements for Co3O4/Cu1-N-C after the electrolysis indicated that the Co3O4 species and Cu-N bonding were preserved (Supplementary Figs. 20 and 21). The stability of Cu single atoms in Co3O4/Cu1-N-C during the electrolysis was further explored by in situ EXAFS measurements, indicating that Cu atoms remained the atomically dispersed state in Co3O4/Cu1-N-C during the NO3− electroreduction (Supplementary Figs. 22 and 23).
To further clarify the synergy effect of Co3O4 on the conversion of NO2−, we conducted a series of control experiments. The catalytic performance of other metal oxides (such as FeOx, CuOx, and NiOx) dispersed on N-doped carbon toward NO2− electroreduction were all lower than that over Co3O4/N-C, suggesting the inferior ability of these metal oxide to facilitate NO2− reduction (Supplementary Figs. 24 and 25). In addition, the loading amount of Co3O4 on Cu1-N-C was vital to the efficient conversion of the accumulated NO2− (Supplementary Fig. 26). Besides, the simply physical mixing of Cu1-N-C and Co3O4/N-C could not sufficiently assure the spatial couple of the adjacent sites, thereby limiting the effective hydrogenation of NO2− into NH3 during NO3− electroreduction (Supplementary Fig. 27). We also exclude the possible ammonia contamination from the self-electrolysis of Co3O4/Cu1-N-C, electrolyte, and carbon paper, respectively (Supplementary Fig. 28). Besides, the catalytic activity of N-doped carbon was much lower compared with that of Co3O4/Cu1-N-C (Supplementary Fig. 29). The possible interference of Co single atoms on Cu1-N-C support could be considered insignificant to the catalytic performance of Co3O4/Cu1-N-C (Supplementary Figs. 30–33). The electroreduction of NO3− was also affected by the diffusion of reactants (Supplementary Fig. 34). Moreover, 15NO3− isotopic labeling measurements for Co3O4/Cu1-N-C was conducted with 1H NMR analysis. Only typical doublet peaks attributed to 15NH4+ were collected with 15NO3− as the N source whereas the triplet peaks of 14NH4+ were detected with 14NO3− as the N source (Supplementary Fig. 35). These results indicated that the NH3 detected in the electrolyte originated from the electroreduction of NO3−.
Mechanistic study on NO3 − electroreduction
To gain more insight into the catalytic process of NO3− electroreduction over Co3O4/Cu1-N-C, we conducted in situ electrochemical Fourier transform infrared spectroscopy (FTIR) and Raman spectroscopy to monitor the reaction process (Supplementary Figs. 36 and 37). Figure 3a displays the in situ FTIR spectra of Co3O4/Cu1-N-C at applied potentials from OCP to −1.0 V vs RHE. The negative peaks at 1382 cm−1 were ascribed to the consumption of NO3− species3. In addition, the emergence of positive peaks located at 1456 cm−1 confirmed that NH4+ was generated during the NO3− electroreduction31. Besides, two peaks at 1541 and 1508 cm−1 were detected, which were assigned to the vibration band of *NO and *NOH, respectively (Supplementary Fig. 38). Figure 3b shows the in situ Raman spectra of Co3O4/Cu1-N-C at all applied potentials. The peaks corresponding to Eg, F2g, and A1g vibration modes of Co3O4 remained unchanged, suggesting that the Co3O4 species was stable during NO3− electroreduction. During the NO3− electroreduction, only the peak at 1049 cm−1 was observed for Co3O4/Cu1-N-C at all applied potentials, assigned to the symmetric stretching vibration of NO3− (Fig. 3c). In the case of Cu1-N-C, apart from the signal of NO3−, a new peak at 810 cm−1 ascribed to the bending vibration of NO2− gradually appeared as the applied potential increased, indicating the accumulation of NO2− for Cu1-N-C during NO3− electroreduction (Fig. 3d). To further probe the variation of local concentration for NO2− near the surface of the catalysts, we designed a Raman cell that allows Raman laser to detect from the surface of catalysts to the electrolyte bulk. The Raman laser was designed to be incident from the back of catalysts to diminish the interference from the strong absorbance of NO3− in the electrolyte. As illustrated in Fig. 3e, the electrocatalysts were deposited on fluorine tin oxide-coated glass (FTO) as the working electrode (WE). The distance from the laser beam to electrode surface is controlled by the mechanical sample stage. Figure 3f, g display the in situ Raman spectra of Co3O4/Cu1-N-C and Cu1-N-C at −0.8 V vs RHE when the laser beam was positioned 0 to 200 μm away from the surface of catalysts, respectively. With the increment of the distance between the focal plane of laser and the surface of catalysts, the signal intensity of graphite carbon for the catalysts gradually decreased. A noticeable peak at 810 cm−1 assigned to NO2− arose near the surface of Cu1-N-C. Whereas, the signal of NO2− for Co3O4/Cu1-N-C was negligible, which was independent of the distance. Furthermore, we determined the local concentration of NO2− near the surface of catalysts based on the integrated areas of NO2− and NO3−, taking the ratio of integrated areas for 1 M NO2− and 1 M NO3− solutions as a correction factor (Supplementary Fig. 39). As the laser beam was set further far away from the surface of catalysts into the electrolyte, the concentration of NO2− for Cu1-N-C gradually decreased from 0.74 to 0.29 M (Fig. 3h). This trend indicates that the NO2− generated at Cu1-N-C/electrolyte interface diffused into the electrolyte due to the sluggish reduction of NO2−. Clearly, the concentration of NO2− for Co3O4/Cu1-N-C was much lower relative to Cu1-N-C, manifesting the facilitated reduction of NO2− with the favor of Co3O4.
a In situ FTIR spectra for Co3O4/Cu1-N-C from OCP to −1.0 V vs RHE in 1 M NO3−. b In situ Raman spectra for Co3O4/Cu1-N-C from OCP to −1.0 V vs RHE in 1 M NO3−. In situ Raman spectra for (c) Co3O4/Cu1-N-C and (d) Cu1-N-C from OCP to −1.0 V vs RHE in 1 M NO3−. e Scheme of the designed Raman cell for detecting from the surface of catalysts to the electrolyte bulk. In situ Raman spectra for (f) Co3O4/Cu1-N-C and (g) Cu1-N-C at −0.8 V vs RHE in 1 M NO3− with different distances ranging from 0 to 200 μm. h The calculated concentration of NO2− for Co3O4/Cu1-N-C and Cu1-N-C with different distances ranging from 0 to 200 μm.
To further understand the synergetic role of Cu1-N-C and Co3O4 in the catalytic process, we calculated the rate constants for NO3− electroreduction (k1) and NO2− electroreduction (k2), respectively (Fig. 4a). The concentration of residual NO3− after the electroreduction process was quantified by UV-Vis spectrophotometry (Supplementary Fig. 40). Compared with Co3O4/N-C, the larger k1 value of Cu1-N-C suggests that Cu1-N-C was more favorable for the conversion of NO3− to NO2−, but the smaller k2 value shows the slower kinetics for NO2− reduction. Accordingly, excessive NO2− would be desorbed into the electrolyte for Cu1-N-C. Notably, the highest k1 and k2 of Co3O4/Cu1-N-C manifested the simultaneous acceleration of the conversion of NO3− to NO2− and NO2− to NH3. Besides, the tafel slopes of Co3O4/Cu1-N-C, Co3O4/N-C, and Cu1-N-C in 1 M NO3− and 1 M NO2− imply that the combination of Co3O4 with Cu1-N-C facilitate the kinetics of NO3− and NO2− reduction during the catalytic process (Supplementary Fig. 41). Figure 4b shows the adsorption capacities (qe) of Co3O4/Cu1-N-C, Cu1-N-C, and Co3O4/N-C for NO3− and NO2−, respectively. It is obvious that Co3O4/Cu1-N-C exhibited the largest qe for both NO3− and NO2− among the three catalysts. As a consequence, combining Cu1-N-C with Co3O4 was conducive to the conversion of both NO3− and NO2−.
a Linearized pseudo first-order kinetic profiles of Co3O4/Cu1-N-C, Cu1-N-C, and Co3O4/N-C in 1 M NO3−/NO2−, respectively. b Adsorption capacities of Co3O4/Cu1-N-C, Cu1-N-C, and Co3O4/N-C for NO3−/NO2−. c Free energy diagram of NO3− electroreduction over CuN4 and Co3O4 (100) slabs. * represents an adsorption site. d Structure models of key intermediates on CuN4 and Co3O4 (100) slabs. The gray, blue, red, yellow, and purple spheres represent C, N, O, Cu, and Co atoms, respectively.
The density functional theory (DFT) calculations were conducted to further interpret the reaction mechanism of NO3− electroreduction over Co3O4/Cu1-N-C catalysts. Based on the results of structural analysis, we adopted CuN4 and Co3O4 (100) slabs as the models to calculate the Gibbs free energies (G) for each step involved in NO3− electroreduction, respectively (Supplementary Fig. 42). After the structure optimization, *NO3 would be adsorbed on CuN4 with O atom. As presented in Fig. 4c, the Gibbs free-energy changes (ΔG) for NO3− adsorption over CuN4 is much lower than that over Co3O4 (100), indicating the stronger binding of NO3− over CuN4. Nevertheless, the ΔG of *NO2 desorption over CuN4 is −1.31 eV, which could be more thermodynamically favorable than the reduction of *NO2 to *NO (−1.20 eV). In this regard, the desorption of *NO2 would give rise to the accumulation of NO2− over CuN4, which was consistent with the high yield for NO2− over Cu1-N-C in NO3− electroreduction (Supplementary Fig. 17). As for the conversion of NO2−, *NO2 would be adsorbed on CuN4 through N atom whereas *NO2 could be connected with Co3O4 (100) through N and O atoms after the structure optimization, leading to the lower ΔG for NO2− adsorption over Co3O4 (100) (Fig. 4d). In addition, the relative high ΔG for *H adsorption over CuN4 and Co3O4 revealed the weak adsorption of *H, indicating that the occurrence of competitive H2 evolution could be depressed (Supplementary Figs. 43 and 44). As a result, Co3O4 would regulate the adsorption configuration of NO2− and possesse an easier binding with NO2−, facilitating the reduction of NO2− to NH3.
Discussion
In summary, we developed a highly efficient catalyst by coupling the separate functions of Cu1-N-C and Co3O4 for the sequential reduction of NO3− to NO2− and NO2− to NH3. The obtained Co3O4/Cu1-N-C catalyst exhibited a superior yield rate for NH3 of 114.0 mg\({}_{{{{{{\rm{NH}}}}}}_3}\) h−1 cm−2, which exceeded all of the reported values for Cu-based catalysts. The mechanism investigations unveiled that the combination of Co3O4 regulated the adsorption configuration of NO2− and strengthened the binding with NO2−, thus accelerating the electroreduction of NO3− to NH3. This work offers a novel guideline for the construction of highly efficient tandem catalysts toward NO3− electroreduction.
Methods
Chemicals and materials
Zinc nitrate hexahydrate (Zn(NO3)2·6H2O, 99.0%), 2-methyl imidazole (2-MeIM, 99.0%), copper(II) acetate monohydrate (Cu(COOCH3)2·H2O, 99.0%), cobalt nitrate hexahydrate (Co(NO3)2·6H2O, 99.0%), iron nitrate nonahydrate (Fe(NO3)3·9H2O, 98.5%), nickel nitrate hexahydrate (Ni(NO3)2·6H2O, 98.0%), copper nitrate trihydrate (Cu(NO3)2·3H2O, 99.0%), methanol (99.5%),ethanol (99.5%), potassium nitrate (KNO3, 99.0%), potassium nitrite (KNO2, 97.0%), potassium hydroxide (KOH, 85%), ammonium sulfate ((NH4)2SO4, 99.0%), sodium hydroxide (NaOH, ≥96.0%), salicylic acid (C7H6O3), sodium hypochlorite solution (NaClO, available chlorine 5.2% of aqueous solution), trisodium citrate dihydrate (C6H5Na3O7·2H2O), sodium nitroferricyanide dihydrate (C5FeN6Na2O·2H2O), hydrochloric acid (HCl, 12 mol L−1), sulfamic acid (99.5%), p-aminobenzenesulfonamide (98.0%), N-(1-Naphthyl) ethylenediamine dihydrochloride (98.0%), phosphoric acid (H3PO4, ≥85.0%), and 1-propanesulfonic acid 3-(trimethylsilyl) sodium salt (DSS) were purchased from Sinopharm Chemical Reagent Co. Ltd. Glyoxylic acid solution (C2H2O3, 50 wt%), dimethyl sulfoxide-d6 (DMSO-d6, 99.9atom% D), (15NH4)2SO4 (99.0 atom% 15N), and K15NO3 (99.0 atom% 15N) were purchased from Aladdin Chemistry Co., Ltd (Shanghai, China). Bipolar membrane (TRJBM) were purchased from Beijing Tingrun Membrane Technology Development Co., Ltd (Beijing, China). The deionized (DI) water was produced using a Millipore Milli-Q grade, with a resistivity of 18.2 MΩ cm. All of the chemicals were used without any further purification.
Instrumentations
TEM images were taken using a Hitachi HT7700 transmission electron microscope at an acceleration voltage of 100 kV. HAADF-STEM and the corresponding EDS elemental mapping were carried out on a Talos F200X field-emission transmission electron microscope operated at an accelerating voltage of 200 kV using Mo-based TEM grids. Aberration-corrected HAADF-STEM images were carried out on Themis Z field-emission transmission electron microscope operating at an accelerating voltage of 300 kV using Mo-based TEM grids. XRD patterns were collected using a Rikagu MiniFlex X-ray diffractometer with Cu-Kα radiation (λ = 1.54059 Å). ICP-OES (Avio 220 MAX, PerkinElmer) analysis was employed to measure the concentration of metal species. XPS measurements were performed using a Kratos Axis supra+ diffractometer with Al-Kα radiation. The Raman spectra were conducted via LabRAM HR Evolution (Horiba) Raman system with a 532 nm excitation laser. The absorbance data was measured on a UV-vis spectrophotometer (Agilent Technologies, Cary 60). The in situ FTIR spectra were acquired by a Nicolet iS50 FTIR spectrometer with a built-in MCT detector.
Synthesis of Cu1-N-C
A mixture of Zn(NO3)2·6H2O (5.6 mmol) and Cu(COOCH3)2·H2O (0.28 mmol) was dissolved in 80 mL of methanol, which was subsequently added into 80 mL of methanol containing 3.70 g of 2-MeIM. Then the mixed solution was kept at 25 °C for 12 h. The as-obtained precipitate (denoted as Cu-doped ZIF-8) was separated by centrifugation and washed subsequently with methanol for five times, and finally dried at 65 °C under vacuum overnight. Next, the obtained Cu-containing derivative of ZIF-8 was heated to 900 °C with a heating rate of 5 °C min−1 in a tube furnace and kept at 900 °C under flowing Ar gas for 3 h. After the tube furnace was naturally cooled to room temperature, Cu1-N-C was obtained and directly used as the catalyst without further treatment. For comparison, Co single atoms anchored on N-doped carbon (denoted as Co1-N-C) and Co single atoms anchored on Cu1-N-C (denoted as Co1Cu1-N-C) were obtained via pyrolyzing the Co-doped ZIF-8 and Cu/Co-doped ZIF-8, respectively. Co-doped ZIF-8 and Cu/Co-doped ZIF-8 prepared with the similar procedure with that of Cu1-N-C except the Co(NO3)2·6H2O and the mixture of Co(NO3)2·6H2O and Cu(COOCH3)2·H2O as the metal precursors, respectively.
Synthesis of Co3O4/Cu1-N-C and Co3O4/N-C
160 mg of Cu1-N-C was dispersed in 20 mL of ethanol by sonication for 30 min. Afterwards, 5 mL of H2O containing 45 mg of Co(NO3)2·6H2O was added into the above solution, which was maintained in an ice-water bath for 1 h with vigorous stirring. Then, 40 mL of freshly prepared NaBH4 (100 mg) with ice-cold H2O was added dropwise into the above suspension, followed by further stirring for 1 h. The as-obtained precipitate was separated by filtration and washed subsequently with water for five times. Finally, Co3O4/Cu1-N-C was obtained by being dried at 65 °C under vacuum overnight. Co3O4/N-C was prepared as a comparison with the similar procedure with that of Co3O4/Cu1-N-C except for the addition of N-doped carbon instead of Cu1-N-C. N-doped carbon was prepared with a similar synthetic procedure with that of Cu1-N-C without the addition of Cu(COOCH3)2·H2O. For comparison, other metal oxides including FeOx, CuOx, and NiOx dispersed on N-doped carbon (denoted as FeOx/N-C, CuOx/N-C, and NiOx/N-C, respectively) were prepared with the similar procedure with that of Co3O4/N-C except for the addition of Fe(NO3)3·9H2O, Cu(NO3)2·3H2O, and Ni(NO3)2·6H2O as the metal precursors, respectively (denoted as FeOx/N-C, CuOx/N-C, and NiOx/N-C, respectively).
X-ray absorption fine structure (XAFS) measurements
The XAFS spectra at Cu K-edge and Co K-edge were performed at 1W1B beamline of Beijing Synchrotron Radiation Facility and BL11B beamline of Shanghai Synchrotron Radiation Facility. The data were obtained in ambient conditions under fluorescence mode for Cu K-edge and transmission mode for Co K-edge, respectively.
The ATHENA module and ARTEMIS codes in the IFEFFIT software packages were employed to extract the data and fitted the profiles32,33,34. The k3-weighted EXAFS spectra were acquired by energy calibration and spectral normalization. For the EXAFS part, the Fourier transformed data in R space of Cu K-edge and Co K-edge were analyzed by applying a hanning windows (dk = 1.0 Å−1) to differentiate the EXAFS oscillation from different coordination shells. Subsequently, we performed the least-squares curve parameter fitting to attain the structural parameters around central atoms. The fitted ranges of k space were set at 3.4–13.2 Å−1 with R range of 1.2–3.0 Å. The four parameters including coordination number (CN), bond length (R), Debye-Waller factor (σ2), and E0 shift (ΔE0) were fitted without anyone being fixed, constrained, or correlated.
The in situ Cu K-edge XAFS measurements were conducted were collected with a home-made XAFS cell. Typically, 8 mg of the catalysts and 40 µL of Nafion were dispersed in 2 mL of ethanol by sonication for 1 h. Then the uniform ink was loaded onto carbon paper with an area of 2 × 2 cm2. The mass loading was calculated to be 2 mg cm−2. The prepared catalysts, a Ag/AgCl electrode, and a Pt wire were used as the working electrode, reference electrode, and counter electrode, respectively. All electrochemical tests were measured in 1 M KOH electrolyte with 1 M KNO3 (45 mL) and controlled by a CHI1140C electrochemical workstation.
Preparation of the working electrodes
8 mg of the catalysts were dispersed in 2 mL of ethanol by sonication for 1 h. Then 40 µL of Nafion solution was added to the mixture and sonicated for 30 min to obtain a uniform ink. Finally, the uniform ink was loaded onto carbon paper with an area of 2 × 4 cm2. The mass loading was calculated to be 1 mg cm−2. The area of working electrodes used in the electrochemical measurements was 0.25 cm2.
Electrochemical measurements
The electrochemical measurements were carried out in an H-cell system which was separated by a bipolar membrane with a CHI1140C electrochemical workstation (Chenhua, Shanghai). Ag/AgCl electrode and graphite rod were used as the reference electrode and counter electrode, respectively. For NO3− electroreduction, 1 M KOH containing 1 M KNO3 solution (60 mL) was evenly distributed to the cathode and anode compartments. The pH value of the electrolyte was determined to be 14 by a FiveEasy Plus pH Meter (METTLER TOLEDO). The applied potentials were measured against the Ag/AgCl reference electrode with 50% iR compensation and converted to the RHE reference scale by E (vs RHE) = E (vs Ag/AgCl) + 0.21 V + 0.0591 × pH – iR. The solution resistance was determined to be 4.4 ± 0.2 ohm in the electrolytes by potentiostatic electrochemical impedance spectroscopy at frequencies ranging from 10 Hz to 100 kHz, which was conducted in a standard three-electrode system at ambient conditions. Before the electroreduction test, CV curves were performed until the polarization curves achieved steady-state ones with a scan rate of 10 mV s−1. Before the electrolysis, Ar gas was delivered into the cathodic compartment at a rate of 10 mL min−1 to remove dissolved O2. The LSVs of the catalysts were recorded at a scan rate of 5 mV s−1 in 1 M KOH containing 1 M KNO3/KNO2. The controlled potential electrolysis was performed at applied potentials for 10 min. NO2− electroreduction was conducted with the same conditions except that the solution of 1 M KOH containing 1 M KNO2 was used as the electrolyte. An absorption cell containing 30 mL of 1 M HCl was set to effectively absorb the possible escaped NH3 from the cathode cell. After the electrolysis at each applied potential, the concentration of NH3 in the absorption cell was lower than 1 μg mL−1. In this case, the volatilization of NH3 from the electrolytes could be negligible. Cyclic voltammetric measurements were conducted in a non-faradaic potential window with various scan rates from 50 to 100 mV s−1. Cdl was calculated by plotting the Δj (Δj = ja – jc) at the middle of the corresponding potential window against scan rates. The ja and jc were the anodic and cathodic current densities, respectively. The slope was twice of Cdl.
The calculation method for FE
The FE for the product (NH3 and NO2−) was calculated at a given potential as follows:
C: the measured concentration of product (mg mL−1),
V: the volume of the electrolyte (mL),
N: the number of electrons transferred for the product, which is 8 for NH3 and 2 for NO2−,
F: Faraday constant, 96,485 C mol−1,
Q: total electric charge (C),
M: the relative molecular mass, which is 17 g mol−1 for NH3 and 46 g mol−1 for NO2−.
The calculation method for the yield rate of NH3 product
The yield rate of NH3 product was calculated at a given potential as follows:
ν\({}_{{{{{{\rm{NH}}}}}}_3}\): the yield rate (mg\({}_{{{{{{\rm{NH}}}}}}_3}\) h−1 cm−2),
C\({}_{{{{{{\rm{NH}}}}}}_3}\): the measured concentration of NH3 (mg mL−1),
V: the volume of the electrolyte (mL),
S: the area of the catalyst (cm2),
t: the reduction reaction time (min).
Determination of ion concentration
Determination of NH3 concentration with indophenol blue method35. After the electroreduction process, a certain amount of electrolyte was taken out from the electrolytic cell and diluted to the detection range. Then, 2 mL of 1 M NaOH solution containing salicylic acid (5 wt%) and sodium citrate (5 wt%) were added into the aforementioned solution, followed by the addition of 1 mL of 0.05 M NaClO and 0.2 mL of C5FeN6Na2O (1 wt%). After standing in darkness for 2 h, the absorption spectra were measured using a UV-vis spectrophotometer. The concentration of indophenol blue was determined using absorbance at the wavelength of 650 nm. The concentration-absorbance curve was calibrated using standard (NH4)2SO4 solution with a series of concentrations.
Determination of NH3 concentration with 1H NMR method. After NO3− electroreduction, a certain amount of electrolyte was taken out for further quantification by 1H NMR (Bruker AVANCE AV III 400). All analyses were performed with 128-time scans. The concentration-integral area curve was calibrated using a standard (NH4)2SO4 solution. Typically, (NH4)2SO4 was dissolved in 20 mL of 1 M KOH electrolyte as a series of standard (NH4)2SO4 solutions with different concentrations. Subsequently, 0.1 mL of dimethyl sulfoxide-d6 (DMSO-d6), 0.1 mL of 6 mM 1-propanesulfonic acid 3-(trimethylsilyl) sodium salt (DSS) solution, and 0.08 mL of 6 M HCl to adjust the pH value were added into 0.32 mL of (NH4)2SO4 standard solutions with different concentrations. The signal appeared at 7.23, 7.10, and 6.97 ppm were attributed to NH4+. The integral areas of the signal of NH4+ were used to determine the concentration of (NH4)2SO4 compared with the as-known DSS reference.
Determination of NO3− concentration36. A certain amount of electrolyte was diluted to the detection range of NO3−. Then, 0.1 mL of 1 M HCl and 0.01 mL of 0.8 wt% sulfamic acid solution were mixed with the diluted electrolyte, followed by shaking for 10 min. Using a UV-vis spectrophotometer, the absorption spectra were collected, obtaining the absorption intensities at a wavelength of 220 and 275 nm. Finally, the calculated absorbance (A = A220nm–2A275nm) was acquired. The concentration-absorbance curve was calibrated using standard KNO3 solutions with a series of concentrations.
Determination of NO2− concentration. 4 g of p-aminobenzenesulfonamide, 0.2 g of N-(1-Naphthyl) ethylenediamine dihydrochloride, and 10 mL of phosphoric acid were mixed with 50 mL of water as the color reagent. A certain amount of electrolyte was taken out from the electrolytic cell and diluted to the detection range. 1 mL of H3PO4 (5 M) was added to the 4 mL of diluted post-electrolysis electrolytes to adjust the pH, followed by the addition of 0.1 mL of color reagent. After standing for 20 min, the absorption spectra were measured using a UV-vis spectrophotometer. The absorption intensity at a wavelength of 540 nm was recorded. The concentration-absorbance curve was calibrated using standard KNO2 solution with a series of concentrations.
Determination of other liquid and gaseous product
The amount of NH2OH was determined by 1H NMR after NH2OH was captured by excess amount of C2H2O3 through oximation process. Specifically, 0.4 mL of the electrolyte after the NO3− electroreduction was mixed with 10 μL of 50% C2H2O3 solution, followed by the addition of 0.1 mL of DMSO-d6 (99%) and 0.1 mL of 6 mM DSS solution. The integral area of the signal appeared at 7.46 ppm were used to determine the concentration of NH2OH compared with the as-known DSS reference. In this work, the amount of NH2OH was below the detection limit for both NO3− and NO2− reduction over Co3O4/Cu1-N-C.
Nitrogen oxides including NO, NO2, and N2O have been detected by an infrared gas analyzer (THA100S). H2 and N2 have been detected by an on-line gas chromatograph (GC-2014) equipped with a flame ionization detector and a thermal conductivity detector.
The FE for gaseous products were calculated by the following equation:
x: the measured mole fraction of product,
Vgas: the total volume of the gas (L),
N: the number of electrons transferred for the product, which is 3 for NO, 8 for N2O, 2 for H2 and 10 for N2,
F: Faraday constant, 96485 C mol−1,
Q: total electric charge (C),
Vm: the molar volume of the gas, 24.5 L mol−1.
Isotope labeling experiments
The isotopic labeling experiment used K15NO3 with 15N enrichment of 99% as the feeding N-source to clarify the source of ammonia. 1 M KOH was used as the electrolyte and K15NO3 with a concentration of 1 M was added into the cathode compartment as the reactant. After the electrolysis, 0.1 mL of DMSO-d6 and 0.1 mL of 6 mM DSS solution were added into 0.4 mL of the electrolyte, followed by adding 0.05 mL of HCl (0.1 M) to adjust the pH of the solutions. Then the obtained 15NH4+ was identified on a Varian 400 MHz NMR spectrometer (Bruker AVANCE AV III 400).
Kinetic evaluation
The electrolysis at −1.0 V vs RHE were conducted for different time to acquire the rate constant in 1 M KOH containing 1 M NO3− or 1 M NO2−. The reaction constant (k1 for NO3− reduction and k2 for NO2− reduction) was calculated by plotting the concentration of NO3− or NO2− against the time of reaction, supposing that the concentrations of NO3− or NO2− declined exponentially as per first-order rate.
C0: initial concentration of NO3− or NO2− (g mL−1),
Ct: the concentration of NO3− or NO2− at time t (g mL−1),
t: the time of reaction (min).
Adsorption experiments
To determine the adsorption capacities of Co3O4/Cu1-N-C, Cu1-N-C, and Co3O4/N-C, 5 mg of catalysts were added to each 25 mL of NO3− or NO2− solutions with the initial concentration of 1 M under stirring for 2 h, respectively. The solutions were separated by filtration using the 0.22 μm microporous membrane filter. For high concentration of NO3− or NO2−, the solution was diluted before absorbance measurements. The adsorption capacity was calculated using the following equation:
qe: the adsorption capacity (g gcat.−1),
C0: the initial concentration of NO3− or NO2− (g mL−1),
Ce: the measured concentration of NO3− or NO2− after the adsorption (g mL−1),
V: the volume of the electrolyte (mL),
m: the mass of the catalyst (g).
In situ FTIR measurements
Using a Nicolet iS50 FTIR spectrometer (Thermo Scientific) with a built-in MCT detector, we obtained the in situ electrochemical FTIR spectra. Typically, 2 mg of catalysts and 20 µL of Nafion dispersed in 2 mL of ethanol were sonicated for 1 h. Then the mixture was loaded onto the Au-coated Si prism to completely cover the Au film. The prepared prism was used as the working electrode after being dried naturally. The reference electrode and counter electrode was a Ag/AgCl electrode and a Pt wire, respectively. The photograph of the in situ FTIR electrochemical cell was shown in Supplementary Fig. 36. All electrochemical tests were measured in 1 M KOH electrolyte with 0.1 M KNO3 (30 mL) and controlled by a CHI1140C electrochemical workstation. All experiments were conducted at room temperature. The background spectra of the working electrode were obtained at an open-circuit potential before the electrochemical tests. All of the spectra were collected in absorbance by averaging 32 scans at a resolution of 4 cm−1.
In situ Raman measurements
In situ Raman was carried out using Lab RAM HR Evolution (Horiba) equipped with a 50× microscope objective. The excitation wavelength was 532 nm with 10% intensity. The photograph of electrochemical cell for in situ Raman measurement was shown in Supplementary Fig. 37. Typically, 2 mg of catalysts and 20 µL of Nafion were dispersed in 2 mL of ethanol by sonication for 1 h. Then the electrocatalysts were deposited on fluorine tin oxide-coated glass as the working electrode. A Ag/AgCl electrode, and a Pt wire were used as the reference electrode, and counter electrode, respectively. All electrochemical tests were measured in 1 M KOH electrolyte with 1 M KNO3/KNO2 (5 mL) and controlled by a CHI1140C electrochemical workstation. All experiments were conducted at room temperature. Each spectrum was collected by integration twice, 60 s per integration. To determine the local concentration of NO2− near the surface of catalysts, we employed an internal standard method during the in situ Raman measurements.
DFT calculations
DFT calculations were performed using the Vienna Ab-Initio Simulation Package (VASP) code at the GGA level within the PAW-PBE formalism37. DFT-D3 method with Becke-Jonson damping is performed for the van der Waals correction. The three-layer Co3O4 (100) slab model was adopted with a vacuum of 15 Å. The total energy calculations were performed using a 2 × 2 × 1 grid and a plane wave cut-off energy of 400 eV. Atoms in the bottom two layers were fixed. A U value of 3.5 eV was applied to the 3d states of Co to describe the strong on-site Coulomb interactions due to the localization of the Co 3d states38. For the model of CuN4 (no atoms were fixed), the total energy calculations were performed using a 3 × 3 × 1 grid and a plane wave cut-off energy of 400 eV. All atoms, which were not fixed, including adsorbates were allowed to relax until the force on each ion was smaller than 0.02 eV/Å.
We calculated the Gibbs free energy (G) for each species as follows:
where EDFT, EZPE, and TS represent the DFT-optimized total energy, zero point energy (ZPE), and entropy contribution, respectively (T is the temperature, 298.15 K). It is assumed that S = 0 for all the adsorbed species.
Data availability
The data that support the findings of this study are available from the corresponding author upon request. The source data underlying Figs. 1–4 and Supplementary Figs. 1–44 are provided as a Source Data file. Source data are provided with this paper.
References
Rosca, V., Duca, M., de Groot, M. T. & Koper, M. T. M. Nitrogen cycle electrocatalysis. Chem. Rev. 109, 2209–2244, (2009).
van Langevelde, P. H., Katsounaros, I. & Koper, M. T. M. Electrocatalytic nitrate reduction for sustainable ammonia production. Joule 5, 290–−294 (2021).
Wang, Y., Wang, C., Li, M., Yu, Y. & Zhang, B. Nitrate electroreduction: Mechanism insight, in situ characterization, performance evaluation, and challenges. Chem. Soc. Rev. 50, 6720–6733 (2021).
Duca, M. & Koper, M. T. M. Powering denitrification: The perspectives of electrocatalytic nitrate reduction. Energy Environ. Sci. 5, 9726–9742 (2012).
Xu, H., Ma, Y., Chen, J., Zhang, W. X. & Yang, J. Electrocatalytic reduction of nitrate - a step towards a sustainable nitrogen cycle. Chem. Soc. Rev. 51, 2710–2758 (2022).
Fan, K. et al. Active hydrogen boosts electrochemical nitrate reduction to ammonia. Nat. Commun. 13, 7958 (2022).
Li, J. et al. Efficient ammonia electrosynthesis from nitrate on strained ruthenium nanoclusters. J. Am. Chem. Soc. 142, 7036–7046 (2020).
Wang, Y. et al. Structurally disordered RuO2 nanosheets with rich oxygen vacancies for enhanced nitrate electroreduction to ammonia. Angew. Chem. Int. Ed. 61, e202202604 (2022).
Deng, X., Yang, Y., Wang, L., Fu, X. Z. & Luo, J. L. Metallic Co nanoarray catalyzes selective NH3 production from electrochemical nitrate reduction at current densities exceeding 2 A cm−2. Adv. Sci. 8, 2004523 (2021).
Zhang, X. et al. Recent advances in non-noble metal electrocatalysts for nitrate reduction. Chem. Eng. J. 403, 126269 (2021).
Wang, X. et al. Free-standing membrane incorporating single-atom catalysts for ultrafast electroreduction of low-concentration nitrate. Proc. Natl Acad. Sci. USA 120, e2217703120 (2023).
Zhang, G. et al. Tandem electrocatalytic nitrate reduction to ammonia on Mbenes. Angew. Chem. Int. Ed. 62, e202300054 (2023).
Fu, Y. F. et al. Enhancing electrochemical nitrate reduction to ammonia over Cu nanosheets via facet tandem catalysis. Angew. Chem. Int. Ed. 62, e202303327 (2023).
Fang, J. Y. et al. Ampere-level current density ammonia electrochemical synthesis using CuCo nanosheets simulating nitrite reductase bifunctional nature. Nat. Commun. 13, 7899 (2022).
Zhu, T. et al. Single-atom Cu catalysts for enhanced electrocatalytic nitrate reduction with significant alleviation of nitrite production. Small 16, e2004526 (2020).
Yang, J. et al. Potential-driven restructuring of cu single atoms to nanoparticles for boosting the electrochemical reduction of nitrate to ammonia. J. Am. Chem. Soc. 144, 12062–12071 (2022).
Li, P. et al. Pulsed nitrate-to-ammonia electroreduction facilitated by tandem catalysis of nitrite intermediates. J. Am. Chem. Soc. 145, 6471–6479 (2023).
Cheng, X. F. et al. Coordination symmetry breaking of single-atom catalysts for robust and efficient nitrate electroreduction to ammonia. Adv. Mater. 34, 2205767 (2022).
He, W. et al. Splicing the active phases of copper/cobalt-based catalysts achieves high-rate tandem electroreduction of nitrate to ammonia. Nat. Commun. 13, 1129 (2022).
Wang, W., Chen, J. & Tse, E. C. M. Synergy between Cu and Co in a layered double hydroxide enables close to 100% nitrate-to-ammonia selectivity. J. Am. Chem. Soc. 145, 26678–26687 (2023).
Liu, Y. et al. A highly efficient metal-free electrocatalyst of f-doped porous carbon toward N2 electroreduction. Adv. Mater. 32, 1907690 (2020).
Li, R. et al. Insights into correlation among surface-structure-activity of cobalt-derived pre-catalyst for oxygen evolution reaction. Adv. Sci. 7, 1902830 (2020).
Wang, J. et al. Electrocatalytic reduction of nitrate to ammonia on low-cost ultrathin CoOx nanosheets. ACS Catal. 11, 15135–15140 (2021).
Gao, Q. et al. Synthesis of core/shell nanocrystals with ordered intermetallic single-atom alloy layers for nitrate electroreduction to ammonia. Nat. Synth. 2, 624–634 (2023).
Song, Z. et al. Efficient electroreduction of nitrate into ammonia at ultralow concentrations via an enrichment effect. Adv. Mater. 34, 2204306 (2022).
Gao, W. et al. Alloying of Cu with Ru enabling the relay catalysis for reduction of nitrate to ammonia. Adv. Mater. 35, e2202952 (2023).
Wang, Y. et al. Enhanced nitrate-to-ammonia activity on copper-nickel alloys via tuning of intermediate adsorption. J. Am. Chem. Soc. 142, 5702–5708 (2020).
Liu, H. et al. Efficient electrochemical nitrate reduction to ammonia with copper-supported rhodium cluster and single-atom catalysts. Angew. Chem. Int. Ed. 61, e202202556 (2022).
Chen, F. Y. et al. Efficient conversion of low-concentration nitrate sources into ammonia on a Ru-dispersed Cu nanowire electrocatalyst. Nat. Nanotechnol. 17, 759–767 (2022).
Wang, Y. T., Zhou, W., Jia, R. R., Yu, Y. F. & Zhang, B. Unveiling the activity origin of a copper-based electrocatalyst for selective nitrate reduction to ammonia. Angew. Chem. Int. Ed. 59, 5350–5354 (2020).
Hao, R. et al. Pollution to solution: A universal electrocatalyst for reduction of all NOx-based species to NH3. Chem. Catal. 2, 622–638 (2022).
Newville, M. IFEFFIT: Interactive XAFS analysis and FEFF fitting. J. Synchrotron Radiat. 8, 322–324 (2001).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541, (2005).
Meng, Q. et al. Sustainable production of benzene from lignin. Nat. Commun. 12, 4534 (2021).
Song, Z. et al. Promoting N2 electroreduction into NH3 over porous carbon by introducing oxygen-containing groups. Chem. Eng. J. 434, 134636 (2022).
Bai, Y. et al. FCF-LDH/BIVO4 with synergistic effect of physical enrichment and chemical adsorption for efficient reduction of nitrate. Green. Energy Environ. https://doi.org/10.1016/j.gee.2023.05.011, (2023).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 6, 15–50 (1996).
Zasada, F. et al. Periodic DFT and HR-STEM studies of surface structure and morphology of cobalt spinel nanocrystals. Retrieving 3d shapes from 2d images. J. Phy. Chem. C. 115, 6423–6432 (2011).
Acknowledgements
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0450401), National Key Research and Development Program of China (2021YFA1500500, 2019YFA0405600), NSFC (22209161, 22302184, 22322901, 22221003, 22250007, and 22361162655), National Science Fund for Distinguished Young Scholars (21925204), CAS project for young scientists in basic research (YSBR-022, YSBR-051), China Postdoctoral Program for Innovative Talents (BX20200324), Collaborative Innovation Program of Hefei Science Center, CAS (2022HSC-CIP004), International Partnership Program of Chinese Academy of Sciences (123GJHZ2022101GC), the Joint Fund of the Yulin University and the Dalian National Laboratory for Clean Energy (YLU-DNL Fund 2022012), Fundamental Research Funds for the Central Universities, the Anhui Natural Science Foundation for Young Scholars (2208085QB41), and the Fellowship of China Postdoctoral Science Foundation (2021M693058). J.Z. acknowledges support from the Tencent Foundation through the XPLORER PRIZE. This work was partially carried out at the Instruments Center for Physical Science, University of Science and Technology of China. This work was also partially carried out at the USTC Center for Micro and Nanoscale Research and Fabrication.
Author information
Authors and Affiliations
Contributions
Z.G. and J.Zeng. supervised this project. Y.L. performed most of the experiments and analyzed the experimental data. J.W. conducted the in situ Raman measurements. Z.Y. and J.Zhao carried out DFT calculations and analyzed the computational data. L.Z. conducted the XAFS measurements and analyzed the results. Z.S., Y.Z., J.C., and J.M. provided help in materials synthesis and characterizations. Y.L., J.W., Z.G., and J.Zeng. wrote the manuscript. All authors discussed the results and assisted during manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Aiqin Wang, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Y., Wei, J., Yang, Z. et al. Efficient tandem electroreduction of nitrate into ammonia through coupling Cu single atoms with adjacent Co3O4. Nat Commun 15, 3619 (2024). https://doi.org/10.1038/s41467-024-48035-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-48035-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.