Abstract
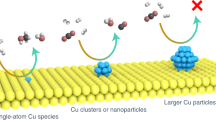
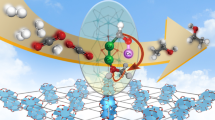
Metal promotion could unlock high performance in zinc-zirconium catalysts, ZnZrOx, for CO2 hydrogenation to methanol. Still, with most efforts devoted to costly palladium, the optimal metal choice and necessary atomic-level architecture remain unclear. Herein, we investigate the promotion of ZnZrOx catalysts with small amounts (0.5 mol%) of diverse hydrogenation metals (Re, Co, Au, Ni, Rh, Ag, Ir, Ru, Pt, Pd, and Cu) prepared via a standardized flame spray pyrolysis approach. Cu emerges as the most effective promoter, doubling methanol productivity. Operando X-ray absorption, infrared, and electron paramagnetic resonance spectroscopic analyses and density functional theory simulations reveal that Cu0 species form Zn-rich low-nuclearity CuZn clusters on the ZrO2 surface during reaction, which correlates with the generation of oxygen vacancies in their vicinity. Mechanistic studies demonstrate that this catalytic ensemble promotes the rapid hydrogenation of intermediate formate into methanol while effectively suppressing CO production, showcasing the potential of low-nuclearity metal ensembles in CO2-based methanol synthesis.
Similar content being viewed by others
Introduction
Mixed zinc-zirconium oxides, ZnZrOx, have emerged as cost-effective and earth-abundant catalysts for CO2 hydrogenation to methanol (CO2 + 3H2 ⇌ CH3OH + H2O), a highly relevant platform chemical and energy vector1,2,3. These systems are particularly attractive owing to their ability to suppress undesired carbon monoxide (CO) formation via the reverse water-gas shift (RWGS) reaction (CO2 + H2 ⇌ CO + H2O), thereby leading to high methanol selectivity2,3,4. Additionally, ZnZrOx catalysts display remarkable on-stream stability even when exposed to impurities such as hydrogen sulfide and sulfur dioxide, which are commonly found in CO2-containing streams2,5. Although coprecipitation (CP) was established as the first method for synthesizing ZnZrOx catalysts1,2,3, a recent study revealed that flame spray pyrolysis (FSP) yields materials with around threefold higher methanol space-time yield compared to coprecipitated systems4. Despite CP and FSP materials possessing similar catalytic ensembles, FSP maximizes surface area and formation of isolated Zn2+ species located at surface positions of the ZrO2 lattice, which foster enhanced performance4. Indeed, the unique architecture of the flame-made catalysts promotes the creation of surface oxygen vacancies, which are key components of the active ensembles that favor methanol formation. Regardless of the preparation method, however, the heterolytic activation of H2 on ZnZrOx is the most demanding step and limits methanol formation rates2,3,4,6,7,8,9.
Metal promotion is a well-established strategy for enhancing the hydrogen splitting ability and overall performance of oxide catalysts in the conversion of CO2 to methanol10,11,12,13,14,15,16. While coprecipitated ZnZrOx catalysts are known to benefit from the addition of small quantities of hydrogenation metals, examples are limited to a few metals (M = Pd, Pt, and Cu), with palladium recognized as the most effective6,7,8,9. However, applying this strategy to flame-made ZnZrOx systems to improve their prospects for industrial implementation is challenging due to limited knowledge and transferability of promotional effects from conventionally synthesized catalysts. The effects of a given metal not only depend on its identity but also on its speciation, which is influenced by various factors, including the synthesis approach, promoter content, catalyst reconstruction under operating conditions, and structure of oxides used6,7,8,9,10,12,13,14,17,18. Despite substantial progress in understanding promotional effects on other oxide catalysts, like indium oxide-based systems10,11,12,16, integrated studies of ZnZrOx catalysts are lacking. Therefore, the exploration of the promotion of flame-made ZnZrOx catalysts to elucidate the active-ensemble structures and associated performance of the resulting systems, including promoter speciation, impact on oxygen vacancy formation, and dynamics under reaction conditions are essential. In particular, operando investigations are key for establishing robust structure-performance relationships to guide the design of practical M-ZnZrOx catalysts19,20,21.
In this study, we systematically investigate the promotion of flame-made ZnZrOx catalysts by relevant hydrogenation metals (0.5 mol% Re, Co, Au, Ni, Rh, Ag, Ir, Ru, Pt, Pd, and Cu) for CO2 hydrogenation to methanol. Standardized catalyst synthesis and evaluation as well as in-depth characterization reveal copper as the most effective promoter for ZnZrOx, as it leads to the largest performance improvement. In addition, Cu-promoted ZnZrOx catalysts comprise an earth abundant and cost-effective alternative to conventional palladium promotion and are compositionally distinct from traditional Cu-ZnO-ZrO2 systems, which generally contain 20–40 mol% of Cu and are significantly less selective to methanol1. Detailed microscopy, kinetic, stability, operando spectroscopy analyses, and theoretical simulations are applied to gather understanding of copper promotion. Specifically, we investigate the speciation under reaction conditions and its effect on oxygen vacancy formation, the architecture of active ensembles, and reactivity. Our study provides valuable insights into ZnZrOx promotion by different metals, and offers unprecedented atomic-level rationalization of the working state of Cu-ZnZrOx catalysts for CO2 hydrogenation to methanol.
Results and discussion
Impact of promoters on performance and catalyst architecture
Metal-promoted ZnZrOx catalysts were synthesized by flame spray pyrolysis (FSP, Supplementary Table 1), which was previously demonstrated to produce ZnZrOx materials with better catalytic performance than coprecipitated analogues for CO2 hydrogenation to methanol4. Accordingly, the optimal zinc content (5 mol%) reported was selected for this study, whereas a nominal amount of 0.5 mol% was chosen for all metal promoters (Re, Co, Au, Ni, Rh, Ag, Ir, Ru, Pt, Pd, and Cu), to produce catalysts with similar composition and thus enable a direct comparison of their promotional effects on ZnZrOx. Both experimental compositions closely matched nominal values, as determined by inductively coupled plasma optical emission spectroscopy (ICP-OES, Supplementary Table 2). Performance evaluation at relevant CO2 hydrogenation to methanol conditions (T = 573 K, P = 5 MPa, GHSV = 24,000 cm3 h−1 gcat−1, and H2/CO2 = 4) showed that, for most M-ZnZrOx systems, methanol space-time yield (STY) remains virtually unchanged (Re- and Co-ZnZrOx), slightly increased (ca. 10–20%, Au-, Ni-, Rh-, Ag-, and Ir-ZnZrOx), or moderately improved (ca. 40–60%, Ru- and Pt-ZnZrOx) in comparison to unpromoted ZnZrOx (STY = 0.25 gMeOH h−1 gcat−1, Fig. 1). This trend in methanol productivity can be traced back to the specific trade-off between CO2 conversion (XCO2) and methanol selectivity (SMeOH) displayed by these catalysts, with Ru- and Pt-ZnZrOx attaining higher XCO2 (ca. 6%) without experiencing significant loss in SMeOH (Supplementary Fig. 1). Remarkably, Cu exerts the highest improvement in the methanol STY (ca. 120%, STY = 0.55 gMeOH h−1 gcat−1, Fig. 1), which surpasses that induced by Pd, the current state-of-the-art promoter for ZnZrOx6,9. The striking methanol productivity of Cu-ZnZrOx is linked to its ability to sustain an outstanding SMeOH (ca. 90%) at high XCO2 levels (ca. 9% Supplementary Fig. 1), a similar behavior also observed for Pd-ZnZrOx. Interestingly, unpromoted and M-ZnZrOx catalysts show very similar specific surface area (SBET) in fresh form (ca. 100 m2 gcat−1, Supplementary Table 3 and Supplementary Fig. 2) which remains virtually unaltered after 20 h equilibration on stream. This indicates that these systems are particularly stable under reaction conditions and their performance difference does not stem from differences in SBET. In addition, this highlights the advantages of using FSP, as it allows the reproducible synthesis of catalysts of varying compositions that can be used and compared without any pre-treatment (washing or calcination) and having to change the manufacturing procedure.
Methanol space-time yield, STY during CO2 hydrogenation M-ZnZrOx (0.5 mol% of metal, M) catalysts prepared by FSP. The horizontal dashed line indicates the methanol STY of the unpromoted ZnZrOx catalyst. Averaged values measured over 20 h on stream are presented with their corresponding error bars. Reaction conditions: T = 573 K, P = 5 MPa, H2/CO2 = 4, and GHSV = 24,000 cm3 h−1 gcat−1.
In-depth characterization was conducted to shed light on the architecture of M-ZnZrOx catalysts. Analysis by X-ray diffraction (XRD, Supplementary Fig. 3) revealed no reflections characteristic of pure metal phases or zinc oxide (ZnO) in the used catalysts, indicating that the promoters and zinc remain well dispersed or form amorphous phases. Concerning the ZrO2 carrier, tetragonal (t) ZrO2 is the predominant crystalline phase present in all fresh materials, which partially transforms into the monoclinic (m) structure upon reaction. This transformation is likely triggered by water formed under CO2 hydrogenation conditions4,10. The partial transformation of t-ZrO2 into m-ZrO2 is a well-documented phenomenon for zirconia-containing materials prepared by FSP4,10. In contrast, ZnZrOx systems reported in the literature are often prepared by coprecipitation, which leads to the incorporation of zinc into the bulk structure of ZrO2, forming a solid solution that stabilizes the tetragonal phase, preventing its transformation into the monoclinic form. Comparatively, FSP typically results in materials with higher surface area and maximized zinc dispersion in the outmost layers of ZrO2. This improved Zn utilization and leads to a less stabilized t-phase, which enables the thermodynamically favored t-to-m transformation, enhancing its CO2 hydrogenation performance.
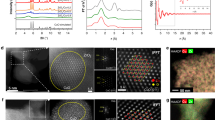
Investigations by scanning transmission electron microscopy (STEM) coupled to energy-dispersive X-ray (EDX) spectroscopy showed that metal nanoparticles with variable sizes (ranging from 5-10 nm) are clearly visible for used Au-, Ag-, Ru-, and Pd-ZnZrOx catalysts (Fig. 2 and Supplementary Fig. 4). For Au and Pd, these observations are corroborated by high-resolution STEM-HAADF analyses, which further revealed that, despite being undetected by EDX maps, nanoparticles of ca. 1 nm are present in Ir- and Pt-containing catalysts (Supplementary Fig. 5). In contrast, other metals like Re, Co, Ni, Rh, and Cu seem well dispersed as low-nuclearity species, as suggested by EDX maps, and, in the case of Re, also by STEM-HAADF (Fig. 2 and Supplementary Fig. 4,5). Interestingly, while zinc appears atomically dispersed over all catalysts, part of it tends to aggregate on Pd-ZnZrOx at regions containing palladium nanoparticles, suggesting that these phases are associated and likely form alloys. Temperature-programmed reduction with hydrogen (H2-TPR, Supplementary Fig. 6) reveals that most M-ZnZrOx catalysts exhibit some distinguishable signals (<600 K) indicative of the reduction of promoters, particularly for Pd-ZnZrOx, suggesting that pure metallic or Zn-containing alloys phases can be formed under reaction conditions6,8,22.
EDX maps of (a) Pd-ZnZrOx and (b) Cu-ZnZrOx catalysts after CO2 hydrogenation for 20 h. Reaction conditions as in the caption of Fig. 1.
X-ray absorption near-edge structure spectra (XANES) of M-ZnZrOx catalysts measured in quasi in situ mode (samples protected in capillaries under air-free environment) evidence differences in the metal promoters’ speciation (Supplementary Fig. 7). Spectral features of the Ag- and Au-ZnZrOx systems probed at the Ag K and Au L2 edges, respectively, closely match those of metallic Ag and Au foils, indicating that Ag and Au form large metallic nanoparticles, in line with microscopy observations (Supplementary Fig. 4). In contrast, while promoters are in a metallic state in Pd-, Ru-, Rh-, Ir-, and Ni-ZnZrOx catalysts, they are likely interacting with zinc and possibly forming alloys, as suggested by the shift in their corresponding spectra compared to that of references. Still, the degree of alloying with zinc is low in several cases, as zinc mostly retains its oxidic character in all M-ZnZrOx catalysts (XANES, Zn K-edge, Supplementary Fig. 8). Indeed, M-Zn contributions can only be reliably assigned in the extended X-ray absorption fine structure spectra (EXAFS, Zn K-edge, Supplementary Fig. 9) of Pd-ZnZrOx. Additionally, no signal characteristic of Zn-Zn scattering paths, generally centered at ca. 2.9 Å for ZnO, could be observed for any M-ZnZrOx catalyst, confirming the high dispersion of zinc species in most systems. Finally, the metals such as Pt, Re, Co, and Cu were mostly in oxidized state (Supplementary Fig. 7). In principle, since metals as cations are less prone to split H223, this could rationalize the limited performance of some of these elements as promoters. Still, it is particularly intriguing that oxidic copper species can effectively split H2, which contrasts with its proven ability to improve the methanol productivity of ZnZrOx (Fig. 1), and therefore further investigation into its speciation under reaction conditions is required.
Overall, considering both microscopic (Fig. 2, and Supplementary Fig. 4,5) and spectroscopic (Supplementary Fig. 7-9) findings, no clear correlation between the speciation and the performance of M-ZnZrOx catalysts is observed, particularly when compared to M-In2O3 systems in which atomically-dispersed metal species are more effective promoters than clusters and nanoparticles6,11. Instead, promotion in ZnZrOx appears more metal-dependent than speciation-dependent, with copper and palladium displaying distinct speciation that lead to similar and superior performance. In the case of Pd, EXAFS fitting of the Pd K-edge spectra reveals a clear Pd-Zn contribution (ca. 6 Zn neighbors, Supplementary Fig. 10 and Supplementary Table 4), confirming the formation of PdZn nanoalloy particles, and in line with H2-TPR (Supplementary Fig. 6). Since palladium is a well-known promoter for ZnZrOx and PdZn alloys are well documented for their favorable methanol synthesis properties6,8,9,24, we have devoted special focus to gather fundamental understanding of ZnZrOx promotion by copper, which represents an earth-abundant, more sustainable, and unexplored promoter class.
Copper speciation and promotional effect
To gain deeper insights into the origin of copper promotion in ZnZrOx, Cu-ZnZrOx and corresponding reference Cu-ZrOx and ZnZrOx materials prepared by FSP were further investigated (Fig. 3a and Supplementary Fig. 11,12). Catalyst durability assessed in a 100-h test (Fig. 3a) confirms that Cu-ZnZrOx shows a significantly higher methanol productivity, which stabilizes after 50 h induction time (STY = ca. 0.55 gMeOH h−1 gcat−1) compared to that of ZnZrOx and Cu-ZrOx combined, indicating that its performance does not result from a simple additive effect of integrating both binary systems. The induction period observed for the Cu-ZnZrOx catalyst relates to the restructuring and equilibration of the catalyst at elevated temperature and pressure. A similar behavior is observed for the Cu-ZrOx catalyst, albeit requiring a shorter time (ca. 20 h), likely due to the less complex architecture of this catalyst. It is important to note that beyond the induction period, Cu-ZnZrOx demonstrates stable performance, greatly surpassing that of the Cu-ZrOx and ZnZrOx systems. The improved methanol STY of Cu-ZnZrOx compared to ZnZrOx and Cu-ZrOx is linked to its higher XCO2 (ca. 9 versus 4 and 3%, respectively) and, in the case of Cu-ZrOx, also SMeOH (ca. 90 versus 70%), as evidenced by SMeOH evaluation carried out at similar XCO2 levels (ca. 5%, Fig. 3b). For completeness, Cu-ZnOx was also synthesized and its performance evaluated (Supplementary Fig. 11), which is comparable to Cu-ZrOx but notably inferior to Cu-ZnZrOx, further highlighting the superior catalytic properties of the ternary system. Interestingly, while all catalysts exhibit a low apparent reaction order with respect to H2 (nH2) for methanol synthesis (Fig. 3c), Cu-ZnZrOx displays a negative nH2 for the RWGS reaction, indicating that CO formation is inhibited over Cu-ZnZrOx at the applied hydrogenation conditions, rationalizing its high methanol selectivity. These findings are in line with copper agglomerating into nanoparticles in Cu-ZrOx upon reaction, which is known to favor CO formation25,26,27 and, although not detected by XRD (Supplementary Fig. 13), it is clearly evidenced by STEM-EDX (Supplementary Fig. 14a). In contrast, copper remains well dispersed as low-nuclearity species in Cu-ZnZrOx (Supplementary Fig. 14b), as copper atoms are most likely associated with zinc, indicating that this speciation favors methanol formation over CO production.
a Methanol space-time yield, STY, (b) selectivity, SMeOH during CO2 hydrogenation over ZnZrOx, Cu-ZrOx, and Cu-ZnZrOx catalysts. (c) Apparent reaction order with respect to H2 (nH2) for CO2 hydrogenation to methanol (blue) and the RWGS reaction (orange). The horizontal line indicates the sum of the methanol STY of ZnZrOx and Cu-ZrOx catalysts. SMeOH was assessed at constant CO2 conversion (ca. 5%) by adjusting the GHSV (24,000–96,000 cm3 h−1 gcat−1). Averaged values measured over 20 h on stream are presented with their corresponding error bars. Reaction conditions as in the caption of Fig. 1.
To shed light into the copper speciation in the Cu-ZnZrOx catalyst at work, operando X-ray absorption spectroscopy (XAS) experiments were conducted under pretreatment in He (1.5 MPa and from 303 to 573 K) and CO2 hydrogenation conditions (1.5 MPa and 573 K) at the Cu and Zn K-edges. Operando XANES reveals that both copper and zinc exist as oxidized phases in the fresh Cu-ZnZrOx catalyst (Fig. 4a, b). Upon thermal treatment in He, both elements undergo partial reduction, likely forming CuOx and ZnOx species. Interestingly, switching to the reaction mixture causes abrupt changes to copper, and more metallic character is evidenced, which contrasts the quasi in situ findings that show oxidized copper species after reaction (Fig. 4a and Supplementary Fig. 8). This apparent discrepancy highlights the importance of operando studies and can be attributed to underlying phenomena such as the highly oxophilic character of copper, which, in the absence of a reducing environment, can promote its reoxidation even by small concentration of oxygen impurities typically present in He streams used for preserving samples before analysis. Still, spectral features of Cu-ZnZrOx under operating conditions deviate from those of Cu-ZrOx, which contains metallic Cu nanoparticles (Fig. 4a). This hints at an effective stabilization of copper as low-nuclearity metallic species on the mixed ZnZrOx surface, owing most likely to a strong interaction with zinc. Nonetheless, while zinc shows further reduction when exposed to the reaction mixture (Fig. 4b), it is not fully reduced and still maintains characteristic features of ZnOx, suggesting that only a small fraction of metallic zinc atoms forms and interacts with copper, while its majority may comprise partially reduced zinc species intermixed on the ZrO2 surface. This rapidly evolving architecture remains unchanged after 5 h on stream (Fig. 4a, b), accounting for the stable performance of Cu-ZnZrOx (Fig. 3a).
Operando (a) Cu and (b) Zn K-edge XANES spectra of Cu-ZnZrOx catalyst (1) during the heating ramp in He (mcat = 0.013 g, heating rate = 5 K min−1, T = 573 K, P = 1.5 MPa, and dwell time = 30 min), (2) under He at 573 K, and under reaction conditions (mcat = 0.013 g, FT = 15 cm3 min−1, T = 573 K, P = 1.5 MPa, H2/CO2 = 4, dwell time = 300 min) after (3) 30 min and (4) 300 min on stream. (c) Fourier-transformed EXAFS spectra of Cu-ZnZrOx catalyst in fresh form and under reaction conditions corresponding to the spectra in (a) and (b). XANES and EXAFS spectra of Cu and Zn foils, ZnO, and activated Cu-ZrOx (mcat = 0.013 g, FT = 15 cm3 min−1, T = 573 K, P = 1.5 MPa, H2/CO2 = 4, dwell time = 300 min) are shown as reference.
In line with XANES findings, EXAFS analysis of the fresh Cu-ZnZrOx and Cu-ZrOx catalysts exhibits strong metal-oxygen (M-O) interactions, with ca. 4 oxygen neighbors (Fig. 4c and Supplementary Table 4). Under the highly reducing CO2 hydrogenation environment, however, a clear difference is observed between these two catalysts. Specifically, Cu-Cu contributions similar to those of Cu foil are present on Cu-ZrOx, with a large number of copper neighbors (ca. 9, Supplementary Table 4), which is consistent with the formation of metallic extended surfaces or nanoparticles. In contrast, while Cu-M signals are evidenced for Cu-ZnZrOx, they are clearly shifted from Cu-Cu foil but distinguishing between Cu-Cu and Cu-Zn interactions is impossible due to their similar scattering factors (Fig. 4c). Regarding the Zn K-edge, the EXAFS spectra shows that Cu-ZnZrOx largely exhibits Zn-O interactions and only a small Zn-M contribution is observed, which is shifted compared to that of the Zn foil (Fig. 4c). Together with XANES and STEM-EDX results (Figs. 2 and 4a), these observations indicate that copper is in a metallic state and strongly associated with partially reduced zinc atoms, most likely forming low-nuclearity zinc-rich CuZn clusters, particularly since the overall zinc content is 10 times higher than that of copper and the latter element is highly dispersed.
Density functional theory (DFT) simulations were performed to rationalize the most likely architectures present on ZnZrOx, Cu-ZrOx, and Cu-ZnZrOx catalysts. For this purpose, more than 200 structures were examined to evaluate the degree of incorporation of Cu and Zn into m-ZrO2 surface sites, formation of oxygen vacancies, Cu adsorption trends, and interaction between Cu and Zn in the ZrO2 matrix. Models were built based on two criteria: (i) the compatibility with the experimental observations (cluster nature); and (ii) the DFT-computed energies were reasonable (i.e., processes thermodynamically favored). The most relevant models and their corresponding potential energies (E) are depicted in Fig. 5, whereas other models are discussed in the Supplementary Information (see Supplementary Methods and Discussion). ZnZrOx is best represented by a model with isolated Zn atoms replacing Zr sites at the ZrO2 surface with a nearby oxygen vacancy, (ZnZrOx, ΔE = –0.04 eV, Fig. 5 and Supplementary Fig. 15)4. In contrast, two independent models, i.e. Cu(111) and m-ZrO2(–111), are considered for the Cu-ZrOx catalyst, particularly since experimental results evidence two separated phases (Supplementary Fig. 14a), and both incorporation and adsorption of a Cu atom on ZrO2 are endothermic (Cu@ZrOx, ΔE = 1.86 eV, Cu/ZrO2, ΔE = 2.63 eV, respectively, see Fig. 5). For CuZnZrOx, we initially explored the incorporation of Cu in the ZnZrOx model with isolated Zn centers (Fig. 5). Cu incorporation (Cu@Zn4ZrOx) is not favored (ΔE = 2.08 eV wrt bulk Cu) even at sites nearby Zn atoms. Interestingly, adsorption of Cu on the surface near Zn sites (Cu/Zn4ZrOx, Fig. 5) is favored compared to zinc-free ZrO2, further confirming the Cu-Zn affinity. Nevertheless, none of the configurations investigated with separated Zn atoms provide suitable nucleation sites for Cu (Fig. 5). Additionally, none of the models fully agree with the EXAFS and STEM-EDX findings, which indicate that Cu is most likely surrounded by a zinc-rich ensemble under reaction conditions (Supplementary Table 4). However, the formation of CuZn clusters under reaction conditions should be traced back to the Cu-Zn affinity, since the formation energy of the bulk Cu5Zn8 (brass) and CuZn alloys are exothermic (ECu5Zn8 = –0.26 eV and ECuZn = –0.17 eV, respectively). Hence, alternative scenarios include that the formation of the Cu-Zn pairs occurs earlier in the preparation. Indeed, the melting temperatures of Cu-Zn and ZrO2 differ significantly (1023 and 2988 K) meaning that the nucleation during cooling of the FSP could occur independently. This would lead to Zn rich environments with Cu and later the stabilization of these clusters by the oxidic ZrO2 phase ensuring that some O atoms bond the oxophilic Zn ones. Thus, we investigated models where 5 Zn atoms aggregate in nearby sites (Zn5ZrOx) within the m-ZrO2 surface with 5 oxygen vacancies. We employed this structure to study the adsorption of a single Cu atom (Cu-Zn5ZrOx) finding that this is more favored than Cu/ZrO2 and Cu/Zn4ZrOx (Fig. 5). Including a second Cu atom is exothermic (Cu2-Zn5ZrOx) and results in a CuZn motif very similar to that found in brass, particularly the Cu5Zn8 (110) surface (Fig. 5). Moreover, larger Cu clusters (containing 3 or 4 atoms) are not favored (Supplementary Fig. 16) further underlying the role of nucleation and alloy properties in the final compound. Furthermore, alternative models (over 150 structures) were evaluated to explore the robustness of the Cu2-Zn5ZrOx ensemble. We assessed different degrees of reduction and the formation of analogous structures with different amount of Zn and Cu, as detailed in the Supplementary Information (see Supplementary Methods and Discussion, Supplementary Fig. 17-21, and Supplementary Table 5). These results indicate that models with 2 copper (possibly forming dimer species) and 5 zinc atoms are more likely thermodynamically and the most compatible with the experimental observations. Therefore, the Cu2-Zn5ZrOx structure was selected as the most representative model for evaluating the catalytic properties of Cu-ZnZrOx catalysts from a computational standpoint.
Snapshots of DFT models with their associated relative potential energy, \(\Delta\)E with respect to Cu and Zn bulk energies and m-ZrO2 surface, were employed to rationalize the tendency of Zn and Cu to incorporate into the ZrO2 surface sites and the deposition of Cu on Zn-doped and pure ZrO2 surfaces. The models employed to assess Cu-ZrOx, ZnZrOx, and Cu-ZnZrOx catalytic systems via DFT simulations are highlighted in light blue, gray, and purple, respectively. The dashed arrows represent the incorporation of n Zn or Cu atoms (blue and light pink, respectively) accompanied with the formation of n oxygen vacancies on the ZrO2 surface. Solid light pink lines denote the deposition of a Cu atom on ZrO2 and ZnZrOx surfaces. The common Zn5Cu2 pattern found in Cu2-Zn5ZrOx and Cu5Zn8(110) is depicted in black. Color code of DFT models: Zn (blue), Zr (green), Cu (light pink), O (red), and O vacancies (doted white circles).
Mechanistic insights into oxygen vacancy formation and dynamics
To investigate the formation and dynamics of oxygen vacancies on Cu-ZnZrOx, in situ electron paramagnetic resonance spectroscopy (EPR) experiments were carried out on this catalyst and reference ZnZrOx and Cu-ZrOx materials (Fig. 6). Fresh ZnZrOx shows a weak and almost isotropic peak (g = 2.003) and an additional anisotropic signal (g║ = 1.959, g┴ = 1.977, Fig. 6a), which are attributed to unpaired electrons trapped in isolated oxygen vacancies (behaving paramagnetically, VO−-p) and Zr3+ sites, respectively, as previously reported4. Upon thermal treatment in He, the intensity of both signals increased (Fig. 6a), indicating the formation of a relatively small amount of thermally-induced vacancies. In contrast, exposure to the reaction mixture (CO2 + H2) led to a decrease in the Zr3+ signal (Fig. 6a and Supplementary Fig. 22a). This is consistent with previous observations indicating that Zr3+ is reoxidized to Zr4+ as electrons are transferred to vacancies (VO2−) formed by reaction with H2 (see Supplementary Discussion for detailed description)4. Still, Zr3+ does not effectively impact the catalytic ensemble properties, acting as a spectator species4,10. Upon switching to reaction mixture a newly broad signal was evidenced and attributed to unpaired electrons delocalized over several oxygen vacancies that interact (exchange-coupled vacancy polarons, VO−-f)4 (Fig. 6a and Supplementary Fig. 22a). Generally, this broad signal appears when the density of vacancies becomes particularly high, which is considered a key performance descriptor, as reported for Pd-In2O3-ZrO2 systems10. For Cu-ZrOx, however, only Cu2+ and VO−-p species are detected under thermal treatment in He, whereas Zr3+ and VO−-f signals are not observed (Fig. 6b and Supplementary Fig. 22b) even under CO2 + H2, suggesting that a low concentration of vacancies is present on Cu-ZrOx, which could in principle explain its inferior performance (Fig. 3a).
In situ EPR spectra measured at room temperature of (a) ZnZrOx, (b) Cu-ZrOx, and (c) Cu-ZnZrOx catalysts in fresh form, after heating ramp in He (mcat = 0.013 g, FT = 15 cm3 min−1, heating rate = 5 K min−1, T = 573 K, P = 1 MPa) and CO2 hydrogenation (mcat = 0.013 g, FT = 15 cm3 min−1, T = 573 K, P = 1 MPa, and H2/CO2 = 4). (d) Operando EPR spectra of key signals for Cu-ZnZrOx catalyst under He (mcat = 0.013 g, FT = 15 cm3 min−1, T = 573 K, P = 1 MPa, and dwell time = 30 min) and under reaction conditions (mcat = 0.013 g, FT = 15 cm3 min−1, T = 573 K, P = 1 MPa, and H2/CO2 = 4) with continuous time on stream. Evolution of Cu2+ and Zr3+ species, as well as paramagnetic and ferromagnetic oxygen vacancies (VO-p and VO-f, respectively) for (e) ZnZrOx, (f) Cu-ZrOx, and (g) Cu-ZnZrOx catalysts under operando conditions described in d and Supplementary Fig. 22a,b.
The EPR spectrum of fresh Cu-ZnZrOx displays a typical Cu2+ signal, with an axial g tensor and a well-resolved hyperfine splitting of the g║ component (Fig. 6c). Interestingly, alike Cu-ZrOx, no Zr3+ signal was observed, indicating that Cu2+ sites are better electron scavengers. Thermal treatment in He led to a strong decrease of the Cu2+ and increase of VO–-p signals, while a new feature appeared around g = 2, partially overlapped with the Cu2+, and consistent with that of surface-adsorbed superoxide ions (Fig. 6c). However, contrary to ZnZrOx, electrons released during these processes are most likely trapped by (i) Cu2+ sites, generating EPR-silent Cu+ species, (ii) empty vacancies, giving rise to VO−-p, and (iii) oxygen released from the surface lattice, leading to the formation of the superoxide. This further corroborates its presence significantly promotes vacancy formation. Exposure to the reaction mixture leads to the complete reduction of Cu2+ and to a significant decrease of the VO−-p signal (Fig. 6c, d). Additionally, a signal assigned to VO−-f is detected but much broader than the one observed for ZnZrOx, indicating that a higher density of vacancies is formed on Cu-ZnZrOx. The decrease of the VO–-p signal is most likely linked to its conversion into the broad feature (VO–-f), as the increased concentration of vacancies leads to ferromagnetically-coupled defect sites and, consequently, depletes the number of isolated vacancies (VO–-p).
To gather additional insights into the dynamics of oxygen vacancies on Cu-ZnZrOx catalyst, operando EPR measurements were performed under He and CO2 hydrogenation conditions (1 MPa and 573 K; Fig. 6d-g, Supplementary Fig. 22a, b, and 23). Evolution of key species over time (Fig. 6e–g) reveals that VO–-p and VO–-f formation rates are mirrored by that of Cu2+ and Zr4+ reduction, suggesting that these processes are linked, thereby validating the above-discussed electron scavenging mechanisms. More importantly, these experiments show that VO–-f signals are better fingerprints compared to VO–-p for monitoring vacancy formation and annihilation (see Supplementary Discussion for details). Additionally, a comparison of the Cu2+ reduction kinetics of Cu-ZnZrOx and Cu-ZrOx shows significant differences (Supplementary Fig. 24), with a monotonous monoexponential decay being observed for Cu-ZnZrOx. In contrast, Cu-ZrOx clearly shows a component that decays with the same rate as the Cu2+ in Cu-ZnZrOx, whereas the other starts decreasing at a later stage and is significantly slower, which is most likely related to the reduction of copper nanoparticles present on Cu-ZrOx, in line with microscopy results (Supplementary Fig. 14a). Furthermore, these observations indicate that the overall Cu2+ reduction is more efficient on Cu-ZnZrOx due likely to a series of coupled factors such as its speciation and strong interaction with zinc and oxygen vacancies. Interestingly, exposing the Cu-ZnZrOx catalyst to oxygen at room temperature after the reaction reveals a full recovery of the Cu2+ signal (Supplementary Fig. 25), hinting at the stable nature of copper sites. Overall, EPR experiments demonstrate that oxygen vacancy generation is augmented on Cu-ZnZrOx upon reaction, which most likely contributes to its enhanced performance. Indeed, these findings strongly suggest that these defect sites are an integral component of the low-nuclearity zinc-rich CuZn catalytic ensembles present on Cu-ZnZrOx, with their role being multifaceted and likely including (i) stabilization of copper atoms and/or reaction intermediates, and (ii) activation of reactants.
Insights on the reaction mechanism
CO2 hydrogenation to methanol requires the efficient activation of CO2 and H2, followed by selective proton-hydride transfers to avoid the CO formation through the RWGS reaction. A bifunctional mechanism combining the properties of oxidic (CO2 activation and hydrogenation) and metallic (H2 activation) phases in close proximity is key to avoid transport of active species and achieve high methanol productivities11,19. Accordingly, adsorption of key reactants, intermediates, and byproduct species (CO2, CO, H2, HCOO*, and CH3O*) on selected models displayed in Fig. 5 was assessed through DFT simulations to rationalize the catalytic properties of Cu-ZrOx, ZnZrOx, and Cu-ZnZrOx. CO2 adsorption is favored on basic sites of m-ZrO2 (−111) whereas H2 activation on this surface is endothermic (Fig. 7), with an activation energy (Ea) of 0.70 eV, and occurs heterolytically, forming hydrides (H–) and protons (H+) on a Zr-O pair (Supplementary Fig. 26). In contrast, Cu (111) poorly activates CO2 but homolytic H2 dissociation takes place (Ea = 0.58 eV) and the adsorption of its products (hydrogen atoms) is highly favored (Fig. 7). Hence, spillover from Cu nanoparticles onto the ZrO2 surface, where CO2 is activated and hydrogenated, is required for the Cu-ZrOx catalyst. Consequently, this leads to a sluggish transport of active species, which hampers methanol synthesis on this system, thereby contributing to its inferior performance. For ZnZrOx, heterolytic H2 activation on a Zn-O pair is more favored (Ea = 0.21 eV and Eads,H2 = 0.02 eV) compared to ZrO2 (Figs. 7 and 9). Additionally, CO2 is activated as carbonate in a nearby site, thus avoiding unnecessary transport of active species onto the catalyst surface, which explains its higher methanol productivity compared to Cu-ZrOx. Adsorption energies for CO2 and H2-dissociated products on the Cu2-Zn5ZrOx model, which better describes active ensembles on the Cu-ZnZrOx system, indicate that this configuration enhances activation of these reactants at spatially resolved sites, thereby also circumventing long-range transport of species (Fig. 7). Moreover, H2 activation is barrierless and found to proceed in a homolytic manner on metallic Cu atoms (Figs. 7 and 9), further corroborating the key role of Cu in improving the H2 splitting ability of ZnZrOx. Similar results were observed for analog models also comprising partially reduced Zn atoms incorporated into surface lattice positions of ZrO2, oxygen vacancies, and metallic Cu atoms deposited on a different arrangement (Cu2-Zn5ZrOx-b, Supplementary Fig. 27). In contrast, adsorption energies of reactant species on the Cu5Zn8 (110) surface exhibit similar trends as Cu(111), favored H2 activation and unfavored CO2 adsorption, suggesting that partially reduced zinc species, present on Cu-ZnZrOx, rather than metallic counterparts are required to enable an efficient activation and subsequent transformation of reactants into methanol (Supplementary Fig. 27).
Adsorption energies, Eads of key reactive species on relevant surfaces representative of Cu-ZrOx, ZnZrOx, and Cu-ZnZrOx catalytic systems. Color code of DFT models: Zn (blue), Zr (green), Cu (light pink), and O (red). While only the most energetically favorable Eads of key reactive species for each model is displayed in Fig. 7, other adsorption structures and Eads and are shown in Supplementary Fig. 26 and 27, respectively.
To gain experimental insights into key reactive intermediate species formed on Cu-ZrOx, ZnZrOx, and Cu-ZnZrOx catalysts under reaction conditions, operando diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) experiments were conducted (Fig. 8 and Supplementary Fig. 28). Upon exposure to the reaction mixture (H2/CO2 = 4, 1.5 MPa, and 573 K; Fig. 8a-c, and Supplementary Fig. 28), all catalysts exhibited bands characteristic of formate (HCOO*, 1586, 1373, 2736, 2875, and 2965 cm−1) and methoxy species (CH3O*, 1051, 1140, 2827, and 2928 cm−1), which can be assigned to distinct vibrational modes (Supplementary Table 6, 7)2,25,28,29,30. These results demonstrate that methanol synthesis via CO2 hydrogenation over all catalysts follows the formate pathway, which is in line with previous studies on ZnZrOx2,6 and indicates that the copper promotion in Cu-ZnZrOx is not governed by a different mechanism. Interestingly, a strong signal attributed to gas-phase CO6,31 (Supplementary Fig. 28) is detected for Cu-ZrOx, which is in agreement with its tendency to promote this undesired product, thus explaining its lower SMeOH compared to ZnZrOx and Cu-ZnZrOx (Fig. 3b). Evolution of HCOO* and CH3O* species assessed by monitoring the vibration bands at 2874 and 2828 cm−1, respectively, over time-on-stream (Fig. 8g–i) revealed that their surface concentration changes simultaneously for all catalysts, suggesting that overall reaction rate is determined by the HCOO* surface concentration. Still, the surface concentration of HCOO* and CH3O* on Cu-ZnZrOx reaches steady state within ca. 30 min, whereas it requires ca. 50 and 70 min for ZnZrOx and Cu-ZrOx, respectively (Fig. 8g–i). This suggests that the catalytic ensemble in Cu-ZnZrOx is more effective in accelerating the formation of these key reaction intermediates. To investigate the dynamics of surface species in pure H2, CO2 was replaced by He and the normalized intensity of the vibration bands at 2874 and 2828 cm−1 was continuously monitored (Fig. 8d–i). The concentration of HCOO* decreases rapidly, gradually, and slowly for Cu-ZnZrOx, ZnZrOx, and Cu-ZrOx, respectively, over 90 min on stream, hinting at a much lower energy barrier for its hydrogenation to CH3O* on the copper-promoted ZnZrOx catalyst (Fig. 8g–i). Still, unlike ZnZrOx and Cu-ZrOx, Cu-ZnZrOx swiftly hydrogenates newly formed CH3O* species, reaching a steady state similar to that in the presence of both CO2 and H2 (Fig. 8i). In this case, further hydrogenation of CH3O* is most likely masked by the dissociative adsorption of methanol on unoccupied sites created by the absence of CO232.
Operando DRIFT spectra of key signals for (a) ZnZrOx, (b) Cu-ZrOx, and (c) Cu-ZnZrOx catalysts under (a-c) CO2 hydrogenation (FT = 40 cm3 min−1, T = 573 K, P = 1.5 MPa, H2/CO2 = 4, and dwell time = 90 min) and (d-f) after switching to a mixture of H2 balanced with He (FT = 40 cm3 min−1, T = 573 K, P = 1.5 MPa, H2/He = 4, dwell time = 90 min). Evolution of evolution of normalized intensity of formate (HCOO*, 2874 cm−1, orange) and methoxy (C H3O*, 2828 cm−1, purple) for (g) ZnZrOx, (h) Cu-ZrOx, and (i) Cu-ZnZrOx catalysts under operando conditions described in (a–f).
To gain further insights into the reaction mechanism, we computed energy profiles for CO2 hydrogenation to methanol and CO on the relevant models presented in Fig. 5 (Supplementary Figs. 29, 30). We followed the formate pathway, which is in line with operando DRIFTS results and previous studies of ZnZrOx2,4,6 and other metal oxide-based catalysts6,11,33. We have also evaluated methanol formation on the Cu(111) surface via CO hydrogenation since gas-phase CO was detected for Cu-ZrOx and Cu-ZnZrOx systems (Supplementary Fig. 28). Methanol formation is favored over CO production on m-ZrO2(–111), ZnZrOx, and Cu2-Zn5ZrOx (Supplementary Fig. 29-30), in agreement with experimental observations (Fig. 3). In contrast, the energy profiles computed on Cu(111) indicate that CO2 hydrogenation to methanol competes with CO formation. Still, CO desorption is favored over being further hydrogenated compatible with the lower SMeOH of Cu-ZrOx. Moreover, DFT simulations indicate that the relative stability between HCOO* and CH3O* is favored on Cu2ZnZrOx over m-ZrO2(–111) and ZnZrOx (Supplementary Table 6). This is in line with the rapid hydrogenation of HCOO* into methanol observed on Cu-ZnZrOx compared to Cu-ZrOx and ZnZrOx (Fig. 8g–i). Overall, based on DFT and DRIFTS, we attribute the high methanol productivity exhibited by Cu-ZnZrOx to the formation of Zn-rich low-nuclearity CuZn clusters intermixed in the ZrO2 matrix. Specifically, this catalytic ensemble, which is likely stabilized by oxygen vacancies formed upon reaction, efficiently combines both oxidic (ZnOx) and metallic (Cu) properties required to effectively activate CO2 and H2 in nearby sites and enable concomitant and fast hydrogenation of key intermediates into methanol (Fig. 9). Finally, given the key role played by copper in promoting ZnZrOx, further optimization of its content in the ternary Cu-ZnZrOx systems should be undertaken in future work.
Summary of key geometric and reactivity features dictating the performance of Cu-ZnZrOx catalysts. Snapshots of the adsorption of CO2 and the product of dissociative H2 adsorption on different surfaces are shown for relevant systems. Color code of DFT models: Zn (blue), Zr (green), Cu (light pink), O (red), H (white), and C (dark gray).
In summary, copper is uncovered as the most effective promoter for flame-made ZnZrOx catalysts for methanol synthesis via CO2 hydrogenation, rivaling state-of-the-art palladium promotion, outperforming various hydrogenation metals (i.e., 0.5 mol% Re, Co, Au, Ni, Rh, Ag, Ir, Ru, or Pt), and leading to a 2-fold increase in methanol productivity compared to unpromoted ZnZrOx. In addition, the Cu-ZnZrOx catalyst exhibits a significantly more stable and superior performance over 100 h on stream compared to Cu-ZrOx, where copper agglomerates into large nanoparticles, leading to inferior reactivity and methanol selectivity. In contrast, copper forms low-nuclearity metallic species on Cu-ZnZrOx upon reaction that strongly interact with zinc ensembles intermixed in the surface of the ZrO2 carrier, as evidenced by combining operando XAS analysis and DFT simulations. Operando EPR spectroscopy shows that oxygen vacancy generation is particularly augmented upon reaction and closely associated with the formation of zinc-rich CuZn clusters. The resulting active catalytic ensemble comprising CuZn clusters and oxygen vacancies in the vicinity of Zn atoms efficiently integrate acid-base and H2 splitting functions that enable CO2 activation and barrierless homolytic H2 dissociation over spatially resolved sites. In fact, this unique geometric configuration greatly fosters methanol formation through the formate (HCOO*) path, as it promotes fast hydrogenation of HCOO* to methanol, while preventing undesired long-range transport of active species and CO formation. This work sets an important milestone in the design of metal-promoted ZnZrOx catalysts and other systems based on reducible oxides, uncovering the promotional effect of a highly effective architecture based on copper, an earth-abundant element, for CO2 hydrogenation to methanol. It also highlights the potential of stable low-nuclearity metal clusters as a promising class of heterogeneous catalytic materials for diverse related applications.
Methods
Catalyst synthesis
M-ZnZrOx (M = Ag, Au, Co, Cu, Ir, Ni, Pd, Pt, Re, Rh, Ru) catalysts with a nominal metal promoter and zinc contents of 0.5 and 5 mol%, respectively, were prepared by FSP. Briefly, a precursor solution of dissolved zinc, zirconium, and promoter complexes (see Supplementary Table 1 for a full list) in the desired ratio was pumped through a 0.4 mm nozzle at a flow rate of 5.0 cm3 min−1 and dispersed into a fine spray by flowing oxygen at 1.5 bar at a flow rate of 5.0 L min−1. The spray was ignited by a supporting flame generated using 2.4 L min−1 of oxygen and 1.2 L min−1 of methane. Such particle-generating flames have been well characterized and reported to reach average flame temperatures of 2500–3000 K, with very fast cooling rates (ca. 106 K s−1)34,35,36. The resulting nanoparticles were collected on a glass fiber filter (GF/A-6) and used in CO2 hydrogenation without further treatment.
Catalyst characterization
Inductively coupled plasma optical emission spectroscopy (ICP-OES) was carried out using a Horiba Ultra 2 instrument equipped with a photomultiplier tube detector. Samples were digested with the aid of microwave irradiation using a mixture of HCl (Alfa Aesar, 36 wt%), H2SO4 (Alfa Aesar, 95 wt%), and HF (Sigma Aldrich, 48 wt%) with a volume ratio of 2:1:0.5, followed by neutralization with a saturated solution of HBO3 (Fluka, 99%). Nitrogen sorption at 77 K was carried out using a Micromeritics TriStar II analyzer. Prior to the measurements, samples were degassed at 473 K under vacuum for 12 h. The specific surface area (SBET) was determined using the Brunauer–Emmett–Teller (BET) method. X-ray diffraction (XRD) was conducted using a Rigaku SmartLab diffractometer with a D/teX Ultra 250 detector using Cu Kα radiation (λ = 0.1541 nm) and operating in the Bragg-Brentano geometry. Data was acquired in the 10°–70° 2θ range with an angular step size of 0.025° and a counting time of 1.5 s per step. Temperature-programmed reduction with hydrogen (H2-TPR) were measured at ambient pressure using a Micromeritics AutoChem HP II analyzer. The effluent stream of the instrument was measured using thermo-conductivity detector. High-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) and energy-dispersive X-ray (EDX) spectroscopy was carried out on a Talos F200X equipped with a Super-X EDS system and high-resolution STEM imaging on a probe-corrected HD2700CS (Hitachi), both operated at 200 kV. Catalyst powder was dispersed in ethanol, ground in a mortar, and dispersed on a copper or nickel STEM-grid covered with a lacey carbon support film. After evaporation of ethanol at room temperature, the specimen was mounted on a single-tilt holder and inserted into the microscope. Selected samples were also analyzed by HAADF-STEM-EDX using a probe-corrected Titan Themis operated at 300 kV. Operando X-ray absorption spectroscopy (XAS) experiments were conducted at the Swiss-Norwegian beamlines (SNBL, BM31) of the European Synchrotron Radiation Facility (ESRF)37. The X-ray beam was collimated using a double-crystal liquid nitrogen cooled Si(111) monochromator37. Μetallic Cu and Zn foils were measured for the absolute energy calibration, using N2-filled ionization chambers for optimal absorption levels. Fluorescence geometry configuration was employed for the analysis under operando conditions, using a one-element Si SDD detector with Peltier cooling. Continuous scanning was performed for both Cu (between 8880 and 9550 eV) and Zn edges (between 9550 and 10650 eV), and the step size was set to 0.5 eV, with a scan duration of 150 s. The beam size was set to 3 × 0.2 mm (horizontally × vertically). The catalyst (mcat = 0.013 g) was placed between two plugs of quartz wool in a quartz capillary reactor cell (1 mm outer diameter, 0.01 mm wall thickness). XAS experiments were carried out in the followings steps: (i) heating to 573 K in He (FT = 15 cm3 min−1, heating rate = 5 K min−1, T = 573 K, P = 1.5 MPa, and dwell time = 30 min), (ii) switching to CO2 hydrogenation conditions (FT = 15 cm3 min−1, T = 573 K, P = 1.5 MPa, H2/CO2 = 4, dwell time = 300 min), (iii) switching to He (FT = 15 cm3 min−1, heating rate = 5 K min−1, T = 573 K, P = 1.5 MPa, and dwell time = 30 min) and (iv) cooling down to room temperature in He (FT = 15 cm3 min−1, heating rate = 5 K min−1, T = 303 K, P = 1.5 MPa, and dwell time = 30 min). Extended X-ray absorption fine structure (EXAFS) measurements were performed at each steady state, including the fresh and used sample, as well as under CO2 hydrogenation reaction conditions. Under all other conditions, including temperature ramping (steps i and iv) and after switching to different conditions (steps ii and iv, as described above), X-ray absorption near-edge structure (XANES) measurements were instead preferred, to capture the dynamic changes of the systems. The high-purity gases were dosed by a set of Bronckhorst digital mass flow controllers and the outcome was monitored on-line via a Pfeiffer Vacuum OmniStar GSD 320 O mass spectrometer with Quadera software. The resulting spectra were energy calibrated, background corrected, and normalized using the Athena program from the Demeter software suite38. k3-weighted EXAFS spectra were fitted in the optimal k- and R-windows (Supplementary Table 5) using the Artemis program. An amplitude reduction factor (S02) of 0.81 and 0.75 were determined by fitting of the EXAFS spectrum of a Cu and Zn foil, respectively. The scattering paths for the fitting were produced using known crystallographic structures of metallic Cu, and Zn, Cu5Zn8, and ZnO. Additional quasi in situ XAS measurements were performed for Re, Ir, and Pt L3 edge, Au L2 edge, and Co, Ni, Rh, Ag, Ru, Pd, and Cu K edge. Samples were prepared under the exclusion of air by transferring catalysts samples after catalytic tests (20 h under standard conditions, see below) into quartz glass capillaries (1 mm outer diameter, 0.01 mm wall thickness, 80 mm length, Hilgenberg GmbH) and sealing of the capillaries in an inert atmosphere. The samples were placed on a stage in place of the setup used for the operando measurements and scanned in fluorescence mode the same Si SDD detector described above. In situ and operando electron paramagnetic resonance (EPR) spectroscopy experiments were performed using a custom-built setup (microwave frequency = 9.2 GHz, center field = 300 mT, sweep width = 570 mT, modulation amplitude = 3 G, modulation frequency = 100 kHz, microwave power = 1.986 mW, power attenuation = 20 dB, conversion time = 86.55 ms, time constant = 20.48 ms). The catalyst sample (mcat = 50 mg) was loaded into a quartz capillary (di = 0.8 mm) and placed inside an EPR quartz tube (Wilmad; di = 2.8 mm). The EPR tube was housed at the center of a homemade water-cooled high-temperature resonator39, which was installed into a continuous wave (CW) EPR spectrometer (Bruker EMX) operating at X-band frequencies. The reactor was pressurized to 1 MPa, heated in a He flow (20 cm3 min−1) to the desired temperature (T = 573 K) and kept at this condition for 2 h, while continuously recording EPR spectra. It was subsequently cooled down to room temperature and an EPR spectrum was recorded. The reactor was then heated up again to 573 K and left to stabilize for 30 min. The reaction mixture (H2/CO2 = 4) was then admitted and was kept flowing (20 cm3 min−1) for 2 h. All gases were dosed by a set of digital mass flow controllers and the reactor outlet was monitored online using a Pfeiffer Vacuum Thermo-Star GSD 320 T1 mass spectrometer. The EPR spectra were continuously acquired upon flowing the gases and separately stored, using a 2D acquisition mode, thus enabling a time-resolved monitoring of the process. The reactor was eventually cooled down and EPR spectra were recorded at room temperature. He and air were then subsequently admitted and the EPR spectrum was monitored until no changes were detected. Operando diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) was conducted in a custom-built setup comprising a gas dosing unit, back-pressure regulators, a Pfeiffer Vacuum OmniStar GSD 350 O2 mass spectrometer for online product analysis, and a Bruker INVENIO-S spectrometer equipped with a liquid nitrogen cooled mercury cadmium telluride (MCT) detector. In a typical experiment, 50 mg of the undiluted sample was packed into a high-temperature reaction chamber with ZnSe windows (Harrick Scientific), which was mounted in a Praying Mantis diffuse reflection attachment (Harrick Scientific). After purging the sample with flowing He (20 cm3 min−1, PanGas, 4.6) for 60 min, the reaction chamber was pressurized to 1.5 MPa and heated to 573 K with a ramp of 5 K min−1. Subsequently, spectra were continuously acquired at a spectral resolution of 2 cm−1 by accumulating 128 scans every 30 s and were normalized against a KBr background taken at 573 K and 1.5 MPa. The gas feed was then switched to a mixture of H2 (PanGas, 5.0) and CO2 (40 vol% in H2, Messer, 4.5) with a molar H2/CO2 ratio of 4 at a total flow rate of 20 cm3 min−1. After 90 min under reaction conditions, the CO2 flow was stopped and H2 (16 cm3 min−1) balanced with He (4 cm3 min−1) was fed into the reaction chamber for an additional 90 min. The raw data obtained with Bruker OPUS 8.2 software were processed and difference spectra as well as intensity profiles were generated with a self-coded Python routine, available as open-source on GitHub (https://github.com/philpreikschas/operando-ir).
Catalyst evaluation
The gas-phase hydrogenation of CO2 to methanol was conducted using a PID Eng&Tech high-pressure continuous-flow setup comprising four parallel fixed-bed reactors, as described elsewhere40. Undiluted catalysts (mass, mcat = 0.1 g; particle size = 0.2-0.4 mm) were loaded into each reactor tube (internal diameter 4 mm), held in place by a quartz-wool bed set on a quartz frit, and purged in flowing He (40 cm3 min−1, PanGas, 4.6) for 30 min. Under the same flow, the pressure was increased to 5 MPa for a leak test, which was followed by heating up the catalyst bed to 573 K (5 K min−1). The reaction was carried out by feeding a mixture of H2 (PanGas, 5.0), CO2 (40 vol% in H2, Messer, 4.5), with a molar H2/CO2 ratio of 4 at 573 K, 5 MPa, and a gas hourly space velocity (GHSV) of 24,000 cm3 h−1 gcat−1, unless stated otherwise. The selectivity of the catalysts was compared at a constant degree of CO2 conversion (\({X}_{{{CO}}_{2}}\)) as described in Fig. 3b by adjusting the GHSV for each system.
The effluent streams were analyzed by gas chromatography every 1 h. Response factors (Fi) for each compound i, respective to the internal standard (20 vol% C2H6 in He, Messer, purity 3.5), in the GC analysis were determined by Eq. (1):
where Ai is the integrated area determined for the peak of compound i and \({n}_{i}^{{{{{{\rm{in}}}}}}}\) is the corresponding known molar flow at the reactor inlet. An average of 5 points around the expected analyte concentration was used. The unknown effluent molar flow of compound i (\({n}_{i}^{{{{{{\rm{out}}}}}}}\)) was determined using Eq. (2):
where \({n}_{{{{{{{\rm{C}}}}}}}_{2}{{{{{{\rm{H}}}}}}}_{6}}^{{{{{{\rm{out}}}}}}}\) is the known flow of C2H6 at the reactor outlet.
Conversion (Xi), selectivity (Si), and methanol production rate (rMeOH) were calculated using Eqs. (3–5):
The methanol space-time yield (STY) is the product of rMeOH and the molar weight (MW) of methanol (32.04 g mol−1) as stated in Eq. (6):
The carbon balance was determined for each experiment according to Eq. (7):
and was always within a 5% margin.
Computational details
Density function theory (DFT) simulations were conducted with Vienna ab initio simulation package (VASP)41 and the Perdew-Burke-Ernzerhof (PBE) density functional42,43. Valence electrons were expanded from a plane-wave basis set with a kinetic cut-off energy of 500 eV while core electrons were described with projector augmented-wave (PAW) pseudopotentials44,45. The Brillouin zone was sampled with a \(\Gamma\)-centered mesh with a reciprocal grid size narrower than 0.037 Å–1, which was obtained with the Monkhorst-Pack method46. Lattice parameters for m-ZrO2, Cu, Zn, CuO, ZnO, and Cu5Zn8 were optimized with a kinetic energy cut-off of at least 700 eV. Then, slab models for m-ZrO2(–111)4,47,48, Cu(111), and Cu5Zn8 (110)49,50 terminations were cleaved from the optimized bulks and represented with p(1 × 1)/p(2 × 2), p(4 × 4), and with p(1 × 1) expansions, respectively. In all cases, the two bottommost layers were kept fixed and the two outermost were allowed to relax. We added a vacuum region of 15 Å between slabs and a dipole correction along the z axes was applied to account for the asymmetry in the relaxations51. The incorporation of Zn and Cu, as well as the formation of oxygen vacancies were assessed at different sites of m-ZrO2(–111) following the procedure employed in our previous work4. Additionally, the deposition of single atoms and small clusters (up to 4 atoms) of Cu was explored in models of pure and Zn-doped m-ZrO2 surfaces. The adsorption energies of key molecules (CO2, CO, H2, HCOO*, and CH3O*) was carried out in relevant surfaces to rationalize the catalytic properties of ZnZrOx, Cu-ZrOx, and Cu-ZnZrOx. Clean surfaces and gas-phase CO2, H2, and CO molecules were employed as thermodynamic sinks. The adsorptions of HCOO* and CH3O* were performed with the adsorption of an H atom in nearby sites as OH or MH to avoid the adsorption of fragments with unpaired electrons that modify the electronic properties of semiconducting metal-oxides52. Furthermore, the effect of weak long-range interactions and the self-interaction error on the adsorption energies were evaluated (Supplementary Tables 8 and 9)53. Dispersion corrections were included via Grimme’s D3 approach while a Hubbard correction54 by means of the Dudarev approach55 with a Ueff value of 4.00 eV was applied on the d-states of Zr atoms56,57,58,59.
Energy profiles for CO2 hydrogenation to methanol and the competitive RWGS to form CO were computed on selected models of the ZnZrOx, Cu-ZrOx, and Cu-ZnZrOx catalysts. Clean surfaces and gas-phase CO2, H2, and H2O molecules were used as thermodynamic sinks. The climbing image nudged elastic band (CI-NEB60) was employed to locate transition states. Their nature was confirmed by computing numerical frequencies with a step size of ± 0.015 Å.
Data availability
Data presented in the main figures of the manuscript are publicly available through the Zenodo repository (entry: 8309938, link: https://doi.org/10.5281/zenodo.8309938)61. Inputs and outputs for all DFT simulations can be found online in the ioChem-BD repository62,63, at https://iochem-bd.iciq.es/browse/review-collection/100/64621/fd52044500da8d298dc081fd. Further data supporting the findings of this study are available in the Supplementary Information and source data. All other relevant source data are available from the corresponding author upon request. Source data are provided with this paper.
Code availability
Source data of the Python application used for processing and analyzing operando infrared spectroscopy data are open-sourced on GitHub (https://github.com/philpreikschas/operando-ir) and additionally available in the Zenodo repository (https://doi.org/10.5281/zenodo.10818472)64
References
Li, K. & Chen, J. G. CO2 Hydrogenation to methanol over ZrO2-containing catalysts: insights into ZrO2 induced synergy. ACS Catal. 9, 7840–7861 (2019).
Wang, J. et al. A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol. Sci. Adv. 3, 1–11 (2017).
Han, Z. et al. CO2 hydrogenation to methanol on ZnO-ZrO2 solid solution catalysts with ordered mesoporous structure. J. Catal. 396, 242–250 (2021).
Pinheiro Araújo, T. et al. Design of flame‐made ZnZrOx catalysts for sustainable methanol synthesis from CO2. Adv. Energy Mater. 13, 2204122 (2023).
Pinheiro Araújo, T. et al. Methanol synthesis by hydrogenation of hybrid CO2-CO feeds. ChemSusChem 14, 2914–2923 (2021).
Lee, K. et al. Atomic Pd-promoted ZnZrO2 solid solution catalyst for CO2 hydrogenation to methanol. Appl. Catal. B: Environ. 304, 120994 (2022).
Xu, D., Hong, X. & Liu, G. Highly dispersed metal doping to ZnZr oxide catalyst for CO2 hydrogenation to methanol: Insight into hydrogen spillover. J. Catal. 393, 207–214 (2021).
Huang, C. et al. CO2 hydrogenation to methanol over PdZnZr solid solution: effects of the PdZn alloy and oxygen vacancy. ACS Appl. Energy Mater. 4, 9258–9266 (2021).
Lee, K. et al. Engineering nanoscale H supply chain to accelerate methanol synthesis on ZnZrOx. Nat. Commun. 14, 819 (2023).
Pinheiro Araújo, T. et al. Flame-made ternary Pd-In2O3-ZrO2 catalyst with enhanced oxygen vacancy generation for CO2 hydrogenation to methanol. Nat. Commun. 13, 5610 (2022).
Pinheiro Araújo, T. et al. Flame spray pyrolysis as a synthesis platform to assess metal promotion in In2O3‐catalyzed CO2 hydrogenation. Adv. Energy Mater. 12, 2103707 (2022).
Wang, J. et al. CO2 Hydrogenation to methanol over In2O3-based catalysts: from mechanism to catalyst development. ACS Catal. 11, 1406–1423 (2021).
Frei, M. S. et al. Atomic-scale engineering of indium oxide promotion by palladium for methanol production via CO2 hydrogenation. Nat. Commun. 10, 1–11 (2019).
Bavykina, A. et al. Turning a methanation Co catalyst into an In-Co methanol producer. ACS Catal. 9, 6910–6918 (2019).
Rui, N. et al. Hydrogenation of CO2 to methanol on a Auδ+-In2O3-x catalyst. ACS Catal. 10, 11307–11317 (2020).
Snider, J. L. et al. Revealing the synergy between oxide and alloy phases on the performance of bimetallic In-Pd catalysts for CO2 hydrogenation to methanol. ACS Catal. 9, 3399–3412 (2019).
Frei, M. S. et al. Nanostructure of nickel-promoted indium oxide catalysts drives selectivity in CO2 hydrogenation. Nat. Commun. 12, 1960 (2021).
Kaiser, S. K. et al. Performance descriptors of nanostructured metal catalysts for acetylene hydrochlorination. Nat. Nanotechnol. 17, 606–612 (2022).
Pinheiro Araújo, T. et al. Reaction‐induced metal‐metal oxide interactions in Pd‐In2O3/ZrO2 catalysts drive selective and Stable CO2 hydrogenation to methanol. Angew. Chem. Int. Ed. 62, 202306563 (2023).
Beck, A. et al. Following the structure of copper-zinc-alumina across the pressure gap in carbon dioxide hydrogenation. Nat. Catal. 4, 488–497 (2021).
Tsoukalou, A. et al. Operando X-ray absorption spectroscopy identifies a monoclinic ZrO2:In solid solution as the active phase for the hydrogenation of CO2 to methanol. ACS Catal. 10, 10060–10067 (2020).
Zabilskiy, M. et al. Mechanistic study of carbon dioxide hydrogenation over Pd/ZnO‐based catalysts: the role of palladium–zinc alloy in selective methanol synthesis. Angew. Chem. Int. Ed. 60, 17053–17059 (2021).
Prins, R. Hydrogen spillover: facts and fiction. Chem. Rev. 112, 2714–2738 (2012).
Bahruji, H. et al. Pd/ZnO catalysts for direct CO2 hydrogenation to methanol. J. Catal. 343, 133–146 (2016).
Zhao, H. et al. The role of Cu1-O3 species in single-atom Cu/ZrO2 catalyst for CO2 hydrogenation. Nat. Catal. 5, 818–831 (2022).
Tada, S. et al. Ag addition to CuO-ZrO 2 catalysts promotes methanol synthesis via CO2 hydrogenation. J. Catal. 351, 107–118 (2017).
Samson, K. et al. Influence of ZrO2 structure and copper electronic state on activity of Cu/ZrO2 catalysts in methanol synthesis from CO2. ACS Catal. 4, 3730–3741 (2014).
Ma, Y. et al. Reactivity of a zirconia-copper inverse catalyst for CO2 hydrogenation. J. Phys. Chem. C 124, 22158–22172 (2020).
Wang, Y. et al. Strong evidence of the role of H2O in affecting methanol selectivity from CO2 hydrogenation over Cu-ZnO-ZrO2. Chem 6, 419–430 (2020).
Yang, C. et al. Strong electronic oxide-support interaction over In2O3/ZrO2 for highly selective CO2 hydrogenation to methanol. J. Am. Chem. Soc. 142, 19523–19531 (2020).
Wu, C. et al. Inverse ZrO2/Cu as a highly efficient methanol synthesis catalyst from CO2 hydrogenation. Nat. Commun. 11, 5767 (2020).
Xu, Y. et al. Insights into the interfacial structure of Cu/ZrO2 catalysts for methanol synthesis from CO2 hydrogenation: Effects of Cu-supported nano-ZrO2 inverse interface. Chem. Eng. J. 470, 144006 (2023).
Frei, M. S. et al. Mechanism and microkinetics of methanol synthesis via CO2 hydrogenation on indium oxide. J. Catal. 361, 313–321 (2018).
Mädler, L. & Pratsinis, S. E. Bismuth oxide nanoparticles by flame spray pyrolysis. J. Am. Ceram. Soc. 85, 1713–1718 (2004).
Kemmler, J. A. et al. Quenched, nanocrystalline In4Sn3O12 high temperature phase for gas sensing applications. Sens. Actuators B: Chem. 161, 740–747 (2012).
Meierhofer, F., Mädler, L. & Fritsching, U. Nanoparticle evolution in flame spray pyrolysis: process design via experimental and computational analysis. AIChE J. 66, 1–14 (2020).
van Beek, W., Safonova, O. V., Wiker, G. & Emerich, H. SNBL, a dedicated beamline for combined in situ X-ray diffraction, X-ray absorption and Raman scattering experiments. Phase Transit. 84, 726–732 (2011).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Zichittella, G., Polyhach, Y., Tschaggelar, R., Jeschke, G. & Pérez‐Ramírez, J. Quantification of redox sites during catalytic propane oxychlorination by operando EPR spectroscopy. Angew. Chem. Int. Ed. 60, 3596–3602 (2021).
Pinheiro Araújo, T. et al. Impact of hybrid CO2-CO feeds on methanol synthesis over In2O3-based catalysts. Appl. Catal. B: Environ. 285, 119878 (2021).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab-initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Ricca, C., Ringuedé, A., Cassir, M., Adamo, C. & Labat, F. A comprehensive DFT investigation of bulk and low-index surfaces of ZrO2 polymorphs. J. Comput. Chem. 36, 9–21 (2015).
Christensen, A. & Carter, E. A. First-principles study of the surfaces of zirconia. Phys. Rev. B 58, 8050–8064 (1998).
Mizutani, U., Takeuchi, T. & Sato, H. Interpretation of the Hume-Rothery rule in complex electron compounds: γ-phase Cu5Zn8 Alloy, FK-type Al30Mg40Zn30 and MI-type Al68Cu7Ru17Si8 1/1-1/1-1/1 approximants. Prog. Mater. Sci. 49, 227–261 (2004).
Jain, A. et al. Commentary: The materials project: a materials genome approach to accelerating materials innovation. APL Mater. 1, 011002 (2013).
Makov, G. & Payne, M. C. Periodic boundary conditions in ab initio calculations. Phys. Rev. B 51, 4014–4022 (1995).
Castelli, I. E. et al. Role of the band gap for the interaction energy of coadsorbed fragments. J. Phys. Chem. C 121, 18608–18614 (2017).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput Chem. 32, 1456–1465 (2011).
Hubbard, J. Electron correlations in narrow energy bands II. the degenerate band case. Proc. R. Soc. A: Math. Phys. Eng. Sci. 277, 237-259 (1963).
Dudarev, S. L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA + U study. Phys. Rev. B 57, 1505–1509 (1998).
Koga, H. et al. Facile NO-CO elimination over zirconia-coated Cu(1 1 0) surfaces: Further evidence from DFT + U calculations. Appl Surf. Sci. 508, 145252 (2020).
Syzgantseva, O. A., Calatayud, M. & Minot, C. Revealing the surface reactivity of zirconia by periodic DFT calculations. J. Phys. Chem. C 116, 6636–6644 (2012).
Ruiz Puigdollers, A., Illas, F. & Pacchioni, G. Effect of nanostructuring on the reactivity of zirconia: a DFT + U study of au atom adsorption. J. Phys. Chem. C 120, 17604–17612 (2016).
Tang, X., Zhang, H., Sun, C., Qiao, X. & Ju, D. Adsorption mechanisms over ZrO2-modified Cu(111) surface for X (CH3OH, H2O and CO): a DFT + U study. Surf. Sci. 716, 121976 (2022).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Pinheiro Araújo, T. et al. In situ formation of low-nuclearity CuZn ensembles on ZnZrOx catalysts promotes CO2 hydrogenation to methanol [Data set]. Zenodo https://doi.org/10.5281/zenodo.8309938 (2023).
Álvarez-Moreno, M. et al. Managing the computational chemistry big data problem: the ioChem-BD platform. J. Chem. Inf. Model. 55, 95–103 (2015).
Bo, C., Maseras, F. & López, N. The role of computational results databases in accelerating the discovery of catalysts. Nat. Catal. 1, 809–810 (2018).
Preikschas, P. In situ formation of low-nuclearity CuZn ensembles on ZnZrOx catalysts promotes CO2 hydrogenation to methanol [Code - philpreikschas/operando-ir: v0.1.1 (v0.1.1)]. Zenodo https://doi.org/10.5281/zenodo.10818472 (2024).
Acknowledgements
This publication was created as part of NCCR Catalysis (grant number 180544), a National Centre of Competence in Research funded by the Swiss National Science Foundation. The Scientific Center for Optical and Electron Microscopy (ScopeM) at ETH Zurich is thanked for access to their facilities. Mr. Dario Faust Akl and Mr. Henrik Eliasson are thanked for acquiring the HAADF-STEM-EDX data. T.Z. thanks the Agency for Science, Technology and Research (A*STAR) Singapore for support through a graduate fellowship. The Spanish Ministry of Science and Innovation is acknowledged for financial support (PRE2019-088791, PID2021-122516OB-I00, and Severo Ochoa Grant MCIN/AEI/10.13039/501100011033 CEX2019-000925-S) and the Barcelona Supercomputing Center-MareNostrum (BSC-RES) for providing generous computer resources. Pol Sanz Berman is thanked for his support with graphic design. The Swiss Norwegian beamlines (SNBL, ESRF) are acknowledged for the provision of beamtime and its staff for invaluable support.
Author information
Authors and Affiliations
Contributions
T.P.A. and J.P.-R. conceived and coordinated all stages of this research. T.P.A., G.G., J.M.V, N.L., S.M., and J.P.-R. wrote the article with input from all other co-authors. T.P.A. and Z.R.B. conducted catalyst characterization and evaluation. J.M.V and N.L. conducted the density functional theory simulations. F.K. conducted the electron microscopy analyses. M.A. and G.J. conducted the electron paramagnetic resonance spectroscopy studies. G.G. evaluated X-ray absorption spectroscopy data. P.O.W., R.N.G., and W.J.S. prepared the catalysts using the flame spray pyrolysis method. T.Z. conducted kinetic analyses. P.P. supervised acquisition and evaluated diffuse reflectance infrared Fourier transform spectroscopy data. J.P.-R. supervised the entire project and managed resources and funding. All the authors provided inputs to the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
.Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pinheiro Araújo, T., Giannakakis, G., Morales-Vidal, J. et al. Low-nuclearity CuZn ensembles on ZnZrOx catalyze methanol synthesis from CO2. Nat Commun 15, 3101 (2024). https://doi.org/10.1038/s41467-024-47447-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-47447-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.