Abstract
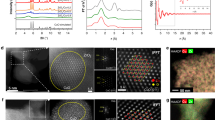
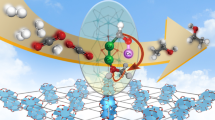
Copper-based catalysts for the hydrogenation of CO2 to methanol have attracted much interest. The complex nature of these catalysts, however, renders the elucidation of their structure–activity properties difficult. Here we report a copper-based catalyst with isolated active copper sites for the hydrogenation of CO2 to methanol. It is revealed that the single-atom Cu–Zr catalyst with Cu1–O3 units contributes solely to methanol synthesis around 180 °C, while the presence of small copper clusters or nanoparticles with Cu–Cu structural patterns are responsible for forming the CO by-product. Furthermore, the gradual migration of Cu1–O3 units with a quasiplanar structure to the catalyst surface is observed during the catalytic process and accelerates CO2 hydrogenation. The highly active, isolated copper sites and the distinguishable structural pattern identified here extend the horizon of single-atom catalysts for applications in thermal catalytic CO2 hydrogenation and could guide the further design of high-performance copper-based catalysts to meet industrial demand.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Data presented in the main figures of the manuscript and Supplementary Information are publicly available through the Zenodo repository (https://zenodo.org/deposit/6874758); all other relevant raw data are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
Code availability
The software code of LASP and NN potential used within the article is available from the corresponding author upon request or on the website http://www.lasphub.com.
References
Li, J. et al. Integrated tuneable synthesis of liquid fuels via Fischer–Tropsch technology. Nat. Catal. 1, 787–793 (2018).
Jenkinson, D. S., Adams, D. E. & Wild, A. Model estimates of CO2 emissions from soil in response to global warming. Nature 351, 304–306 (1991).
Kang, X. et al. Highly efficient electrochemical reduction of CO2 to CH4 in an ionic liquid using a metal–organic framework cathode. Chem. Sci. 7, 266–273 (2016).
Zhong, J. et al. State of the art and perspectives in heterogeneous catalysis of CO2 hydrogenation to methanol. Chem. Soc. Rev. 49, 1385–1413 (2020).
Yao, B. et al. Transforming carbon dioxide into jet fuel using an organic combustion-synthesized Fe–Mn–K catalyst. Nat. Commun. 11, 6395 (2020).
Hu, J. et al. Sulfur vacancy-rich MoS2 as a catalyst for the hydrogenation of CO2 to methanol. Nat. Catal. 4, 242–250 (2021).
Tan, L. et al. Development of soluble UiO-66 to improve photocatalytic CO2 reduction. Catal. Today https://doi.org/10.1016/j.cattod.2022.05.001 (2022).
Ferri, P. et al. Chemical and structural parameter connecting cavity architecture, confined hydrocarbon pool species, and MTO product selectivity in small-pore cage-based zeolites. ACS Catal. 9, 11542–11551 (2019).
Ilias, S. & Bhan, A. Mechanism of the catalytic conversion of methanol to hydrocarbons. ACS Catal. 3, 18–31 (2013).
Tan, L. et al. Bifunctional capsule catalyst of Al2O3@Cu with strengthened dehydration reaction field for direct synthesis of dimethyl ether from syngas. Ind. Eng. Chem. Res. 58, 22905–22911 (2019).
Tan, L. et al. Design of a core–shell catalyst: an effective strategy for suppressing side reactions in syngas for direct selective conversion to light olefins. Chem. Sci. 11, 4097–4105 (2020).
Tan, L. et al. Direct CO2 hydrogenation to light olefins by suppressing CO by-product formation. Fuel Process. Technol. 196, 106174 (2019).
Ma, S., Huang, S.-D. & Liu, Z.-P. Dynamic coordination of cations and catalytic selectivity on zinc–chromium oxide alloys during syngas conversion. Nat. Catal. 2, 671–677 (2019).
Behrens, M. et al. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science 336, 893–897 (2012).
Beck, A. et al. Following the structure of copper–zinc–alumina across the pressure gap in carbon dioxide hydrogenation. Nat. Catal. 4, 488–497 (2021).
Shi, Z. et al. CO2 hydrogenation to methanol over Cu–In intermetallic catalysts: effect of reduction temperature. J. Catal. 379, 78–89 (2019).
Li, K. & Chen, J. G. CO2 hydrogenation to methanol over ZrO2-containing catalysts: insights into ZrO2 induced synergy. ACS Catal. 9, 7840–7861 (2019).
Samson, K. et al. Influence of ZrO2 structure and copper electronic state on activity of Cu/ZrO2 catalysts in methanol synthesis from CO2. ACS Catal. 4, 3730–3741 (2014).
Wu, C. et al. Inverse ZrO2/Cu as a highly efficient methanol synthesis catalyst from CO2 hydrogenation. Nat. Commun. 11, 5767 (2020).
Bahruji, H. et al. Pd/ZnO catalysts for direct CO2 hydrogenation to methanol. J. Catal. 343, 133–146 (2016).
Wang, J. et al. A highly selective and stable ZnO–ZrO2 solid solution catalyst for CO2 hydrogenation to methanol. Sci. Adv. 3, e1701290 (2017).
Martin, O. et al. Indium oxide as a superior catalyst for methanol synthesis by CO2 hydrogenation. Angew. Chem. Int. Ed. 55, 6261–6265 (2016).
Wang, J. et al. High-performance MaZrOx (Ma = Cd, Ga) solid-solution catalysts for CO2 hydrogenation to methanol. ACS Catal. 9, 10253–10259 (2019).
Sharafutdinov, I. et al. Intermetallic compounds of Ni and Ga as catalysts for the synthesis of methanol. J. Catal. 320, 77–88 (2014).
Kong, H., Li, H.-Y., Lin, G.-D. & Zhang, H.-B. Pd-decorated CNT-promoted Pd-Ga2O3 catalyst for hydrogenation of CO2 to methanol. Catal. Lett. 141, 886 (2011).
Bai, S., Shao, Q., Feng, Y., Bu, L. & Huang, X. Highly efficient carbon dioxide hydrogenation to methanol catalyzed by zigzag platinum–cobalt nanowires. Small 13, 1604311 (2017).
Graciani, J. et al. Highly active copper–ceria and copper–ceria–titania catalysts for methanol synthesis from CO2. Science 345, 546–550 (2014).
Yu, J. et al. Stabilizing Cu+ in Cu/SiO2 catalysts with a shattuckite-like structure boosts CO2 hydrogenation into methanol. ACS Catal. 10, 14694–14706 (2020).
Yang, H. et al. A highly stable copper-based catalyst for clarifying the catalytic roles of Cu0 and Cu+ species in methanol dehydrogenation. Angew. Chem. Int. Ed. 57, 1836–1840 (2018).
Karelovic, A. & Ruiz, P. The role of copper particle size in low pressure methanol synthesis via CO2 hydrogenation over Cu/ZnO catalysts. Catal. Sci. Technol. 5, 869–881 (2015).
Rong, W. et al. Size-dependent activity and selectivity of atomic-level copper nanoclusters during CO/CO2 electroreduction. Angew. Chem. Int. Ed. 60, 466–472 (2021).
Zhu, Y. et al. Copper–zirconia interfaces in UiO-66 enable selective catalytic hydrogenation of CO2 to methanol. Nat. Commun. 11, 5849 (2020).
Zhou, H. et al. Engineering the Cu/Mo2CTx (MXene) interface to drive CO2 hydrogenation to methanol. Nat. Catal. 4, 860–871 (2021).
Tada, S. et al. Design of interfacial sites between Cu and amorphous ZrO2 dedicated to CO2-to-methanol hydrogenation. ACS Catal. 8, 7809–7819 (2018).
Tada, S. et al. Cu species incorporated into amorphous ZrO2 with high activity and selectivity in CO2-to-methanol hydrogenation. J. Phys. Chem. C. 122, 5430–5442 (2018).
Ma, Y. et al. Reactivity of a zirconia–copper inverse catalyst for CO2 hydrogenation. J. Phys. Chem. C. 124, 22158–22172 (2020).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Nguyen, L. et al. Ir1Znn bimetallic site for efficient production of hydrogen from methanol. ACS Sustain. Chem. Eng. 7, 18793–18800 (2019).
Tang, Y. et al. Synergy of single-atom Ni1 and Ru1 sites on CeO2 for dry reforming of CH4. J. Am. Chem. Soc. 141, 7283–7293 (2019).
Li, H. et al. Synergetic interaction between neighbouring platinum monomers in CO2 hydrogenation. Nat. Nanotechnol. 13, 411–417 (2018).
Ye, X. et al. Highly selective hydrogenation of CO2 to ethanol via designed bifunctional Ir1–In2O3 single-atom catalyst. J. Am. Chem. Soc. 142, 19001–19005 (2020).
Han, Z., Tang, C., Wang, J., Li, L. & Li, C. Atomically dispersed Ptn+ species as highly active sites in Pt/In2O3 catalysts for methanol synthesis from CO2 hydrogenation. J. Catal. 394, 236–244 (2021).
Witoon, T., Chalorngtham, J., Dumrongbunditkul, P., Chareonpanich, M. & Limtrakul, J. CO2 hydrogenation to methanol over Cu/ZrO2 catalysts: effects of zirconia phases. Chem. Eng. J. 293, 327–336 (2016).
Chen, C. et al. The significant role of oxygen vacancy in Cu/ZrO2 catalyst for enhancing water–gas-shift performance. Int. J. Hydrog. Energy 39, 317–324 (2014).
Ikuno, T. et al. Methane oxidation to methanol catalyzed by Cu-oxo clusters stabilized in NU-1000 metal–organic framework. J. Am. Chem. Soc. 139, 10294–10301 (2017).
Wang, L.-C. et al. Structural evolution and catalytic properties of nanostructured Cu/ZrO2 catalysts prepared by oxalate gel-coprecipitation technique. J. Phys. Chem. C. 111, 16549–16557 (2007).
Velu, S., Suzuki, K., Gopinath, C. S., Yoshida, H. & Hattori, T. XPS, XANES and EXAFS investigations of CuO/ZnO/Al2O3/ZrO2 mixed oxide catalysts. Phys. Chem. Chem. Phys. 4, 1990–1999 (2002).
Qian, J. et al. Exploration of CeO2–CuO quantum dots in situ grown on graphene under hypha assistance for highly efficient solar-driven hydrogen production. Inorg. Chem. 57, 14532–14541 (2018).
Yuan, L. et al. Dynamic evolution of atomically dispersed Cu species for CO2 photoreduction to solar fuels. ACS Catal. 9, 4824–4833 (2019).
Qiu, X. et al. Hybrid CuxO/TiO2 nanocomposites as risk-reduction materials in indoor environments. ACS Nano 6, 1609–1618 (2012).
Yang, J. et al. Dynamic behavior of single-atom catalysts in electrocatalysis: identification of Cu-N3 as an active site for the oxygen reduction reaction. J. Am. Chem. Soc. 143, 14530–14539 (2021).
Nosaka, Y., Takahashi, S., Sakamoto, H. & Nosaka, A. Y. Reaction mechanism of Cu(II)-grafted visible-light responsive TiO2 and WO3 photocatalysts studied by means of ESR spectroscopy and chemiluminescence photometry. J. Phys. Chem. C 115, 21283–21290 (2011).
Chusuei, C. C., Brookshier, M. A. & Goodman, D. W. Correlation of relative X-ray photoelectron spectroscopy shake-up intensity with CuO particle size. Langmuir 15, 2806–2808 (1999).
Sato, A. G. et al. Effect of the ZrO2 phase on the structure and behavior of supported Cu catalysts for ethanol conversion. J. Catal. 307, 1–17 (2013).
Lamberti, C. et al. XAFS, IR, and UV–vis study of the CuI environment in CuI–ZSM-5. J. Phys. Chem. B 101, 344–360 (1997).
Zhang, Z. et al. Transfer hydrogenation of fatty acids on Cu/ZrO2: demystifying the role of carrier structure and metal–support interface. ACS Catal. 10, 9098–9108 (2020).
Cui, G. et al. ZrO2-x modified Cu nanocatalysts with synergistic catalysis towards carbon–oxygen bond hydrogenation. Appl. Catal. B 280, 119406 (2021).
Gao, J. et al. Cu2In nanoalloy enhanced performance of Cu/ZrO2 catalysts for the CO2 hydrogenation to methanol. Ind. Eng. Chem. Res. 59, 12331–12337 (2020).
Zhang, Z. et al. The most active Cu facet for low-temperature water gas shift reaction. Nat. Commun. 8, 488 (2017).
Ladera, R. et al. Catalytic valorization of CO2 via methanol synthesis with Ga-promoted Cu–ZnO–ZrO2 catalysts. Appl. Catal. B 142-143, 241–248 (2013).
Zhang, X. et al. Reaction-driven surface reconstruction of ZnAl2O4 boosts the methanol selectivity in CO2 catalytic hydrogenation. Appl. Catal. B 284, 119700 (2021).
Yan, G. et al. Reaction product-driven restructuring and assisted stabilization of a highly dispersed Rh-on-ceria catalyst. Nat. Catal. 5, 119–127 (2022).
Dandekar, A. & Vannice, M. A. Determination of the dispersion and surface oxidation states of supported Cu catalysts. J. Catal. 178, 621–639 (1998).
Pokrovski, K., Jung, K. T. & Bell, A. T. Investigation of CO and CO2 adsorption on tetragonal and monoclinic zirconia. Langmuir 17, 4297–4303 (2001).
Yang, C. et al. Strong electronic oxide–support interaction over In2O3/ZrO2 for highly selective CO2 hydrogenation to methanol. J. Am. Chem. Soc. 142, 19523–19531 (2020).
Wang, Y. et al. Strong evidence of the role of H2O in affecting methanol selectivity from CO2 hydrogenation over Cu–ZnO–ZrO2. Chem 6, 419–430 (2020).
Li, H. et al. CO2 activation on ultrathin ZrO2 film by H2O co-adsorption: in situ NAP-XPS and IRAS studies. Surf. Sci. 679, 139–146 (2019).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P. & Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 45, 13244–13249 (1992).
Huang, S.-D., Shang, C., Kang, P.-L., Zhang, X.-J. & Liu, Z.-P. LASP: fast global potential energy surface exploration. WIREs Comput. Mol. Sci. 9, e1415 (2019).
Guan, S.-H., Zhang, K.-X., Shang, C. & Liu, Z.-P. Stability and anion diffusion kinetics of yttria-stabilized zirconia resolved from machine learning global potential energy surface exploration. J. Chem. Phys. 152, 094703 (2020).
Ma, S., Shang, C., Wang, C.-M. & Liu, Z.-P. Thermodynamic rules for zeolite formation from machine learning based global optimization. Chem. Sci. 11, 10113–10118 (2020).
Guan, S.-H., Shang, C., Huang, S.-D. & Liu, Z.-P. Two-stage solid-phase transition of cubic ice to hexagonal ice: structural origin and kinetics. J. Phys. Chem. C 122, 29009–29016 (2018).
Huang, S.-D., Shang, C., Zhang, X.-J. & Liu, Z.-P. Material discovery by combining stochastic surface walking global optimization with a neural network. Chem. Sci. 8, 6327–6337 (2017).
Behler, J. & Parrinello, M. Generalized neural-network representation of high-dimensional potential-energy surfaces. Phys. Rev. Lett. 98, 146401 (2007).
Acknowledgements
This work was supported by the National Natural Science Foundation of China under grant numbers 22172032, 21902027, 51701201 and U19B2003, the National Key Research and Development Program of China under grant number 2018YFA0208600, the Natural Science Foundation of Fujian Province under grant numbers 2020J05121 and 2020J01443, and the DNL Cooperation Fund, CAS (DNL201903). The X-ray experiment was supported by BL14W1, Shanghai Synchrotron Radiation Facility (j21sr0041). We thank the staff at the BL14W1 beamline of the Shanghai Synchrotron Radiation Facility and M. Shakouri at the Canadian Light Source for assistance with the EXAFS and XANES measurements.
Author information
Authors and Affiliations
Contributions
L.T. conceived and designed the experiments. H.Z. performed the catalyst synthesis, characterization and performance experiments. Z.L. and S.M. contributed to the DFT calculation and wrote the related section of the manuscript. R.Y., K.X., Y.C., K.J., Y.F., C.Z. and X.L. assisted with the synthesis and performance testing of the catalysts. Y.T. and L.W. helped to analyse the XPS and XAS data. Q.J. conducted the HAADF-STEM experiments. P.H. and Y.W. assisted with the in situ DRIFT experiments. Data were discussed among all coauthors. L.T. and H.Z. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Xiaodong Wen, Shohei Tada and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–33 and Tables 1–9.
Supplementary Data 1
All the relevant structure of CAZ-1.
Supplementary Data 2
Gibbs free energy profile of CO2 hydrogenation to CH3OH/CO for CAZ-1 catalyst.
Supplementary Data 3
The variations of reaction intermediates concentrations during microkinetics simulation on CAZ-1 catalyst.
Supplementary Data 4
The variations of reaction rate during microkinetics simulation on CAZ-1 catalyst.
Supplementary Data 5
Source data of Supplementary Information.
Source data
Source Data Fig. 1
HAADF-STEM and TEM images, k2-weighted Fourier transform spectra and wavelet transform spectroscopy.
Source Data Fig. 2
Activity
Source Data Fig. 3
Cu K-edge XANES spectra, first derivative of the XANES spectra and fitting results of k2-weighted EXAFS data.
Source Data Fig. 4
HAADF-STEM images and in situ XRD.
Source Data Fig. 5
TOF-SIMS images and semiquantitative analysis.
Source Data Fig. 6
In situ DRIFT.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, H., Yu, R., Ma, S. et al. The role of Cu1–O3 species in single-atom Cu/ZrO2 catalyst for CO2 hydrogenation. Nat Catal 5, 818–831 (2022). https://doi.org/10.1038/s41929-022-00840-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00840-0
This article is cited by
-
Solvent-free selective hydrogenation of nitroaromatics to azoxy compounds over Co single atoms decorated on Nb2O5 nanomeshes
Nature Communications (2024)
-
Low-nuclearity CuZn ensembles on ZnZrOx catalyze methanol synthesis from CO2
Nature Communications (2024)
-
Homolytic H2 dissociation for enhanced hydrogenation catalysis on oxides
Nature Communications (2024)
-
Silica mesoporous nanosphere-loaded single-atom Cu as highly efficient peroxymonosulfate activator for phenazopyridine hydrochloride degradation at all pH values
Journal of Materials Science: Materials in Electronics (2024)
-
Nickel-modified In2O3 with inherent oxygen vacancies for CO2 hydrogenation to methanol
Science China Chemistry (2024)