Abstract
Forest soils harbor hyper-diverse microbial communities which fundamentally regulate carbon and nutrient cycling across the globe. Directly testing hypotheses on how microbiome diversity is linked to forest carbon storage has been difficult, due to a lack of paired data on microbiome diversity and in situ observations of forest carbon accumulation and storage. Here, we investigated the relationship between soil microbiomes and forest carbon across 238 forest inventory plots spanning 15 European countries. We show that the composition and diversity of fungal, but not bacterial, species is tightly coupled to both forest biotic conditions and a seven-fold variation in tree growth rates and biomass carbon stocks when controlling for the effects of dominant tree type, climate, and other environmental factors. This linkage is particularly strong for symbiotic endophytic and ectomycorrhizal fungi known to directly facilitate tree growth. Since tree growth rates in this system are closely and positively correlated with belowground soil carbon stocks, we conclude that fungal composition is a strong predictor of overall forest carbon storage across the European continent.
Similar content being viewed by others
Introduction
Forests are home to roughly 80% of terrestrial biodiversity1 and represent one of the world’s largest carbon sinks2,3,4. Perhaps the least understood and most complex component of forest biodiversity is the soil microbiome. With a growing need to offset the effects of climate change, there is a rising interest to discover how the biodiversity of Earth’s most diverse lifeforms – microbes5 – affects terrestrial carbon storage6,7,8,9,10. Soil microbes mediate unique aspects of the forest carbon cycle. Microbial life is responsible for over 50% of soil respiration11, most plant litter decomposition8, and steers tree growth and death via mutualisms and pathogen infections12,13,14. Soil microbial community composition is a measure of the identity and relative abundance of microbial species within communities. While it is well known that the composition of tree species strongly impacts forest processes such as growth15, albedo16, and carbon sequestration17, a comparable effort to understand how soil microbial community composition impacts whole forest-scale processes is urgently needed.
A growing body of experimental and observational studies suggest that microbial composition can affect entire forest functioning by influencing key forest carbon pools, fluxes, and process efficiencies. For example, dark septate fungal endophytes, an ubiquitous group of biotrophic plant root symbionts, stimulate plant growth 52-138% depending on plant and fungal species18, and similar observations have been made for different rhizosphere bacterial19, endophytic bacterial20, and ecto- and arbuscular mycorrhizal fungal species21. Belowground, bacterial, and fungal diversity promotes soil respiration10, microbial carbon use efficiency22, and overall decomposition rates23. Some of these experimental discoveries also seem to generalize to carbon cycle outcomes in actual forest systems. For example, differences in ectomycorrhizal fungal composition have been linked to a three-fold variation in tree growth rates across Europe7, an observation consistent with decades of mesocosm experiments24,25,26. Studies on soil biogeochemistry have observed that variation in decomposition rates are linked to differences in bacterial composition23,27,28 and fungal richness29, and that these different community types likely explain important variation in soil organic carbon storage30,31. All these signatures highlight the potential importance of microbial biodiversity, but how they translate to total forest carbon storage remains unknown.
Globally, forest tree biomass and soil organic carbon represent most of the total forest carbon stock2, and these two pools may positively32,33 or negatively34,35 interact. Soil organic carbon storage is balanced by inputs and outputs, with most carbon inputs derived from net primary production36. For this reason, plant growth and soil organic carbon stocks are positively linked in commonly used carbon models such as RothC37 and CENTURY38. Yet, experimental research suggests that this connection is often more complex due to priming39, mineralogy40, forest management and disturbance32, soil carbon saturation41, and mycorrhizal symbiosis42. A recent meta-analysis found that the positive effects of elevated CO2 on plant growth are negatively correlated with changes in soil organic carbon stocks across the globe35. This was attributed to enhanced nutrient scavenging from organic matter by ectomycorrhizal fungi that can boost plant growth. Many ectomycorrhizal fungi, but not all, also decay soil organic matter to mine for nitrogen43, a function that is expected to increase soil organic carbon stocks under nitrogen-limiting conditions42. Therefore, due to the context-dependency of plant biomass-soil carbon storage relationships, the link between forest soil microbiomes (i.e., a community of microorganisms) and total forest carbon storage requires explicit examination of both above- and belowground carbon pools.
In this study, we explored how in situ forest properties and processes are linked to soil microbiome composition at a large spatial scale across Europe. We then modeled the extent to which features of the soil microbiome are correlated to forest carbon accumulation and storage, both above- and belowground, and we identified which constituents of the microbiome explain these patterns. Until now, efforts to link soil microbial composition to the major components of forest carbon storage have been limited by a lack of paired data on microbial composition, tree biomass carbon stocks, tree growth, and soil organic carbon stocks. We used DNA sequencing to generate soil microbiome profiles of bacteria and fungi across 238 forest monitoring plots spanning 15 European countries (Fig. 1a). All forest monitoring plots are part of the International Cooperative Programme on Assessment and Monitoring of Air Pollution Effects on Forests (ICP Forests) network and have extensive data on forest carbon cycling and storage above and belowground. This microbiome survey allowed us to generate a unique analysis between forest microbiome profiles and paired, co-located observations of total forest carbon balance signatures at a continental scale. We show that soil fungal communities, especially tree-associated ectomycorrhizal and endophytic guilds, are strong predictors of forest tree growth and biomass. Because tree growth is also positively correlated with soil carbon stocks in this system, fungal composition and diversity are prominent bioindicators of overall forest carbon storage.
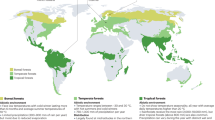
Soil was collected from 285 forest monitoring plots across 15 European countries participating in the International Co-operative Programme on Assessment and Monitoring of Air Pollution Effects on Forests (ICP Forests) network (a). Forests were classified as either broadleaves or conifers based on the dominant tree types at each site (≥50% cover). Fungal (b, d) and bacterial (c, e) community compositions in the organic (b; n = 209; c; n = 255) and mineral (d; n = 195, e; n = 266) soil horizons. Sample size variations result from samples unable to be amplified for fungal and/or bacterial profiling or those not meeting quality control standards of sequencing depth. Vectors show correlations with forest abiotic and biotic variables with principal coordinate analysis (PCoA) axes 1 and 2 where each environmental variable is predicted by PCoA axes 1 and 2 using multiple regression. Solid lines with black labels show significant correlations (P ≤ 0.05) while dashed arrows with gray text show non-significant correlations. The significance of fitted vectors and factors was tested using 999 permutations of environmental variables. Squared correlation coefficients for each variable with respect to PCoA axes 1 and 2 for fungi (panel with left-orientation bars) and bacteria (panel with right-orientation bars) (f). N dep. nitrogen deposition, MAT mean annual temperature, MAP mean annual precipitation, C stocks soil carbon stocks.
Results and discussion
Variation in forest biotic conditions is linked to fungal versus bacterial composition
Forest biotic variables were correlated with fungal versus bacterial community composition (Fig. 1b–e). Fungal composition was specifically correlated with the dominant tree type, forest age, and tree growth rate (Fig. 1b, d, f). Since tree growth and tree biomass stocks were also positively correlated themselves (r = 0.7, P < 0.001), we only included tree growth to avoid issues of co-linearity and redundancy. Conversely, neither forest age nor tree growth rate were correlated with bacterial community composition (Fig. 1f). While patterns were similar between soil horizons for both groups, there was a stronger effect of dominant tree type and forest age on fungal composition in the organic versus mineral soil horizon, while variation in tree growth rate was more tightly linked to mineral than organic horizon fungal community composition. Fungal communities typically differ between broadleaves and conifers, especially in the organic horizon44,45, and fungal composition specifically varies with forest age46,47,48. While we observed the same dissimilarly patterns in fungal communities with forest type and age, fungal composition varied even more strongly with tree growth rate. It is possible that variation across specific tree species would reveal even finer-scale differences, but exploring this was beyond the goal of our study since most sites were dominated by a single species. Fungal, not bacterial, composition is therefore a uniquely informative marker of forest productivity in addition to forest type and age.
Soil and remaining geographic variables captured similar variation in bacterial and fungal community compositions. Soil clay content, soil pH, and soil carbon stocks were significantly correlated with both fungal and bacterial community compositions, though correlations to soil pH and carbon stocks were stronger for bacteria than fungi (Fig. 1f). For both groups, correlations with soil pH and carbon stocks were twice as strong in the organic compared to mineral soil horizon. These are expected results since soil pH and soil carbon content often co-vary with microbial composition46,49,50,51,52,53,54,55. However, few microbiome studies measure soil carbon stocks - an actual metric of forest carbon storage because carbon content alone does not account for the quantity of soil in a system. We suggest that previous focuses on carbon content have obscured the link between microbial composition and soil carbon storage because carbon content was only weakly correlated with microbiome composition in our study (Supplementary Fig. 1). The remaining geographic characteristics were not tightly linked to microbial composition, except for mean annual precipitation and geographic space. Total variation explained in microbiome composition by all environmental variables based on distance-based redundancy analysis ranged between 22.8–28.2%, the typical amount of variation explained in microbiome composition at large spatial scales56,57,58.
Digging deeper: using microbiome metrics to predict forest tree growth and biomass
Our analyses of microbial community composition indicate that fungal and bacterial communities are differentially linked to multiple metrics of forest carbon storage. Since forest carbon pools and microbial communities interact, this cause-and-effect conundrum could not be resolved in our observational study. But we could investigate these linkages deeper to evaluate which components of the microbial community best captured variation in above and belowground carbon storage. To do this, we also took into consideration known factors affecting forest tree growth and biomass across the ICP Forest network where this work was conducted. Earlier research showed that the best non-microbial predictors of tree growth include nitrogen deposition, stand age, and multiple aspects of climate (see ref. 59). Here, we show that none of these non-microbial predictor variables are strongly multicollinear with microbiome composition and diversity (variance inflation values ≤ 5 in all models sensu60), but they are important predictors of tree growth in our study (see supplementary tables and datasets referenced throughout the results section). Our analyses of the environmental predictors of microbial composition also demonstrate that non-microbial predictors of tree growth are only partially linked to microbial composition, which highlights that there is unique variation in soil microbiomes that could contribute to variation in carbon storage outcomes that cannot be explained by the environment alone. We therefore built and compared statistical models to explore which dimensions of microbiome biodiversity are most strongly linked to forest tree growth and biomass carbon stocks after accounting for other important co-variables.
We demonstrate that both fungal community composition (principal coordinate analysis axis 1; PCoA1; Fig. 2a, e), and now fungal richness (Fig. 2c, g) are correlated with tree biomass and rates of tree growth, even after statistically controlling and accounting for the influence of other important environmental co-variables (Supplementary Data 1, Supplementary Table 1). Tree growth was more strongly linked to fungal composition compared to fungal richness, indicating that which species are present could have larger impacts on tree growth than the overall number of species in a community. These links were also stronger in conifer versus broadleaf forests, but comparable correlations were observed in both stand types (Supplementary Fig. 2). Tree growth was not correlated with bacterial composition (Fig. 1b, f) nor richness (Fig. 1d, h). Tree growth was also more strongly correlated with fungal composition in the mineral versus organic horizon (Supplementary Data 1 and Supplementary Table 2), potentially because most tree roots grow in the mineral horizon61 and symbiotic, ectomycorrhizal fungal relative abundances were higher in mineral compared to organic horizon soils (Supplementary Table 3). To that end, the composition of ectomycorrhizal fungi followed by endophytes was most tightly linked to tree growth rates (Fig. 3a). This is consistent with an earlier root tip survey of ectomycorrhizal fungal communities7 based on an entirely independent sampling effort and medium (individual root-tip sequencing rather than whole soil DNA sequencing). We also discovered that fungal endophyte richness was strongly and positively linked to tree growth rates (Fig. 3a, b), followed by richness of saprotrophs, wood-decomposing fungi (a subgroup within the saprotroph community), plant pathogens, and ericoid but not ectomycorrhizal fungi. The endophyte richness effect size was approximately one third higher than all other groups, even when accounting for other co-variables (Supplementary Table 4). Unlike other fungal guilds, endophytic and ectomycorrhizal fungi are both mutualistic, tree-biotrophic groups in these forests, which might explain why both groups were more connected to forest tree growth compared to other fungi.
Panels showing that total fungal composition (principal coordinate analysis axis 1; PCoA1; a, e; n = 112) and richness (c, g; n = 112), but not bacterial composition (b, f) and richness (d, h), are correlated with tree biomass and tree growth rates. Plotted lines show linear correlations, shaded areas around each line are 95% confidence intervals, and r values are Pearson correlation coefficients. Communities from the mineral soil are shown here because they were more tightly correlated than organic horizon communities. The full statistical models, including all co-variates, for each correlation are shown in Supplementary Data 1, Supplementary Table 1. Only significant correlation coefficients are shown (P ≤ 0.05).
Generalized additive model coefficients showing the slope of the linear relationship between tree growth and fungal richness or fungal composition that is independent of all other co-variables (a). The standardized model coefficient (bars) and standard error (error bars) are shown so each predictor is on the same scale but note that the effect size of richness and composition are on different scales and should not be directly compared. Separate models were made for each fungal functional group. ‘Pure’ refers to analyses with fungi only identified to one versus multiple functional categories and asterisks (*) indicates a significant effect (*P ≤ 0.05, **P < 0.01; see Supplementary Table 4 for full statistical model summaries, including exact P-values). Correlation between endophyte fungal richness and tree growth rate (b; n = 112). The plotted line shows the linear correlation, shaded area around the line shows the 95% confidence interval, and the r value is the Pearson correlation coefficient. The top biotrophic fungal indicator species of variation in tree growth rates (c). The top five positively and negatively correlated ectomycorrhizal fungi and all endophytic fungal indicators are visualized. We only show the top ten ectomycorrhizal fungi because there were too many ectomycorrhizal indicator species to fit in one graphic (see Supplementary Data 2 for a complete list). And we only show ectomycorrhizal fungi and endophytes because both are the major biotrophic fungal group in European forest soils, and their compositions both predicted tree growth rates. Bars with a genus level designation are OTUs that could not be identified at the species level. For example, there are two Russula OTUs with opposite directional effect sizes distinguished as species (spp) 1 and 2. Indicator species were identified as those having significant differential relative abundances based on the negative binomial distribution. Values are reported on a logarithmic scale to base 2 and represent changes in relative abundance for a unit change in tree growth (bars) and their standard error (error bars). Volcano plot showing the strength of all endophytic and ectomycorrhizal fungal OTUs significantly correlated to tree growth (d). Values less and more than 0 indicate negative and positive correlations with tree growth, respectively.
All plants in the environment associate with fungal endophytes that can profoundly impact plant fitness. Yet, the ecological significance of fungal endophytes in forests is surprisingly understudied compared to pathogens and mycorrhizae. Most endophyte research is conducted in grasslands62,63, but it is possible that some of these findings can be generalized to forest systems. Endophytes can promote plant growth via phytohormone production, protection against pathogens, increased nutrient uptake, and abiotic stress alleviation64. Nevertheless, the benefits provided by endophytes to plants might not always be reciprocal with plant investments, especially for some groups such as dark-septate endophytes which were common in our study system65. However, it is particularly interesting that the endophytic indicator species we identified in our study (Fig. 3c, d) include Trichoderma citrinoviridie and T. koningii which produce high levels of the plant growth promoting hormone, indole acetic acid, compared to other fungi with known endophytic life cycles66, and their relative abundances were positively correlated with broadleaf tree growth in our study (Supplementary Data 2). We also identified four putatively endophytic Mortierella indicators of fast conifer and broadleaf tree growth. In agricultural systems, many Mortierella stimulate indole acetic acid production, reduce abiotic stress levels, and improve access to phosphorus and iron67, with similar positive effects recently observed in tree seedlings68. This highlights these taxa as important for future research in forestry applications. Our results suggest that forest fungal endophyte richness and species identity may be key components of forest biodiversity-ecosystem function relationships.
It is important to note that most endophytic fungi, including Mortierella, can live saprotrophically, which is one reason we detect them in soil samples where roots were removed. However, much like ectomycorrhizal fungi, some dark-septate endophytes also grow hundreds of meters of extraradical hyphae per gram of soil69, including endophytes present in our soil samples. Taxa annotated as endophytes in our study most likely have mixed ecological strategies and were detected in both biotrophic and saprotrophic states, a limitation of our study since we cannot identify the precise trophic strategy employed by fungi with endophytic capacities in our samples. This is why we did not separate endophytes into “pure” and “mixed” ecological groups, as we did for ectomycorrhizal fungi where there is clearer albeit still ambiguous trophic division. However, the distinction between soil and roots is constantly obscured as roots modify nearby soil, giving rise to conditions where similar endophyte communities may reside inside roots and the surrounding soil70. This suggests that even though some of the endophytic fungi we observed were probably living saprotrophically, they are still indicators of taxa that form symbioses with trees.
While every significant endophyte indicator was positively correlated with tree growth, the other ubiquitous biotrophic group – ectomycorrhizal fungi – included species linked to both slow and fast tree growth (Fig. 3c, d; Supplementary DatV). This is consistent with our earlier root tip survey of ectomycorrhizal fungi7. We identified numerous Russula and Cortinariaceae (including the entire genus Cortinarius) taxa significantly linked to variation in tree growth. In general, the most indicative species of fast tree growth were Russula species (Fig. 3c) whereas Cortinarius and Inocybe indicator species were the topmost negatively correlated OTUs with tree growth. Cortinarius are energy demanding species that produce extensive biomass71, fungal rhizomorphs72, and extracellular enzymes73. In contrast to some Russula, both Cortinarius and Inocybe also actively assimilate nitrogen from organic sources as deep as 30 cm belowground74, which not only requires producing exploratory mycelium to vertically tunnel deeper into denser soil but also requires costly oxidases and proteases to access organically bound nitrogen. Because mycorrhizal fungi obtain their energy from host-trees, these “costly” traits may constrain tree growth compared to certain, less energy-demanding Russula species. Russula itself is a large genus containing nitrophobic and nitrophilic species75, which could explain why we detected Russula indicators of both slow and fast tree growth rates for both conifer and broadleaf forests in our study. Importantly, local environmental conditions such as forest succession76, soil pH77, drought78, and nitrogen deposition79 also select for these particular ectomycorrhizal taxa, which in turn shapes their distributions and potential impacts on forest tree growth. Thus, any potential effects of these fungi on tree growth are likely modulated by environmental conditions. Our results provide support for the idea that ectomycorrhizal symbiosis spans a large spectrum of outcomes for plant growth in forest ecosystems80.
Which microbiome metrics predict soil organic carbon stocks?
Fungal (Fig. 4a) and bacterial (Fig. 4c) community compositions were correlated with organic horizon carbon stocks, though this correlation was more than two times stronger for bacteria compared to fungi, even after accounting for other co-variables of known importance (see Supplementary Data 3), including soil clay content81, climate80, forest type82, and nitrogen deposition83. Like microbiome composition, both fungal (Fig. 4b) and bacterial (Fig. 4d) richness were also negatively correlated with organic horizon carbon stocks, and this effect was relatively larger for bacteria compared to fungi and less driven by dominant tree type differences (i.e., broadleaf forests possessing higher soil microbial diversity and lower organic horizon carbon stocks than coniferous forests). Like tree growth, organic horizon carbon stocks were more tightly linked to microbiome composition than richness. Bacterial, but not fungal, composition was also correlated with mineral horizon carbon stocks (P = 0.006), but this effect size was approximately one sixth of that observed in the organic horizon (Supplementary Fig. 3). Neither bacterial richness nor fungal composition/richness were correlated with mineral horizon carbon stocks. This shows that while fungal composition is correlated with mineral horizon carbon stocks alone (Fig. 1f), this correlation is not robust when we account for other co-variables. Thus, in contrast to tree growth, bacterial communities were more tightly correlated with carbon stocks in the organic horizon compared to fungal communities, and bacteria were the only group correlated with mineral horizon carbon stocks.
Panels showing that total fungal composition (principal coordinate analysis axis 2; PCoA2; a; n = 106) and richness (b; n = 106) as well as bacterial composition (PCoA1; c; n = 104) and richness (d; n = 104) are correlated with tree growth rates. Because there were no correlations in the mineral horizon (except for a weak correlation between bacterial composition and soil organic carbon stocks, see Supplementary Fig. 3), communities and carbon stocks from the organic horizon alone are shown. Plotted lines show linear correlations, shaded areas around each line are 95% confidence intervals, and r values are Pearson correlation coefficients. The full statistical models, including all co-variates, for each correlation are shown in Supplementary Data 3. Only significant correlation coefficients are shown (P ≤ 0.05).
The relationship between biodiversity and ecosystem functioning is of long-standing ecological and conservation interest. An open question is why fungal and bacterial richness is negatively correlated with organic horizon carbon stocks in our study system. On one hand, this is surprising because the species-energy hypothesis predicts that increasing carbon inputs to a system should boost biodiversity84, and thus, we should expect microbial richness to positively co-vary with carbon stocks. We would also expect this based on the species-area relationship85, where less organic horizon carbon means less habitat for species co-existence. In contrast, the biodiversity-ecosystem function concept challenges the idea that environmental conditions alone determine species diversity86 and argues that higher levels of diversity increase rates of emergent biological processes such as productivity and decomposition. A major soil carbon loss pathway is decomposition, which can be enhanced by microbial richness in European forests under certain scenarios87. A recent meta-analysis also shows that experimental reduction of bacterial and fungal diversity decreases soil respiration10, and microbial richness is positively correlated with decomposition rate in multiple observational studies23,29. We will not disentangle the directionality or causality of these correlations in our study, we can raise this as a subject for future investigation. A notable starting point would be to explore the links between microbial richness and soil carbon storage while experimentally removing confounding effects of soil pH. Microbial richness is often linked to soil pH46,49, and soil pH is correlated with soil carbon stocks in our study (Supplementary Data 4). While soil pH was not so strongly correlated with microbial richness in our study system, and the statistical effects of richness and soil pH on soil carbon stocks were independent, we cannot disentangle the possibility that microbial richness is largely structured by soil pH and thus, only indirectly linked to soil carbon stocks.
While the link between microbial richness and carbon storage is particularly interesting in the context of conflicting ecological theories, it is important to emphasize that bacterial composition was more strongly linked to carbon stocks than species richness. We therefore explored which bacterial lineages were positively and negatively linked to organic horizon carbon stocks. At the OTU-level, there were both positive and negative indicators of organic horizon carbon stocks within most major phyla. Eighty percent of the Proteobacteria [Pseudomonadota] indicator OTUs were negatively correlated with carbon stocks in conifer forests (Supplementary Fig. 4). Relative abundances of Proteobacteria in conifer stands was also positively correlated with soil pH (r = 0.24, P = 0.02; Supplementary Fig. 5), which is negatively correlated with organic horizon carbon stocks independent of the microbiome (Supplementary Data 4). An indirect tie to soil pH could further explain this lineage’s link to organic horizon carbon stocks. For fungi, we identified two ectomycorrhizal fungal Russulaceae OTUs (Lactifluus vellereus and Russula rhodopus) positively linked to organic horizon carbon stocks in conifer stands (Supplementary Fig. 6). These ectomycorrhizal fungi might slow decomposition via the Gadgill effect88, an expected outcome of some ectomycorrhizal fungi in conifer forests89. Though other ectomycorrhizal lineages with stronger decomposing potentials, such as Cortinarius or Piloderma, would be the more anticipated fungi responsible for the Gadgill effect, ectomycorrhizal genera with weaker decomposing potentials have been recently linked to slower carbon cycling90. Many more fungi were indicators of organic horizon carbon stocks in broadleaf versus conifer forests (120 versus 11 OTUs). Most top positive indicators were ectomycorrhizal fungi, whereas the top negative indicators were mostly saprotrophs, with or lacking mixed trophic assignments (e.g., saprotroph-pathogen), implying that ectomycorrhizal fungal indicators are more strongly tied to higher organic horizon carbon stocks. Among the top indicators were various Inocybe, Sebacina, and Russula OTUs (Supplementary Data 5), all ectomycorrhizal fungi that we also found to be strongly linked to variation in tree growth (Fig. 2c). These OTUs, many of which we classified at the species-level so they can be investigated more directly, are unique since they are indicators of carbon cycling both above- and belowground.
Conclusion and limitations
In this study, we linked soil microbiome composition and diversity to three major forest carbon storage metrics across Europe (tree growth, tree biomass, and soil organic carbon stocks). We show that fungal, but not bacterial, composition and richness are correlated with tree growth rates and tree biomass carbon stocks, when controlling for the effects of climate, dominant tree type, and other important co-variables. We suspect a major reason for opposing fungal and bacterial signals aboveground is the ubiquity of biotrophic fungal groups in the forest mycobiome and key groups of symbiotic endophytic and ectomycorrhizal fungi. Fungal endophyte biodiversity was positively linked to tree growth rates above and beyond any other microbial group we studied, a surprising discovery since most work in forests highlights mycorrhizal fungi7,12,30,31,91,92. It is important to note that there are also many biotrophic bacteria in forests, including endophytes93, but these are especially difficult to identify based on DNA sequencing alone and could not be confidently separated from the entire bacterial community in our study. Future work will need to further investigate our fungal endophyte-tree growth results to explore any causal relationship between the two. That said, the biotechnology sector has already begun widely capitalizing on fungal endophytes for applied tree growth promotion94. By sourcing specific endophytes or communities from forested areas in the wild, locatable using observational studies like ours, it would be possible to test whether even more powerful plant biostimulants can be developed.
Even though tree growth is a major component of forest carbon cycling, an equal or even greater quantity of carbon is stored belowground2. Both fungal and bacterial composition and richness were negatively correlated with organic horizon carbon stocks, implying a potential direct link between the two that will need to be disentangled further with experimental studies to investigate any cause-effect interactions. In the mineral soil horizon, where most carbon is stored, the microbiome was not tightly linked to soil organic carbon stocks. However, tree growth and biomass were tightly linked to mineral horizon organic carbon stocks in our study system (Fig. 5) even after controlling for other co-variables (Supplementary Data 4). Since the mycobiome was tightly correlated with tree growth/tree biomass, it is therefore indirectly linked to mineral horizon organic carbon stocks and in turn total ecosystem carbon storage. This establishes that the soil mycobiome is a unique biological indicator of forest carbon storage across Europe.
Panels show the correlation between tree biomass (a) and growth (b) with soil organic carbon stocks in the mineral horizon (0−10 cm depth; n = 88). Plotted lines show linear correlations, shaded areas around each line are 95% confidence intervals, and r values are Pearson correlation coefficients. The full statistical models, including all co-variates, for each correlation are shown in Supplementary Data 4. Only significant correlation coefficients are shown (P ≤ 0.05).
Methods
Study sites
This work was conducted across the ICP Forests network which has been monitoring hundreds of permanent forest plots across Europe since the 1990’s95. We sampled level II plots that are intensively monitored, at least 0.25 ha, and where almost all trees with a > 5 cm diameter at breast height (DBH) are measured approximately every five years, a common interval for estimating tree growth. At each plot, we measured tree species membership and whether the plot was dominated by conifer versus broadleaf trees (>50% cover). There were 21 tree species included in the survey, and tree richness ranged from 1 to 9 tree species. Most plots were between 1 and 5 tree species. Forest age ranged from <30 years old to >120 years old with an average age of 90 years. The locations spanned a -2.5 to 15.5 °C mean annual temperature range, a 443 to 2,082 mm year-1 mean annual precipitation range, and a 0.10 to 50.11 t C ha-1 year-1 productivity range.
Soil sampling
Soil was sampled between July-August in 2019 and 2020 from 285 level II plots across 18 European countries. We were only able to include 238 plots from 15 countries in our study due to issues extracting DNA and amplifying microbial marker genes. A 30 ×30 m subplot was established inside the plot or in the buffer zone, and nine samples were collected in a grid-design (Supplementary Fig. 7). The organic horizon was first removed using a serrated knife and spatula (only at sites where it was formed and separable), and mineral soil was collected to a 10 cm depth using a soil corer (5 cm diameter). Soil samples were pooled within horizon, homogenized, and dried in an oven at 40 °C or air-dried for at least 48 hours, depending on whether a drying oven was available. Fully dried samples were then shipped to ETH Zürich and stored at -20 °C prior to analysis.
Carbon cycling and meta-data measurements
Each level II monitoring plot collects in situ tree, vegetation, soil, climate, and atmospheric chemistry data. Tree growth was calculated using periodic DBH measurements and allometric equations for each tree species and DBH size range. In short, we removed any dead trees, trees with <5 cm DBH, trees that shrank over the growth period, and then used the first and last DBH measurement to calculate diameter growth increment. The mean growth interval was 5.5 years, the mean initial year was 2005, and the mean final year was 2008. While this varies marginally from the time of soil sampling, previous work has demonstrated that year-to-year variation in microbiome composition is rather low96,97. Next, we used species specific allometric equations from publications made studying trees in Europe within the size range of those observed in our dataset to compute tree mass at the first and final census (see7), computed tree growth mass, and assumed a 50% C content across all species98. Because every tree in a plot is not measured for DBH, we could not strictly sum the mass of all measured trees to go from the tree to stand level. We therefore randomly sampled with replacement trees which are periodically measured until reaching in situ stem density 1,000 times and used the mean value to estimate stand-level tree growth rates (tonnes C ha-1 yr-1) and live tree biomass (tonnes C ha−1).
Soil carbon and nitrogen stocks were calculated using measurements of elemental content (%), bulk density, and sampling depth (tonnes C ha-1) determined from field-based measurements. Soil carbon and nitrogen contents were measured using dry combustion on finely ground soil samples. Soil pH was measured in soil slurries with DI water (10 g soil: 20 mL DI water) using a pH probe. Soil clay content was measured in situ and also estimated using SoilGrids at a 250 m resolution99. However, this data-product-derived estimate was only used after we assessed the accuracy of these estimates using in situ data. We compared estimated values to those collected in the lab for a subset of the plots where data was available across the entire ICP level II network. The two values were strongly correlated (r = 0.51, P < 0.0001, n = 321), so we used data from SoilGrids to have more complete observations except when modeling soil carbon storage. For soil carbon storage, we used in situ clay data due to the importance of clay in stabilizing soil carbon. Finally, we obtained mean annual temperature and precipitation measurements from WorldClim100, and N deposition predictions for 2019 at a 1 km resolution from EMEP101.
Molecular analyses
DNA was extracted from frozen soil (250 mg) using the DNeasy PowerSoil Pro kit (Qiagen, Hilden, Germany). Template DNA was then used to amplify the variable regions 4 and 5 of the 16S rRNA gene using the primers 515F (GTGYCAGCMGCCGCGGTAA) + 926R (GGCCGYCAATTYMTTTRAGTTT)102 to study prokaryotes and the entire ITS region using the primers ITS9munngs (GTACAC ACCGCCCGTCG)+ ITS4ng (CGCCTSCSCTTANTDATATGC)103 to study fungi. The 16S primers were selected because they offer improved phylogenetic resolution compared to the use of alternative reverse primers104, and the ITS primers were selected because they also span a wide phylogenetic range of fungi and best recapitulate mock communities compared to other primer combinations103. Each primer contained a 12 bp index sequence in the 5’ position. PCR reactions were performed in duplicate 25 μL reactions (13 μL of PCR grade water, 10 μL of Phusion Flash High-Fidelity PCR Master Mix, 1 μL 12.5 μM forward primer, 1 μL 12.5 μM reverse primer, and 1 μL of template DNA). 16S amplicon thermocycler conditions were 94 °C for 3 min followed by 30 cycles of 94 °C for 45 s, 50 °C for 60 s, and 72 °C for 90 s, then 72 °C for 10 min, and finally a 4 °C hold. ITS amplicon thermocycler conditions were 95 °C for 15 min followed by 30 cycles of 95 °C for 30 s, 57 °C for 30 s, and 72 °C for 60 s, then 72 °C for 10 min, and finally a 4 °C hold.
The success and relative quantity of PCR product was assessed using agarose gel electrophoresis. We then pooled samples based on band intensity and removed remaining PCR reagents, short DNA and PCR products, and PCR primer dimers using AMPure beads for specific size selection. The ITS amplicons averaged ca. 750 bp whereas the 16S amplicons averaged 300 bp. Pooled products were then quantified on a Qubit using the dsDNA BR Assay Kit (Invitrogen, Waltham, Massachusetts, USA) and sent for library preparation and sequencing at the Functional Genomics Center Zürich. 16S libraries were sequenced using four Illumina MiSeq Runs with v3 chemistry (2 × 300 bp). ITS libraries were sequenced using four PacBio Sequel IIe SMRT Cell 8 M (15 h movie lengths).
Bioinformatics
Raw sequences were first demultiplexed using Cutadapt105 allowing for 0.10% mismatch, no insertions or deletions, and using the –pair-adapters function. 16S reads included (forward) F and (reverse) R reads whereas ITS sequences were HiFi reads produced using the circular consensus sequencing mode. The accuracy of HiFi reads provides a base-level resolution of 99.9% accuracy. Demultiplexed sequences were then imported into QIIME2 (v2021.8) for downstream processing106. However, prior to importing the ITS sequences, we first extracted the complete ITS region using ITSx (v1.1.3)107. 16SF and R reads were first merged using the vsearch join-pairs plug-in derived from USEARCH108. Because the ITS reads are single end, there was no need for pairing. We then QC filtered all reads using the quality-filter q-score command removing reads with average PHRED scores <4, truncating reads if >3 successive base call PHRED scores were <3, and removing all sequences with ambiguous base calls. We then dereplicated sequences and clustered de novo operational taxonomic units (OTUs) at 97% sequence similarity for 16S sequences and 98% sequence similarity for ITS sequences to account for variation in sequence conservation and better capture species identities compared to computing amplicon sequence variants or ASVs109 using the dereplicate-sequences and cluster-features-de-novo functions, respectively. Previous research also shows that computing ASVs or OTUs makes little difference when detecting patterns in community composition, species richness, and relative abundances of taxa for both 16 S and ITS DNA metabarcoding110. Singletons were later removed from the dataset in R. Finally, we assigned taxonomy to representative 16 S and ITS OTUs using Greengenes111 (2019-05 release) and UNITE112 (v8, 2021-10 release), respectively. We used the naïve Bayes machine-learning classifier and the feature-classifier fit-classifier-I-bayes function to train the classifier. We then assigned taxonomy using the classify-sklearn function and used the default confidence parameter of 0.7. ITS OTUs were also assigned functional guild annotations at the genus level using FUNGuild113, accepting all ‘probable’ or higher level annotations. Where multiple functional annotations were assigned, we grouped them together (e.g., ectomycorrhizal-saprotroph). We also calculated metrics for taxa strictly identified to one trophic group for ectomycorrhizal fungi and saprotrophic fungi as ‘pure’ ectomycorrhizal fungi and ‘pure’ saprotrophic fungi. We did not do this for pathogens or endophytes because these groups are generally expected to harbor >1 trophic strategy. See Supplementary Table 3 for a summary of the relative abundance of different fungal functional group annotations.
Statistical analyses
Microbiome diversity and composition
Samples with low sequencing depth were first removed from the dataset (<5000 sequences for 16S analysis; <500 sequences for ITS analysis). Because PacBio Sequel IIe sequencing is much shallower than Illumina MiSeq (2 × 300 bp) sequencing, we rarified the fungal dataset to a lower depth than the bacterial dataset. Although, sequencing depth in the ITS dataset is relatively low, we find similar correlations with environmental variables when removing low-depth samples and rarefying to 3000 sequences. We therefore opted to retain more samples and rarefied to <500 sequences (see Supplementary Data 6 for raw sequence counts). For estimating alpha and beta diversity, we rarified the datasets to the lowest sequencing depth using the rrarefy function in vegan (2.6-4)114. We then calculated relative abundance of OTUs and measured the correlation between microbiome composition and environmental variables used to predict tree growth and soil organic carbon stocks (in addition to latitude referred to as ‘geographic space’ in Fig. 1) using distance-based redundancy analysis and the capscale function in the vegan package. Analyses were performed separately for bacteria and fungi using Bray–Curtis dissimilarities, and predictor variables were scaled to directly compare effect sizes. We also estimated species richness and Shannon Diversity using the specnumber and diversity functions in vegan, respectively. We then estimated community composition (i.e., beta diversity) based on OTU relative abundances converted to Bray–Curtis dissimilarities to have a metric of community composition for our statistical models of tree growth and SOC stocks. Bray–Curtis dissimilarities were analyzed using principal coordinate analysis (PCoA) and the pcoa function in the ape package (5.6-2)90 (see Supplementary Table 5 for eigenvalues). We also fit environmental variables to PCoA1 and 2 using the envfit function in the vegan package. Analyses were conducted separately for bacteria and fungi, and then the fungal dataset was split into functional guilds and analyses were repeated separately for each guild. Microbiome alpha and beta diversity (PCoA1 and 2) were then used to predict tree growth, tree biomass, and soil carbon stocks in subsequent regression analyses (see below).
Indicator species analysis of discrete and continuous variables
We identified which taxa were linked to continuous variables of C cycling (tree growth rate and soil organic carbon stocks stocks) using analysis of differential relative abundances and negative binomial models. We used the non-rarified OTU table and DESeq2 package (1.34.0)115. We used the estimateSizeFactors function with type = ‘poscounts’ and then the DESeq function with test = ‘Wald’ and fitType = ‘parametric’. Estimated values from this analysis represent a log change in sequence abundance for a unit change in the response variable. Taxa with significant correlations were identified as “indicators species”, defined as those with significant p-values and log two-fold change >0.6 or <-0.6, consistent with most RNA sequencing workflows. The direction of the model coefficient was used to assess whether they were linked to low or high values of each response variable. Although we use the term “indicator species”, this is distinct from those species identified using traditional indicator species analysis116 which only identifies indicator species linked to discrete groups versus continuous variables.
Regression analyses
All statistical analyses were conducted in R117 and significance was set to P ≤ 0.05. We used generalized additive modeling (GAMs) to account for the linear and non-linear effects of predictors on tree growth and soil carbon stocks. We used the gam function from the mgcv package (1.8–38)118 and used REML estimation of the smoothing parameters. We predicted tree growth, tree biomass carbon stock, and soil carbon stocks using nitrogen deposition, soil nitrogen stocks (for tree growth only), mean annual temperature, mean annual precipitation, soil pH, soil clay content, stem density, forest age, and a categorical predictor of broadleaf versus conifer stand type. Each model also contained one microbiome predictor (e.g., PCoA1, PCoA2, species richness) to maintain independence and facilitate model comparisons across different microbiome predictors. We diagnosed model fit based on the distribution of the residuals and confirmed that predictors were not too strongly multi-collinear based on variance inflation factors ≤560 (see Supplementary Data 7 for results). To emphasize the correlational nature of our work in the display items, we computed Pearson correlation coefficients (r) versus the coefficient of determination from regression analysis (r2).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Full access to raw ICP Forest datasets is available via the ICP Forests network upon request (http://icp-forests.net/page/data-requests). Restrictions apply to the availability of these data without a formal data request. Raw microbiome datasets can be downloaded from the NCBI SRA using accession numbers PRJNA1068067, PRJNA639984, PRJNA644776, and PRJNA1068308. Microbiome and other data products can be downloaded in the following repository https://gitlab.com/fungalecology/icpf.micro. The fungal taxonomic database UNITE can be accessed here: https://unite.ut.ee/index.php; the bacterial taxonomic database Greengenes can be accessed here: https://greengenes.secondgenome.com/. The fungal functional group database FUNGuild can be accessed here: http://www.funguild.org/.
Code availability
All scripts used for the analysis are openly available at the following repository: https://gitlab.com/fungalecology/icpf.micro.
References
Watch, Global Forest. “Global forest watch.” World Resources Institute, Washington, DC Available from http://www.globalforestwatch.org (2002).
Pan, Y. et al. A large and persistent carbon sink in the world’s forests. Science 333, 988–993 (2011).
Crowther, T. W. et al. Mapping tree density at a global scale. Nature 525, 201–205 (2015).
McNeely, J. A. Lessons from the past: forests and biodiversity. Biodivers. Conserv. 3, 3–20 (1994).
Anthony, M. A., Bender, S. F. & van der Heijden, M. G. A. Enumerating soil biodiversity. Proc. Natl Acad. Sci. 120, e2304663120 (2023).
Schimel, J. & Schaeffer, S. Microbial control over carbon cycling in soil. Front. Microbiol. 3, 348 (2012).
Anthony, M. A. et al. Forest tree growth is linked to mycorrhizal fungal composition and function across Europe. ISME J. 16, 1327–1336 (2022).
Schneider, T. et al. Who is who in litter decomposition? Metaproteomics reveals major microbial players and their biogeochemical functions. ISME J. 6, 1749–1762 (2012).
Averill, C. et al. Defending Earth’s terrestrial microbiome. Nat. Microbiol. 7, 1–9 (2022).
De Graaff, M.-A., Adkins, J., Kardol, P. & Throop, H. A meta-analysis of soil biodiversity impacts on the carbon cycle. Soil 1, 257–271 (2015).
Bond-Lamberty, B., Wang, C. & Gower, S. T. A global relationship between the heterotrophic and autotrophic components of soil respiration? Glob. Change Biol. 10, 1756–1766 (2004).
Read, D. & Perez‐Moreno, J. Mycorrhizas and nutrient cycling in ecosystems–a journey towards relevance? N. Phytologist 157, 475–492 (2003).
Brundrett, M. C. & Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. N. Phytologist 220, 1108–1115 (2018).
Bennett, J. A. et al. Plant-soil feedbacks and mycorrhizal type influence temperate forest population dynamics. Science 355, 181–184 (2017).
Chamagne, J. et al. Forest diversity promotes individual tree growth in central European forest stands. J. Appl. Ecol. 54, 71–79 (2017).
Halim, M. A., Chen, H. Y. H. & Thomas, S. C. Stand age and species composition effects on surface albedo in a mixedwood boreal forest. Biogeosciences 16, 4357–4375 (2019).
Vesterdal, L., Clarke, N., Sigurdsson, B. D. & Gundersen, P. Do tree species influence soil carbon stocks in temperate and boreal forests? For. Ecol. Manag. 309, 4–18 (2013).
Newsham, K. K. A meta-analysis of plant responses to dark septate root endophytes. N. Phytologist 190, 783–793 (2011).
Singh, M. et al. Complementarity among plant growth promoting traits in rhizospheric bacterial communities promotes plant growth. Sci. Rep. 5, 1–8 (2015).
Hannula, S. E. et al. Persistence of plant-mediated microbial soil legacy effects in soil and inside roots. Nat. Commun. 12, 5686 (2021).
Hoeksema, J. D. et al. A meta‐analysis of context‐dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 13, 394–407 (2010).
Domeignoz-Horta, L. A. et al. Microbial diversity drives carbon use efficiency in a model soil. Nat. Commun. 11, 3684 (2020).
Wagg, C., Schlaeppi, K., Banerjee, S., Kuramae, E. E. & van der Heijden, M. G. A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 10, 4841 (2019).
Alberton, O., Kuyper, T. W. & Gorissen, A. Competition for nitrogen between Pinus sylvestris and ectomycorrhizal fungi generates potential for negative feedback under elevated CO2. Plant Soil 296, 159–172 (2007).
Sim, M.-Y. & Eom, A.-H. Effects of ectomycorrhizal fungi on growth of seedlings of Pinus densiflora. Mycobiology 34, 191–195 (2006).
Jonsson, L. M., Nilsson, M., Wardle, D. A. & Zackrisson, O. Context dependent effects of ectomycorrhizal species richness on tree seedling productivity. Oikos 93, 353–364 (2001).
Glassman, S. I. et al. Decomposition responses to climate depend on microbial community composition. Proc. Natl Acad. Sci. 115, 11994–11999 (2018).
Chiba, A. et al. Soil bacterial diversity is positively correlated with decomposition rates during early phases of maize litter decomposition. Microorganisms 9, 357 (2021).
van der Wal, A., Ottosson, E. & de Boer, W. Neglected role of fungal community composition in explaining variation in wood decay rates. Ecology 96, 124–133 (2015).
Jörgensen, K., Granath, G., Strengbom, J. & Lindahl, B. D. Links between boreal forest management, soil fungal communities and below-ground carbon sequestration. Funct. Ecol. 36, 392–405 (2022).
Lindahl, B. D. et al. A group of ectomycorrhizal fungi restricts organic matter accumulation in boreal forest. Ecol. Lett. 24, 1341–1351 (2021).
Jandl, R. et al. How strongly can forest management influence soil carbon sequestration? Geoderma 137, 253–268 (2007).
Liski, J., Perruchoud, D. & Karjalainen, T. Increasing carbon stocks in the forest soils of western Europe. For. Ecol. Manag. 169, 159–175 (2002).
Chen, G. et al. Accelerated soil carbon turnover under tree plantations limits soil carbon storage. Sci. Rep. 6, 19693 (2016).
Terrer, C. et al. A trade-off between plant and soil carbon storage under elevated CO2. Nature 591, 599–603 (2021).
Powlson, D., Smith, P. & Nobili, M. D. in Soil Conditions and Plant Growth (eds Gregory, P. J. & Nortcliff, S.) 86–131 (Blackwell Publishing Ltd. 2013).
Jenkinson, D. S. The turnover of organic carbon and nitrogen in soil. Philos. Trans. R. Soc. Lond. Ser. B: Biol. Sci. 329, 361–368 (1990).
Parton, W. J., Schimel, D. S., Cole, C. V. & Ojima, D. S. Analysis of factors controlling soil organic matter levels in Great Plains grasslands. Soil Sci. Soc. Am. J. 51, 1173–1179 (1987).
Kuzyakov, Y., Friedel, J. K. & Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 32, 1485–1498 (2000).
Cotrufo, M. F., Wallenstein, M. D., Boot, C. M., Denef, K. & Paul, E. The M icrobial E fficiency‐M atrix S tabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Glob. Change Biol. 19, 988–995 (2013).
Stewart, C. E., Paustian, K., Conant, R. T., Plante, A. F. & Six, J. Soil carbon saturation: evaluation and corroboration by long-term incubations. Soil Biol. Biochem. 40, 1741–1750 (2008).
Baskaran, P. et al. Modelling the influence of ectomycorrhizal decomposition on plant nutrition and soil carbon sequestration in boreal forest ecosystems. N. Phytologist 213, 1452–1465 (2017).
Frey, S. D. Mycorrhizal fungi as mediators of soil organic matter dynamics. Annu. Rev. Ecol. Evol. Syst. 50, 237–259 (2019).
Prescott, C. E. & Grayston, S. J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 309, 19–27 (2013).
Urbanová, M., Šnajdr, J. & Baldrian, P. Composition of fungal and bacterial communities in forest litter and soil is largely determined by dominant trees. Soil Biol. Biochem. 84, 53–64 (2015).
Tedersoo, L. et al. Regional-scale in-depth analysis of soil fungal diversity reveals strong pH and plant species effects in Northern Europe. Front. Microbiol. 11, 1953 (2020).
Tedersoo, L. et al. Towards global patterns in the diversity and community structure of ectomycorrhizal fungi. Mol. Ecol. 21, 4160–4170 (2012).
Wallander, H., Johansson, U., Sterkenburg, E., Brandström Durling, M. & Lindahl, B. D. Production of ectomycorrhizal mycelium peaks during canopy closure in Norway spruce forests. N. Phytologist 187, 1124–1134 (2010).
Glassman, S. I., Wang, I. J. & Bruns, T. D. Environmental filtering by pH and soil nutrients drives community assembly in fungi at fine spatial scales. Mol. Ecol. 26, 6960–6973 (2017).
Lauber, C. L., Hamady, M., Knight, R. & Fierer, N. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl. Environ. Microbiol. 75, 5111–5120 (2009).
Liu, S. et al. Phylotype diversity within soil fungal functional groups drives ecosystem stability. Na. Ecol. Evol. 6, 900–909 (2022).
Feng, X., Simpson, A. J., Schlesinger, W. H. & Simpson, M. J. Altered microbial community structure and organic matter composition under elevated CO2 and N fertilization in the duke forest. Glob. Change Biol. 16, 2104–2116 (2010).
Tripathi, B. M. et al. Soil pH mediates the balance between stochastic and deterministic assembly of bacteria. ISME J. 12, 1072–1083 (2018).
Anthony, M. A., Stinson, K. A., Trautwig, A. N., Coates-Connor, E. & Frey, S. D. Fungal communities do not recover after removing invasive Alliaria petiolata (garlic mustard). Biol. Invasions 21, 3085–3099 (2019).
Delgado-Baquerizo, M. et al. Carbon content and climate variability drive global soil bacterial diversity patterns. Ecol. Monogr. 86, 373–390 (2016).
van der Linde, S. et al. Environment and host as large-scale controls of ectomycorrhizal fungi. Nature 558, 243–248 (2018).
Tedersoo, L. et al. Global diversity and geography of soil fungi. Science 346, 1256688 (2014).
Davison, J. et al. Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism. Science 349, 970–973 (2015).
Etzold, S. et al. Nitrogen deposition is the most important environmental driver of growth of pure, even-aged and managed European forests. For. Ecol. Manag. 458, 117762 (2020).
O’brien, R. M. A caution regarding rules of thumb for variance inflation factors. Qual. Quant. 41, 673–690 (2007).
Claus, A. & George, E. Effect of stand age on fine-root biomass and biomass distribution in three European forest chronosequences. Can. J. For. Res. 35, 1617–1625 (2005).
Rodriguez, R. J., White, J. F. Jr, Arnold, A. E. & Redman, R. S. Fungal endophytes: diversity and functional roles. N. Phytologist 182, 314–330 (2009).
Moller, L., Lerm, B. & Botha, A. Interactions of arboreal yeast endophytes: an unexplored discipline. Fungal Ecol. 22, 73–82 (2016).
Joubert, P. M. & Doty, S. L. in Endophytes of Forest Trees. Forestry Sciences (eds. Pirttilä, A. & Frank, A.) 3–14 (Springer, 2018).
Ruotsalainen, A. L. et al. Dark septate endophytes: mutualism from by-products? Trends Plant Sci. 27, 247–254 (2022).
Chen, L. et al. Towards the biological control of devastating forest pathogens from the genus Armillaria. Forests 10, 1013 (2019).
Ozimek, E. & Hanaka, A. Mortierella species as the plant growth-promoting fungi present in the agricultural soils. Agriculture 11, 7 (2020).
Moreno, J., León, J. D. & Osorio, N. W. Tree seedling growth promotion by dual inoculation with Rhizoglomus fasciculatum (Thaxt.) Sieverding, Silva & Oehl and Mortierella sp., rhizosphere fungi for reforestation purposes, to promote plant P uptake and growth at the nursery state. Acta Agron.ómica 65, 239–247 (2016).
Alberton, O., Kuyper, T. W. & Summerbell, R. C. Dark septate root endophytic fungi increase growth of Scots pine seedlings under elevated CO2 through enhanced nitrogen use efficiency. Plant Soil 328, 459–470 (2010).
Sieber, T. N. in Plant Roots (eds Waisel, Y., Eshel, A., Beeckman, T. & Kafkafi, U.) 1369−1418 (CRC Press, 2002).
Agerer, R. Exploration types of ectomycorrhizae. Mycorrhiza 11, 107–114 (2001).
Deslippe, J. R., Hartmann, M., Grayston, S. J., Simard, S. W. & Mohn, W. W. Stable isotope probing implicates a species of Cortinarius in carbon transfer through ectomycorrhizal fungal mycelial networks in Arctic tundra. N. Phytologist 210, 383–390 (2016).
Bödeker, I. T. et al. Ectomycorrhizal C ortinarius species participate in enzymatic oxidation of humus in northern forest ecosystems. N. Phytologist 203, 245–256 (2014).
Hobbie, E. A. et al. Fungal carbon sources in a pine forest: evidence from a 13C-labeled global change experiment. Fungal Ecol. 10, 91–100 (2014).
Looney, B. P. et al. Russulaceae: a new genomic dataset to study ecosystem function and evolutionary diversification of ectomycorrhizal fungi with their tree associates. N. Phytologist 218, 54–65 (2018).
Gao, C. et al. Community assembly of ectomycorrhizal fungi along a subtropical secondary forest succession. N. Phytologist 205, 771–785 (2015).
Ge, Z.-W., Brenneman, T., Bonito, G. & Smith, M. E. Soil pH and mineral nutrients strongly influence truffles and other ectomycorrhizal fungi associated with commercial pecans (Carya illinoinensis). Plant Soil 418, 493–505 (2017).
Long, D., Liu, J., Han, Q., Wang, X. & Huang, J. Ectomycorrhizal fungal communities associated with Populus simonii and Pinus tabuliformis in the hilly-gully region of the Loess Plateau, China. Sci. Rep. 6, 24336 (2016).
Moore, J. A. M. et al. Fungal community structure and function shifts with atmospheric nitrogen deposition. Glob. Change Biol. 27, 1349–1364 (2021).
Jones, M. D. & Smith, S. E. Exploring functional definitions of mycorrhizas: Are mycorrhizas always mutualisms? Can. J. Bot. 82, 1089–1109 (2004).
Georgiou, K. et al. Global stocks and capacity of mineral-associated soil organic carbon. Nat. Commun. 13, 3797 (2022).
De Vos, B. et al. Benchmark values for forest soil carbon stocks in Europe: Results from a large scale forest soil survey. Geoderma 251, 33–46 (2015).
Frey, S. D. et al. Chronic nitrogen additions suppress decomposition and sequester soil carbon in temperate forests. Biogeochemistry 121, 305–316 (2014).
Kriegel, P. et al. Ambient and substrate energy influence decomposer diversity differentially across trophic levels. Ecol. Lett. 26, 1157–1173 (2023).
MacArthur, R. H., Wilson, E. O. & Wilson, E. O. in The Theory of Island Biogeography 8–18 (Princeton University Press, 1967).
Picasso, V. D. The “biodiversity–ecosystem function debate”: an interdisciplinary dialogue between ecology, agricultural science, and agroecology. Agroecology Sustain. Food Syst. 42, 264–273 (2018).
Purahong, W., Kahl, T., Krüger, D., Buscot, F. & Hoppe, B. Home-field advantage in wood decomposition is mainly mediated by fungal community shifts at “home” versus “away”. Microb. Ecol. 78, 725–736 (2019).
Fernandez, C. W. & Kennedy, P. G. Revisiting the ‘Gadgil effect’: do interguild fungal interactions control carbon cycling in forest soils? N. Phytologist 209, 1382–1394 (2016).
Smith, G. R. & Wan, J. Resource-ratio theory predicts mycorrhizal control of litter decomposition. N. Phytologist 223, 1595–1606 (2019).
Fernandez, C. W., See, C. R. & Kennedy, P. G. Decelerated carbon cycling by ectomycorrhizal fungi is controlled by substrate quality and community composition. N. Phytologist 226, 569–582 (2020).
Hupperts, S. F. & Lilleskov, E. A. Predictors of taxonomic and functional composition of black spruce seedling ectomycorrhizal fungal communities along peatland drainage gradients. Mycorrhiza 32, 67–81 (2022).
Clemmensen, K. E. et al. Carbon sequestration is related to mycorrhizal fungal community shifts during long‐term succession in boreal forests. N. Phytologist 205, 1525–1536 (2015).
Pirttilä, A. M. in Endophytes of Forest Trees: Biology and Applications (eds Pirttilä, A. M. & Caroline Frank, A.) 139–149 (Springer, 2011).
Ortega, H. E., Torres-Mendoza, D. & Cubilla-Rios, L. Patents on endophytic fungi for agriculture and bio- and phytoremediation applications. Microorganisms 8, 1237 (2020).
Ferretti, M. & Fischer, R. Forest Monitoring: Methods for Terrestrial Investigations in Europe with an Overview of North America and Asia 12 (Elsevier, 2013).
Averill, C., Cates, L. L., Dietze, M. C. & Bhatnagar, J. M. Spatial vs. temporal controls over soil fungal community similarity at continental and global scales. ISME J. 13, 2082–2093 (2019).
Pellitier, P. T., Ibáñez, I., Zak, D. R., Argiroff, W. A. & Acharya, K. Ectomycorrhizal access to organic nitrogen mediates CO2 fertilization response in a dominant temperate tree. Nat. Commun. 12, 1–10 (2021).
Penman, J. et al. Good practice guidance for land use, land-use change and forestry. Good practice guidance for land use, land-use change and forestry. Institute for Global Environmental Strategies (IGES) for the IPCC (2003).
Hengl, T. et al. SoilGrids250m: Global gridded soil information based on machine learning. PLoS ONE 12, e0169748 (2017).
Fick, S. E. & Hijmans, R. J. WorldClim 2: new 1‐km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017).
Tørseth, K. et al. Introduction to the European Monitoring and Evaluation Programme (EMEP) and observed atmospheric composition change during 1972–2009. Atmos. Chem. Phys. 12, 5447–5481 (2012).
Walters, W. et al. Improved bacterial 16S rRNA gene (V4 and V4-5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. Msystems 1, e00009–e00015 (2016).
Tedersoo, L. & Anslan, S. Towards PacBio‐based pan‐eukaryote metabarcoding using full‐length ITS sequences. Environ. Microbiol. Rep. 11, 659–668 (2019).
Parada, A. E., Needham, D. M. & Fuhrman, J. A. Every base matters: assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 18, 1403–1414 (2016).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 17, 10–12 (2011).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019).
Bengtsson‐Palme, J. et al. Improved software detection and extraction of ITS1 and ITS 2 from ribosomal ITS sequences of fungi and other eukaryotes for analysis of environmental sequencing data. Methods Ecol. Evol. 4, 914–919 (2013).
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010).
Tedersoo, L. et al. Best practices in metabarcoding of fungi: From experimental design to results. Mol. Ecol. 31, 2769–2795 (2022).
Glassman, S. I. & Martiny, J. B. H. Broadscale ecological patterns are robust to use of exact sequence variants versus operational taxonomic units. mSphere 3, e00148–18 (2018).
DeSantis, T. Z. et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72, 5069–5072 (2006).
Abarenkov, K. et al. The UNITE database for molecular identification of fungi–recent updates and future perspectives. N. Phytologist 186, 281–285 (2010).
Nguyen, N. H. et al. FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 20, 241–248 (2016).
Dixon, P. VEGAN, a package of R functions for community ecology. J. Vegetation Sci. 14, 927–930 (2003).
Love, M., Anders, S. & Huber, W. Differential analysis of count data–the DESeq2 package. Genome Biol. 15, 10–1186 (2014).
De Caceres, M., Jansen, F. & De Caceres, M. M. Package ‘indicspecies’. indicators 8 (2016).
Team, R. C. R.: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2013).
Wood, S. & Wood, M. S. Package ‘mgcv’. R. Package Version 1, 729 (2015).
Acknowledgements
This work was supported by an Ambizione grant (PZ00P3_179900 awarded to C.A.) from the Swiss National Science Foundation (SNSF). M.A.A. was also partially supported by an Ambizione grant (PZ00P3_208648 awarded to M.A.A.) from the SNSF. The evaluation was based on data (Data Request No 168) that was collected by partners of the official UNECE ICP Forests Network (http://icp-forests.net/contributors). Part of the data was co-financed by the European Commission (Data achieved at “15.02.2022”).
Author information
Authors and Affiliations
Contributions
Conceptualization, M.A.A. and C.A.; Formal Analysis, M.A.A.; Investigation, M.A.A., C.A.; Resources, C.A., T.W.C., M.A.A.; Writing—Original Draft, M.A.A.; Review & Editing, M.A.A., L.T., B.D.V, L.C., H.Mee., M.W., H.A., F.J., P.L., A.K., M.G., G.Pop., B.F., A.G., M.S., M.F., P.W., V.C., R.C., G.Pap., A.M., M.I., L.V., P.R., H.Mei., V.T., M.D., N.E., A.S., N.v.T., T.C., C.A.; Supervision, C.A., T.W.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anthony, M.A., Tedersoo, L., De Vos, B. et al. Fungal community composition predicts forest carbon storage at a continental scale. Nat Commun 15, 2385 (2024). https://doi.org/10.1038/s41467-024-46792-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-46792-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.