Abstract
Hemorrhagic toxin (TcsH) is a major virulence factor produced by Paeniclostridium sordellii, which is a non-negligible threat to women undergoing childbirth or abortions. Recently, Transmembrane Serine Protease 2 (TMPRSS2) was identified as a host receptor of TcsH. Here, we show the cryo-EM structures of the TcsH-TMPRSS2 complex and uncover that TcsH binds to the serine protease domain (SPD) of TMPRSS2 through the CROP unit-VI. This receptor binding mode is unique among LCTs. Five top surface loops of TMPRSS2SPD, which also determine the protease substrate specificity, constitute the structural determinants recognized by TcsH. The binding of TcsH inhibits the proteolytic activity of TMPRSS2, whereas its implication in disease manifestations remains unclear. We further show that mutations selectively disrupting TMPRSS2-binding reduce TcsH toxicity in the intestinal epithelium of the female mice. These findings together shed light on the distinct molecular basis of TcsH-TMPRSS2 interactions, which expands our knowledge of host recognition mechanisms employed by LCTs and provides novel targets for developing therapeutics against P. sordellii infections.
Similar content being viewed by others
Introduction
Paeniclostridium sordellii (also known as Clostridium sordellii), is an anaerobic, spore-forming, and gram-positive bacterium commonly found in the soil and the gastrointestinal tracts of animals1. In humans, the bacterium opportunistically colonizes the gastrointestinal and genital tracts and releases devastating toxins, resulting in acute symptoms including peritonitis, myonecrosis, gangrene, sepsis, toxic shock syndrome, and fatality1,2,3. Approximately 3–4% of women carry P. sordellii and thus are particularly vulnerable to acute infections after gynecologic procedures4. It is reported that unsafe abortion leads to septicemia complicated by toxic shock with a mortality of 100%2.
Hemorrhagic toxin (TcsH, ~300 kDa) and lethal toxin (TcsL, ~270 kDa), which were first described in 1969, are two major virulence factors of P. sordellii5,6. Both toxins belong to the large clostridial toxin (LCT) family, which is a group of potent exotoxins secreted by clostridial species with large protein sizes. Among major LCT family members, including Clostridioides difficile toxin A (TcdA) and toxin B (TcdB), P. sordellii TcsL and TcsH, Clostridium novyi alpha-toxin (Tcnα), and Clostridium perfringens large cytotoxin (TpeL), TcsH shares the highest homology with TcdA, with a sequence identity of ~77%7.
Similar to other LCTs, TcsH comprises four structural domains: an N-terminal glucosyltransferase domain (GTD), a cysteine protease domain (CPD), a combined transmembrane delivery and receptor-binding domain (DRBD), and a C-terminal combined repetitive oligopeptides (CROPs) domain (Fig. 1a)8,9. These domains together contribute to a multi-step intoxication process: the toxin initially recognizes the receptor(s) of target cells and undergoes endocytosis; low pH inside the endosome triggers a yet unclear conformational change of the DRBD, which allows the GTD and CPD across the membrane10,11, followed by a CPD-mediated and Inositol hexakisphosphate (InsP6)-induced autocleavage in the cytosol12,13,14,15; the released GTD then glucosylates and inactivates Rho GTPases, leading to cytopathic and cytotoxic effects7,16,17. The CROPs are special elements exclusively found in the LCT family, which may have carbohydrate-binding capacity18,19. For TcsH, the CROPs domain consists of twenty-nine 20 to 24-amino acid short repeats (SRs) interspersed with six 31-amino acid long repeats (LRs).
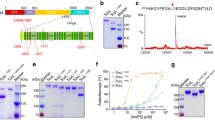
a The schematic diagram showing the domain structures of TcsH and TMPRSS2, as well as the domain boundaries for TcsH2236-2618 and TMPRSS2ECD used for cryo-EM studies. GTD glucosyltransferase domain, CPD cysteine protease domain, DRBD delivery and receptor-binding domain, CROPs combined repetitive oligopeptides domain. The CROP unit-I to VI were marked in the enlarged CROPs domain; blue and pink bars represent SRs (short repeats) and LRs (long repeats) respectively. TM transmembrane domain, LDLRA low-density lipoprotein receptor class A domain, SRCR scavenger receptor cysteine-rich domain, SPD serine protease domain. b The EM map of the full-length TcsH in complex with TMPRSS2ECD is composed of two parts: a 3.2 Å map for the core region of TcsH holotoxin and colored as shown in Fig. 1a, and a 6.0 Å map for the C-terminal region of TcsH is colored in gray. c Superposition of the structure of TcsH2-2387 (blue) onto a cryo-EM structure of TcdA (gray; PDB: 7POG). d Cartoon representation of the core region of TcsH holotoxin. e CROP unit-IV bridges to DRBD in the full-length TcsH. LRs are highlighted in pink. f The pull-down experiment showed that His-tagged TcsH2210-2618, but not TcsH1832-2209, binds to immobilized Fc-TMPRSS2ECD. Source data are provided as a Source Data file.
Researchers have made encouraging progress in resolving the full-length or near-complete structures of LCTs including TcdA20,21, TcdB22,23,24,25, and TcsL26. At neutral pH, TcdA displays in a “closed” conformation that the CROPs domain curves downward alongside the DRBD, while TcdB and TcsL present in an “open” conformation that the CROPs domain curves upward around the GTD-CPD head. Under the low-pH condition, the structures of TcdB and TcsL would convert from the open to closed conformation22,26. Little is known about the full-length structure of TcsH.
Following the recent progress in defining LCT receptors, structures of some LCT-receptor complexes have been resolved, including TcdB1-FZD227, TcsL-SEMA6A28, TcdB1-CSPG429, and TcdB4-TFPI25. On the other hand, the structural basis of toxin recognition for other receptors, including low-density lipoprotein receptor (LDLR) family proteins for TcdA and Tcnα30,31,32,33, low-density lipoprotein receptor-related protein 1 (LRP1) for TpeL34, and poliovirus receptor-like 3 (PVRL3) for TcdB35, remain to be investigated.
Recently, we reported that Transmembrane Serine Protease 2 (TMPRSS2) is a gastrointestinal epithelial receptor for TcsH36. TMPRSS2 is a type II transmembrane protein that contains an extracellular domain consisting of a low-density lipoprotein receptor class A (LDLRA) domain, a scavenger receptor cysteine-rich (SRCR) domain, and a serine protease domain (SPD)37. TcsH recognizes the SPD of TMPRSS2 (TMPRSS2SPD) in a CROPs-dependent manner, but the exact molecular mechanism is unknown. TcsH also binds to fucosylated glycans on the cell surface, as knocking out key genes for fucosylation, such as GMDS, FUT, or SLC35C138, results in drastically reduced TcsH binding/entry in the MCF-7 cells36.
Here, we determined the cryo-electron microscopy (cryo-EM) structure of the full-length TcsH in complex with its protein receptor TMPRSS2. We also demonstrate that TcsH binding potently inhibits the proteolytic activity of TMPRSS2, which may lead to potential applications in related biological studies on cancer signaling and anti-viral therapies.
Results
Cryo-EM structure of the full-length TcsH
To understand how TcsH recognizes TMPRSS2, we set out to determine the structure of full-length TcsH in complex with the ectodomain of TMPRSS2. For ease of description, we define each LR surrounded by several adjacent SRs as a “CROP unit” (Fig. 1a, Supplementary Fig. 1). Because TMPRSS2 simultaneously undergoes autoproteolysis39, we introduced an R255Q mutation that prevents the self-cleavage and generated an extracellular domain fragment of TMPRSS2 namely TMPRSS2106-492/R255Q or TMPRSS2ECD (Fig. 1a). TMPRSS2ECD was used for forming the complex with TcsH at neutral pH (Supplementary Fig. 2). The cryo-EM analysis reconstituted a complex map: 3.2 Å for residues 1-2387 including the “core” domains (GTD, CPD, and DRBD) and 6.0 Å for the rest part (Fig. 1b, Supplementary Fig. 3). Notably, an extra lobe of EM density was observed at the C-terminal tip of the CROPs domain and inferred as the bound TMPRSS2ECD (Fig. 1b).
The structure of the TcsH part presents a TcdA-like “closed” conformation20, of which the CROPs domain curves downward alongside the DRBD domain. This structure exhibits an RMSD of about 7.0 Å with TcdA (PDB:7POG) between 2,365 pruned atom pairs (Fig. 1c). The CROPs domain is bridged to the DRBD domain through the CROP unit-IV (Fig. 1d, e), which likely forms a relatively stable configuration of the “core” domains and the CROP unit-I to IV. However, the CROP unit-V and VI along with the presumable TMPRSS2ECD were in low resolution, possibly due to the flexibility of these regions in the full-length toxin.
Structure of the TcsH2236-2618-TMPRSS2ECD complex
To acquire accurate information regarding the TcsH-TMPRSS2 binding interface, we managed to recruit smaller CROPs fragments for the cryo-EM study. We generated three CROPs constructs: TcsH1832-2209, TcsH2210-2618, and TcsH2236-2618. The first two are His-tagged proteins and the latter one is fused with an MBP tag. As expected, the pull-down assay showed that TcsH2210-2618 but not TcsH1832-2209 robustly binds to Fc-fused TMPRSS2ECD (Fig. 1f), supporting the structural observation that TMPRSS2 binds to the C-terminus of the CROPs domain. Using the BLI assay, we confirmed that both TcsH2210-2618 and TcsH2236-2618 bound to Fc-TMPRSS2ECD with similar affinity (KD of 0.37 and 0.75 nM, respectively, Supplementary Fig. 4). MBP-fused TcsH2236-2618 was selected as a substitutive TcsH fragment to form a complex with TMPRSS2ECD for the cryo-EM study.
We then determined the cryo-EM structure of the TcsH2236-2618-TMPRSS2ECD complex, resulting in a density map of intermediate resolution. To represent a more detailed interface, we masked out the marginal parts of the EM-map, and the local resolution of the TcsH-TMPRSS2 interface (residues 2460-2616 for TcsH and residues 142-492 for TMPRSS2) was further improved to 3.0 Å (Supplementary Fig. 5). This structure exactly shows that the SPD of TMPRSS2 binds to the CROP unit-VI of TcsH (Fig. 2a, b and Supplementary Table 1).
a The 2.95 Å resolution cryo-EM map of the TcsH2236-2618-TMPRSS2ECD complex in two views and colored as shown in Fig. 1a. b Cartoon representation of the structure of the TcsH2460-2616-TMPRSS2ECD complex that is shown in similar orientations and color schemes as that in (a). c Left panel: EM map of the complete TcsH-TMPRSS2ECD complex; right panel: cartoon representation of the complete TcsH-TMPRSS2ECD complex. d Ribbon diagram showing the structure of the CROPs domain with LR1-6 colored in pink. The entire CROPs domain is divided into six CROP units by dashed lines.
Structure of the TcsH-TMPRSS2ECD complex
The newly determined TcsH2236-2618-TMPRSS2ECD complex map together with the earlier 3.2 Å EM map for TcsH allows us to reconstitute the atomic model of the TcsH-TMPRSS2 complex (Fig. 2c, Supplementary Fig. 6). This practice also generates a complete TcsH CROPs domain from unit-I to VI, exhibiting a long curved β-solenoid fold pinched at LR4 with each LR/SR consisting of a single β-hairpin followed by a loop (Fig. 2d).
TcsH recognizes TMPRSS2 through interactive networks
The TMPRSS2ECD adopts a conserved chymotrypsin/trypsin fold with two six-strand β barrels and a catalytic center containing the canonical Ser441-His296-Asp345 catalytic triad. This structure is almost identical to the previously reported one40 (PDB: 7MEQ) with an RMSD of 1.025 Å between 322 pruned atom pairs (Supplementary Fig. 7a). The top surface of TMPRSS2SPD contains eight surface loops, namely LA, LB, LC, LD, LE, L1, L2, and L3 (Supplementary Fig. 7b), which determine the substrate specificity of the protease41. In the TcsH-TMPRSS2ECD complex, LA, LB, LE, L2, and L3 from TMPRSS2 together hold the CROP unit-VI of TcsH (Fig. 3a), like a big hand grabbing a guinea pig from the top-view (Fig. 3b). Interactive networks are observed predominantly through hydrophobic interactions and a few hydrogen bonds with a buried interface at about 1130 Å2 (Fig. 3c–e). Particularly, side chains of H2489 from SR25, E2544 from LR6, and Q2520 from SR26 form three hydrogen bonds with Y322 from LE and Q276 from LA (Fig. 3c). L2558 from SR27 and F2605/I2608 from SR29, together with V280 from LA, L302 from LB, L419 from L3, and W461 from L2 contribute to extensive hydrophobic interactions (Fig. 3d, e). In addition, the backbone amino groups of G2559 from SR27 and F2596 from SR29 form two hydrogen bonds with E299 from LB and S463 from L2 of TMPRSS2, respectively (Fig. 3d, e).
a Hydrophobicity of the TcsH-TMPRSS2 interface. The SRs and LRs of TcsH and surface loops of TMPRSS2 that participate in the interactions are colored and labeled as follows: LR: pink; SR: dark blue; LE: magenta; LA: yellow; LB: orange; L2: purple; L3: brown; (b) The top view of the complex shows that TMPRSS2SPD interacts with TcsH through five loops. c–e Close-up views of the TcsH-TMPRSS2 interface with interacting side chains shown as sticks and colored as shown in (a). Hydrogen bonds are shown as blue dotted lines.
Validation of TcsH-TMPRSS2 interactions by site-specific mutagenesis
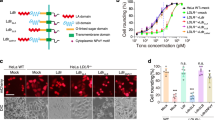
To confirm the TcsH-TMPRSS2 interface, we performed structure-guided mutagenesis on TMPRSS2ECD. Site-directed point mutations on different loops, including V275R and Q276 A at LA, L302R and H307A at LB, and D417A and L419R at L3, all attenuate the TcsH-TMPRSS2 interaction, as demonstrated by the pull-down assay (Fig. 4a). We also generated several mutations on TcsH2210-2618 according to the structure. Some of them, such as Q2520A/F2521A, Q2520F/F2521R, E2544R, and F2557N/L2558N, substantially impair the binding capability of TcsH2210-2618, as demonstrated by the pull-down assay (Fig. 4b). Consistently, these TcsH2210-2618 mutants also showed reduced binding to the surface of MCF7 GMDS‒/‒ cells (Fig. 4c), which expresses TMPRSS2 but not fucosylated glycans. We further introduced mutations, including Q2520A/F2521A, E2544R, F2557N/L2558N, and Q2520A/F2521A/E2544R into the full-length TcsH. The circular dichroism (CD) spectra analysis showed that all these mutant toxins, including TcsHQ2520A/F2521A, TcsHE2544R, TcsHF2557N/L2558N, and TcsHQ2520A/F2521A/E2544R (referred to as TcsHAAR thereafter), were properly folded (Supplementary Fig. 8). When applied to MCF7 cells, all the mutants were less toxic than TcsH (~8 to 50-fold) to the WT cells (Fig. 4d) but equally potent to the TMPRSS2‒/‒ cells (Fig. 4e), indicating that these designed mutations specifically impair the TMPRSS2 recognition.
a Binding of the wild-type TcsH2210-2618 to Fc-His-TMPRSS2ECD variants immobilized on Protein A resins was examined using pull-down assays. b The binding of TcsH2210-2618 variants to Fc-His-TMPRSS2ECD immobilized on Protein A resins was examined using pull-down assays. c Confocal fluorescence images show the binding of different GFP-fused TcsH2210-2618 variants to the MCF-7 GMDS‒/‒ cells. Cell nuclei were stained by DAPI (blue). The blots and images are representatives of three independent experiments. The scale bar represents 50 μm. d, e The sensitivities of the MCF-7 GMDS‒/‒ and TMPRSS2‒/‒ cells to TcsH or its mutants were tested using the cytopathic cell-rounding assays. The percentages of the rounded cells were plotted over the toxin concentrations. Error bars (n = 5 biologically independent samples) indicate mean ± SD. Source data are provided as a Source Data file.
Albeit the TcsH CROP unit-VI and its homologous fragment in TcdA (CROP unit-VII) are sequentially and structurally close42 (Supplementary Fig. 9), it was reported that the TcdA CROPs domain does not bind to TMPRSS236. By interrogating the different residues between the TcsH CROP unit-VI and TcdA CROP unit-VII, we identified that F2521I, a single-point substitution in TcsH, is capable of abolishing the TMPRSS2 binding of TcsH2210-2618 where some other mutations also partly affect the interaction (Supplementary Fig. 10a). On the other hand, reversely mutating the according residue in TcdA (residue position 2613) from I to F did not render the TcdA CROPs overt binding to TMPRSS2 (Supplementary Fig. 10b).
TcsH binding inhibits the proteolytic activity of TMPRSS2
The proteolytic activity of TMPRSS2 was achieved by the catalytic triad composed of S441-H296-D34543 (Fig. 5a). Based on the structure of TcsH-TMPRSS2ECD complex, the TcsH CROP unit-VI largely covered the flattop surface of TMPRSS2SPD, with the β-hairpin of SR27 and the loop of SR29 stretching into the catalytic pocket (Fig. 5b, Supplementary Fig. 11a).
a The top view of TMPRSS2SPD structure with the catalytic triad (H296, D345, S441) in the serine protease domain highlighted with sticks and green color. b In the TcsH-TMPRSS2 complex, CROP unit-VI covers the catalytic pocket of TMPRSS2. Loops of SR27 and SR29 stretch into the catalytic pocket and possibly block the substrate entrance. c Schematic diagram illustrating the principle of TMPRSS2 protease activity assay. TMPRSS2 cleaves at the bond after the Arginine of a fluorogenic peptide substrate Boc-QAR-AMC, releasing AMC with bright fluorescence emitted. d TcsH2210-2618 blocks the proteolytic activity of TMPRSS2ECD in a concentration-dependent manner. The relative fluorescence value (proportional to the proteolytic activity of TMPRSS2) generated from released AMC is shown in a bar chart. The concentration of the substrate, TMPRSS2ECD, and TcsH1832-2209 is 10 μM, 30 nM, and 2 μM, respectively. TcsH2210-2618 concentration gradient: 3.2 nM, 16 nM, 80 nM, 400 nM, and 2 μM. e TcsH2210-2618 mutants show the attenuated ability to block the proteolytic activity of TMPRSS2ECD. The concentrations of Boc-QAR-AMC, TMPRSS2ECD, and TcsH2210-2618 fragments are 10 μM, 30 nM, and 400 nM, respectively. In (d, e), data are shown as mean ± SD, n = 3 independent samples, two-tailed Student’s t test. Source data are provided as a Source Data file.
We then tested whether the binding of TcsH inhibits its proteolytic activity of TMPRSS2. A fluorogenic peptide Boc-Gln-Ala-Arg-7-amino-4-methylcoumarin (Boc-QAR-AMC) was used as a reporting substrate for the proteolytic activity of TMPRSS244. Active TMPRSS2 can cleave the peptide right after Arg thus the fluorescent signals are emitted (Fig. 5c). We showed that the proteolytic activity of TMPRSS2 could be well suppressed by the addition of TcsH2210-2618 (Fig. 5d) but not TcsH2210-2618 mutants lacking TMPRSS2-binding ability (Fig. 5e). These results suggest that the TcsH CROPs-derived fragments may serve as potent protease inhibitors specific for TMPRSS2.
We also tested three serine protease inhibitors of TMPRSS2, including Bromhexine, Nafamostat, and Avoralstat. Nafamostat modifies Ser441 on TMPRSS240, Bromhexine, and Avoralstat may also target the catalytic triad of TMPRSS2 in the same way45. However, all three chemicals failed to protect cells from TcsH (Supplementary Fig. 11b), likely because the modification moiety in the catalytic triad does not interfere with the toxin-receptor binding interface (Supplementary Fig. 11c).
TcsH mutant with impaired TMPRSS2 binding causes less epithelial damages
Lastly, we analyzed the toxicity of a TMPRSS2-binding defective mutant (TcsHAAR) in comparison with the WT toxin by injecting them into the ligated mouse colons. The dissected colon tissues were processed and stained using hematoxylin and eosin (H&E) to examine the toxin-induced damage in the intestinal epithelium. We observed that the WT TcsH induced overt epithelial damage to the colon tissue, resulting in inflammatory cell infiltration, epithelial disruption, and hemorrhage. TcsHAAR showed attenuated potency and caused greatly reduced epithelial disruption and hemorrhage to the colonic epithelium (Fig. 6). These results further confirmed our TMPRSS2-TcsH structure as well as the role of TMPRSS2 in TcsH-induced colonic epithelial lesions.
a H&E-stained mouse colonic tissue sections harvested after colon-loop ligation assays. Representative images are shown. The scale bar represents 200 μm. b Histological scores for (a) were assessed based on inflammatory cell infiltration, hemorrhage, epithelial disruption, and overall. N = 6 mice per group, error bars indicate mean ± SEM, Mann–Whitney test. Source data are provided as a Source Data file.
Discussion
The host receptor is a primary determinant of the specificity and efficacy of a toxin that further affects the manifestations of related bacterial infection-associated diseases. Recently, TMPRSS2 and fucosylated glycans were identified as cellular receptors for TcsH, a major toxin produced by P. sordellii36. Here, we report the cryo-EM structures of the full-length TcsH and the partial CROPs in complex with the ectodomain of TMPRSS2, revealing the underlying mechanism of host recognition by this notorious toxin (Supplementary Fig. 12).
We show that TcsH binds to TMPRSS2 through the end of the CROPs domain, or more precisely the CROP unit-VI. Whereas the CROPs domains of LCTs are generally thought to bind sugar moieties on the cell surface, TMPRSS2 is the first protein receptor reported that solely binds to the CROPs in this toxin family. Each LCT recognizes distinct host receptors, while two types of protein receptor-binding interfaces were well-characterized before. The first one is located at the convex edge of the DRBD, which is thought to be evolutionarily developed for receptor recognition46. Several LCT receptors bind to this region, including Frizzled proteins (FZDs) for TcdB1 and TcdB327,47, Semaphorin 6A and 6B (SEMA6A/6B) for TcsL28,48, and tissue factor pathway inhibitor (TFPI) for TcdB2 and TcdB425,49. The second interface lies in an area where CPD, DRBD, and CROPs converge; chondroitin sulfate proteoglycan 4 (CSPG4) for TcdB is the only reported receptor that binds to this region29,50. Our finding of the C-terminus of CROPs as a new protein receptor-binding region for LCTs is intriguing but also puzzling: while a distinct receptor-binding domain can usually be defined in most toxins, LCTs seem to have various receptor-binding interfaces/regions. Nevertheless, the presence of multiple receptor-binding regions potentiates LCTs to explore a broader spectrum of host recognition.
Recent studies have resolved the high-resolution structures of full-length TcdB22,24,25, full-length TcsL26, and near-complete TcdA20,21. Despite these important achievements, architectural insights into other LCT members are still lacking. Our current structure of the TcsH-TMPRSS2 complex provides a snapshot of TcsH. Since the TMPRSS2 binding site in TcsH is far away from the core domains and would unlikely cause conformational change to the toxin, we believe that it could represent the structure of native TcsH at neutral pH. The overall structure and conformation of TcsH resemble that of TcdA, indicating the evolutionarily close relationship and homology between these two toxins. TcdA and TcsH contain the longest CROPs among LCTs for unknown reasons. Notably, the reported TcdA structures miss the C-terminal part of the CROPs20,21, likely due to the high flexibility. Hence, the structure of complete TcsH CROPs may be helpful to study the architecture and orientation of the TcdA CROPs domain. The C-terminal regions beyond the CROP unit-IV hang freely in the full-length structures of TcdA and TcsH, which seem to be functionally mysterious. Our structural study now provides a rational explanation for these extra-long CROPs: the extra CROP units may serve as variable platforms to explore host recognition. Besides, it would be interesting to inspect whether TcdA also adopts these CROP units to bind uncharacterized receptor(s).
TMPRSS2 recognizes TcsH via multiple loops on the top surface of the SPD, which are also responsible for the recognition of its intrinsic substrates. These loops, including LA, LB, LE, L2, and L3, are highly variable among the TTSP family members51, explaining why TMPRSS2 is specifically recognized by TcsH. On the other hand, the CROPs of LCTs are also less conserved, implying that this toxin-receptor recognition might still undergo rapid evolutionary adaptation. TMPRSS2 is mainly expressed in the epithelium of the prostate, gastrointestinal tracts, kidney, and pancreas39,52. Dysregulated TMPRSS2 activity is related to the proliferation, invasiveness, and metastasis of prostate tumor cells53,54. TMPRSS2 is also well-known for facilitating the cellular entry of several viruses, such as influenza viruses and coronaviruses, through cleaving glycoproteins on the viral envelope to activate membrane fusion55,56,57,58. We demonstrate that the TcsH CROP unit-VI tightly binds to the SPD of TMPRSS2 with sub-nanomolar affinity, masks the catalytic pocket in SPD, and inhibits the enzymatic activity of TMPRSS2. Particularly, the CROP unit-VI exhibits a straightforward and well-organized structural configuration, making it amenable to modifications and rational engineering. Therefore, we also propose that the TcsH CROP unit-VI could be a potential precursor to develop anti-cancer and anti-viral mediations. Taken together, our study on the molecular basis of recognition between TcsH and its receptor TMPRSS2 would help to understand the bacterial pathogenesis, portray the host-pathogen coevolution, unveil the vulnerability of the devastating toxin, and provide potential therapeutic avenues for the related diseases.
Methods
Ethics statement
All animal procedures reported herein were performed following the institutional guidelines and approved by the Institutional Animal Care and Use Committee at Westlake University (IACUC Protocol #22-018-2-TL). To minimize the distress and pain, the mice injected with toxins were monitored every hour. Animals with signs of pain or distress such as labored breathing, inability to move after gentle stimulation, or disorientation were euthanized immediately. This method was approved by the IACUC and monitored by a qualified veterinarian.
Cell lines and antibodies
MCF-7 (HTB-22) cells were originally obtained from ATCC and Expi293F cells were purchased from ThermoFisher Scientific (U.S.). MCF-7 TMPRSS2‒/‒ and GMDS‒/‒ cells were previously generated laboratory stocks36. Expi293F cells were cultured in SMM 293-T II Expression Medium (Sino Biological, Beijing, China) under 95% air and 5% CO2 in a Multitron-Pro shaker (Infors) at 37 °C. MCF-7 cells were cultured in DMEM media plus 10% fetal bovine serum and 1% penicillin-streptomycin in a humidified atmosphere of 95% air and 5% CO2 at 37 °C.
The following antibodies were purchased from commercial vendors: mouse monoclonal anti-6×His tag antibody (Proteintech, 66005-1-Ig, 1:5000), goat monoclonal anti-human IgG-Fc antibody (Sino Biological, SSA001, 1:10000), and horseradish peroxidase-labeled goat monoclonal anti-mouse IgG antibody (H + L, PI-1000, Vector Labs, 1:10000).
Mice
C57BL/6 mice were purchased from the Laboratory Animal Resources Center at Westlake University (Hangzhou, China). Female, 6–8 weeks C57BL/6 mice were used in this study. Mice were housed in specific-pathogen-free micro-isolator cages with free access to drinking water and food and monitored under the care of full-time staff. All mice had a 12-h cycle of light/darkness (7 a.m. to 7 p.m.), housed at 20–24 °C with 40-60% humidity.
Cloning of DNA constructs and site-directed mutagenesis
DNA encoding full-length of TcsH (reference sequence: P. sordellii 9048) was codon-optimized, synthesized by Genscript (Nanjing, China), and inserted into pHT01 via the sites of PstI/XbaI with an additional C-terminal His-tag. The DNA fragments encoding TcsH1832-2246, TcsH2236-2618, TcsH1832-2209, and TcsH2210–2618 were PCR amplified and inserted into pET28a with a His-tag at the C-terminus. For cryo-EM sample preparation, TcsH2236-2618 was cloned into a pET21a vector with a His-MBP tag introduced at the N-terminus. The gene encoding the ectodomain of TMPRSS2 (amino acids 106-492) was PCR amplified from the cDNA library and inserted into the AgeI/XhoI sites of a pHLsec vector with an Fc-His, or His-tag fused to their N-terminus. The point mutations of TMPRSS2 and TcsH were generated using QuickChange II Site-Directed Mutagenesis Kit (Agilent Technologies, 200523) or Q5 Site-Directed Mutagenesis Kit (New England Biolabs, E0554S) following the manufacturer’s protocol.
Expression and purification of recombinant proteins
The recombinant full-length TcsH was expressed in Bacillus subtilis SL401 as described previously59. The fresh transformants were inoculated into 5 mL 2×YT medium supplemented with 50 µg/mL chloramphenicol and allowed to grow overnight at 37 °C. The overnight culture was transferred to one liter of 2×YT medium containing 50 µg/mL chloramphenicol. The culture was grown at 37 °C until an OD600 of 0.8, then the temperature was set up to 16 °C and the cell culture was induced with 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) for 36 h. Cells were thawed and resuspended in lysis buffer (50 mM Tris-HCl, pH 7.4, 300 mM NaCl, 10% glycerol, 5 mM imidazole) supplemented with 0.5 mg/mL lysozyme (Macklin, L6051), 0.5% Triton X-100 (Sigma, T9284), 5 units/mL DNAse I (Biorigin, BN20219), and 1 µg/mL RNAse A (ZF-50-0002, Multi Sciences). After rocking for 2 hours at room temperature, the insoluble fraction was removed by centrifugation at 20,000 g for 1 hour at 4 °C. The soluble fraction was applied to TALON superflow metal affinity resin (Takara, 635507) and washed with lysis buffer supplemented with 10 mM imidazole (Vetec, V900153). The protein was eluted with a buffer of 20 mM Tris-HCl, pH 7.4, 150 mM NaCl (Buffer A), and 100 mM imidazole. Then, the protein was further purified by size-exclusion chromatography on a Superdex 200 increase 10/300 GL column (GE Healthcare) in Buffer A. Protein fractions were confirmed by SDS-PAGE, concentrated, and stored at −80 °C.
TcsH CROPs fragments were expressed in E. coli BL21(DE3) and purified as His- or MBP-tagged proteins. E. coli was grown in the Luria-Bertani medium containing 50 µg/mL kanamycin and the protein expression was induced with 0.5 mM IPTG at 16 °C for 16 hours. Bacterial cells were collected by centrifugation, resuspended in Buffer A supplemented with protease inhibitors, and then lysed by passing through an LM20 microfluidizer (Microfluidics) once. Cell lysates were subjected to high-speed centrifugation at 12,000 × g for 30 minutes to remove unbroken cells and debris. His-tagged TcsH1832-2246, TcsH2236-2618, TcsH1832-2209, and TcsH2210–2618 were purified by Ni-affinity chromatography. MBP-tagged TcsH2236-2618 was purified using Amylose resin (NEB, E8021L) followed by size-exclusion chromatography on a Superdex 200 increase 10/300 GL column (GE Healthcare). Protein fractions were confirmed by SDS-PAGE, concentrated, and stored at −80 °C.
Recombinant TMPRSS2ECD and its variants were expressed in Expi293F cells and purified as His-tagged proteins. Expi293F cells were transfected with the plasmids when the cell density reached 2 × 106 cells/mL. Approximately 2 mg of plasmid was preincubated with 4 mg of polyethyleneimine (Polysciences) in 50 mL of fresh medium for 15 minutes. The mixture was added to one liter of cell culture for transfection. After 72 hours, the cell pellets were removed by centrifugation at 4000 × g for 10 minutes at 4 °C. The supernatant was collected and concentrated to approximately 200 mL using 10 kDa filters (Sartorius Stedim) and then loaded to a gravity flow column packed with TALON superflow metal affinity resin. The resin was washed extensively with Buffer A supplemented with 10 mM imidazole and the protein was eluted with Buffer A supplemented with 100 mM imidazole. The eluted protein was concentrated and further applied to size-exclusion chromatography on a Superdex 200 increase 10/300 GL column (GE Healthcare) equilibrated with Buffer A supplemented with 10% glycerol. The target peak fractions were confirmed by SDS-PAGE, then concentrated, and stored at −80 °C.
Biolayer interferometry (BLI) assay
The binding affinities between toxin fragments and TMPRSS2 proteins were measured using the BLI assay with the Octet RED96e system and analyzed with the Octet Data Analysis software (version 12.0.1.2, ForteBio, Fremont, CA, USA). Briefly, Fc-tagged proteins were immobilized onto the capture biosensors (AHC biosensor, ForteBio) and balanced with binding buffer (20 mM Tris-HCl pH 7.4, 150 mM NaCl). The biosensors were then exposed to the indicated concentrations of MBP-TcsH2236-2618 or TcsH2210-2618, followed by dissociation in the binding buffer.
Pull-down assays
Pull-down assays were performed using Protein A agarose beads (Thermo Fisher Scientific). Briefly, Fc-tagged TMPRSS2ECD (50 nM) were mixed with TcsH2210-2618 or TcsH1832-2209 (10 nM) in 300 μL of binding buffer (20 mM Tris-HCl pH 7.4, 150 mM NaCl). The mixtures were incubated at 4 °C for 2 hours and co-precipitated by Protein A agarose beads. Beads were washed, pelleted, boiled in SDS sample buffer, and subjected to SDS-PAGE or immunoblot analysis.
Assembly of the TcsH-TMPRSS2 complex
To assemble the TcsH-TMPRSS2ECD and TcsH2236-2618-TMPRSS2ECD complexes, purified full-length TcsH or TcsH2236-2618 were incubated with TMPRSS2ECD in the presence of 10% glycerol on ice for one hour in molar ratios of 1:5 and 1:1, respectively. These protein complexes were further purified by size-exclusion chromatography on a Superdex 200 increase 10/300 GL column (GE Healthcare) in a buffer containing 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, and 2% glycerol. Protein fractions were confirmed by SDS-PAGE. Peak fractions containing the TcsH-TMPRSS2ECD complex and the TcsH2236-2618-TMPRSS2ECD complex were pooled and concentrated to 5 mg/mL and 1 mg/mL for cryo-EM analysis.
Cryo-EM specimen preparation and data acquisition
For cryo-EM sample preparation, three microliters of each protein were placed on glow-discharged holey carbon grids (Quantifoil Au R2.1/3.1, 300 mesh). The grid was blotted with filter paper for 3.5 s in a chamber set with 100% humidity at 8 °C to remove the excess sample and then plunge-frozen in liquid ethane cooled by liquid nitrogen with the Vitrobot Mark IV system (ThermoFisher Scientific). Cryo-EM specimens were imaged on a 300-kV Titan Krios electron microscope (ThermoFisher Scientific) using a normal magnification of 81,000 rpm. Movies were recorded using a Gatan K3 detector equipped with a GIF Quantum energy filter (slit width 20 eV) at the super-resolution mode, with a physical pixel size of 1.087 Å. Each stack of 32 frames was exposed for 2.56 s, with a dose rate of ∼23 counts/second/physical-pixel (∼19.5 e-/second/Å2) for each frame using EPU (ThermoFisher Scientific). All 32 frames in each stack were aligned and summed using the whole-image motion correction program MotionCor260 and binned to a pixel size of 1.087 Å. The defocus value for each image varied from −1.5 to −2.0 μm and was determined by Gctf61.
Cryo-EM data processing
For the TcsH-TMPRSS2ECD complex, a total of 1300 micrographs were collected, of which 1,118 micrographs were selected for further processing. All the processing steps were carried out in cryoSPARC62 except that especially mentioned. A total of 879,891 particles were extracted with 2× binning (pixel size: 2.174 Å) (Round 1) and subjected to multiple two-dimensional (2D) classifications, resulting in 225,500 good particles. These particles were further classified using Heterogeneous refinement. 148,184 particles from the good class were then re-extracted (pixel size: 1.087 Å) (Round 2) for further 2D classification and Non-uniform refinement, resulting in a final EM reconstruction for the TcsH-TMPRSS2ECD complex at 3.21 Å from 87,378 particles. The 3.21-Å EM density map displays clear features for amino acid side chains in the core region of TcsH. These particles were further locally refined using a soft mask around the C-terminal TcsH and TMPRSS2, which yielded a reconstruction at an average resolution of 5.95 Å.
For the TcsH2236-2618-TMPRSS2ECD complex, a total of 3,341 micrographs were collected. A total of 2× binned 8,005,902 particles (pixel size: 2.174 Å) were extracted and applied to multiple rounds of 2D classifications, resulting in 1,975,699 good particles. Followed by Heterogeneous refinement, 887,779 good particles were selected. Further 2D classifications of re-extracted particles (pixel size: 1.087 Å) yielded 760,140 particles. These particles further go through Non-uniform refinement and Local refinement, which generated a reconstruction of the TcsH2236-2618-TMPRSS2ECD complex at an average resolution of 2.95 Å.
The reported resolutions were calculated based on the gold-standard Fourier shell correlation (FSC) = 0.143 criteria. Local resolution volumes were estimated using cryoSPARC. The angular distributions of the particles used for the final reconstructions are reasonable. The workflows of cryo-EM data processing are illustrated in Supplementary Fig. 3, 5.
Model building and refinement
The cryo-EM structure of C. difficile toxin A (PDB code: 7POG) was used as a template to generate a homology model for TcsH using CHAINSAW63. The homology model was fitted into the cryo-EM map for the TcsH-TMPRSS2ECD complex using UCSF Chimera64. Manual adjustment of the model was performed in COOT65, followed by iterative rounds of real-space refinement in PHENIX66 and manual adjustment in COOT. Similarly, the crystal structure of the ectodomain of TMPRSS2 (PDB: 7MEQ) was fit into the cryo-EM map and manually adjusted in COOT. The structures were further validated through examination of the Molprobity scores and statistics of the Ramachandran plots. Molprobity scores were calculated as described67.
CD spectra analysis of full-length TcsH and mutants
For CD spectroscopy, the sample buffer of the toxin was changed to 10 mM potassium phosphate and 100 mM (NH4)2SO4 (pH 7.4). The protein sample was concentrated to a final concentration of 1 mg/ml for each measurement. CD spectra were recorded using a Chirascan V100 (Applied Photophysics Inc.) in the wavelength range of 190 to 260 nm, with a bandwidth of 1.0 nm and scan step of 0.5 nm using a 0.05-cm path length quartz cuvette at 18 °C. In each case, three spectra were collected, averaged, baseline-corrected, smoothed, and converted with the Chirascan software.
Cell surface binding assay
MCF-7 GMDS‒/‒ cells were incubated with 10 nM WT or mutant GFP-TcsH2210–2618 in the medium on ice for 20 min. Cells were washed three times with ice-cold PBS, fixed with 4% paraformaldehyde (PFA) for 15 minutes at room temperature, and stained with DAPI, followed by fluorescence microscopy. Fluorescent images were captured using an Olympus FV3000-BX63 LSCM Confocal System with the software FV31S-SW v2.3.2.169.
The cytopathic cell-rounding assay
The cytopathic effect of the toxin was analyzed using the cell-rounding assay. Cells were exposed to toxins for 12 hours. The phase-contrast images of the cells were captured by a microscope (Olympus IX73; ×10 objectives) with the software Olympus CellsSens Standard 2.1. Six zones of 200 μm × 200 μm were selected randomly, with each zone containing ~50–150 cells. Round-shaped and normal-shaped cells were counted manually. The percentage of round-shaped cells was analyzed using GraphPad Prism (ver. 9.0.0, GraphPad Software, LLC).
TMPRSS2 enzymatic activity assay
Peptide substrate N-tert-butoxycarbonyl-Gln-Ala-Arg-7-amino-methylcourarin (Boc-QAR-AMC, Bachem, catalog no. I-1550) was diluted into assay buffer (20 mM Tris-HCl, 150 mM NaCl, 0.1% Triton X-100, pH 8.0) to a final concentration of 10 μM. TcsH2210-2618, TcsH1832-2209, TcsH2210-2618/Q2520A/F2521A, TcsH2210-2618/E2544R, TcsH2210-2618/F2557N/L2558N, and/or TMPRSS2ECD of indicated concentrations were added into the reaction system with a final volume of 120 μL. After incubation for 2 hours at room temperature, 100 μL of the mixtures were dispensed into 96-well plates (WHB Scientific) and read on a microplate reader (Thermo Varioskan LUX) with fluorescence module at 340 nm excitation and 440 nm emission.
Colon-loop ligation assay
Six- to eight-week-old female mice were anesthetized by intraperitoneal injection of 1% pentobarbital sodium. A midline-right laparotomy was performed to locate the ascending colon and seal a ~ 2 cm loop with 4–0 surgical suture ligatures. Six micrograms of TcsH or TcsHAAR in 100 μL of normal saline or 100 μL of saline alone was injected into the sealed colon segment using an insulin syringe, followed by suturing of the skin incision. Mice were allowed to recover in the 37 °C thermostatic plates. After 8 hours, mice were euthanized, and the ligated colon segments were excised. The colon segments were fixed, paraffin-embedded, sectioned, and subjected to either H&E staining for histological scoring.
H&E staining and histopathological analysis
Mouse colon specimens were fixed with 4% formaldehyde for 12 hours before dehydration with gradient alcohol. The samples were then cleared with xylene, embedded in paraffin, and cut into 5 μm thick sections. The tissue sections were stained with H&E. The H&E stains were scored blinded by a pathologist based on inflammatory cell infiltration, hemorrhage, and epithelium disruption on a scale of 0 to 3 (mild to severe).
Statistics and reproducibility
Data are presented as mean ± standard deviation (SD) for biochemical experiments and mean ± standard error of the mean (SEM) for pathological experiments. The number of the sample size (n) and statistical hypothesis testing method are described in the legends of the corresponding figures. Statistical analyses of data were performed with GraphPad Prism v9.3 or OriginPro v8.5. Experiments in Fig. 1f, Fig. 4a, b, Fig. 5d, e, and Supplementary Fig. 10a, b have been repeated at least twice with similar results.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The atomic coordinates for the TcsH-TMPRSS2ECD complex and TcsH2236-2618-TMPRSS2ECD complex have been deposited in the Protein Data Bank (PDB) under the accession code 8JHZ and 8JI0, respectively. The EM maps of the TcsH-TMPRSS2ECD complex and TcsH2236-2618-TMPRSS2ECD complex have been deposited in the Electron Microscopy Data Bank (EMDB) with the accession codes EMD-36301, and EMD-36303, respectively. The local EM map of the C-terminal part of the TcsH-TMPRSS2ECD complex has been deposited in the EMDB with accession code EMD-36302. All other data are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Vidor, C., Awad, M. & Lyras, D. Antibiotic resistance, virulence factors and genetics of Clostridium sordellii. Res. Microbiol. 166, 368–374 (2015).
Aronoff, D. M. & Marrazzo, J. M. Infections caused by Clostridium perfringens and Paeniclostridium sordellii after unsafe abortion. Lancet Infect. Dis. 23, e48–e55 (2023).
Aldape, M. J., Bryant, A. E. & Stevens, D. L. Clostridium sordellii infection: epidemiology, clinical findings, and current perspectives on diagnosis and treatment. Clin. Infect. Dis. 43, 1436–1446 (2006).
Fischer, M. et al. Fatal toxic shock syndrome associated with Clostridium sordellii after medical abortion. N. Engl. J. Med. 353, 2352–2360 (2005).
Arseculeratne, S. N., Panabokke, R. G. & Wijesundera, S. The toxins responsible for the lesions of Clostridium sordelli gas. gangrene. J. Med. Microbiol. 2, 37–53 (1969).
Popoff, M. R. & Bouvet, P. Clostridial toxins. Future Microbiol 4, 1021–1064 (2009).
Orrell, K. E. & Melnyk, R. A. Large clostridial toxins: Mechanisms and roles in disease. Microbiol. Mol. Biol. Rev. 85, e0006421 (2021).
Jank, T. & Aktories, K. Structure and mode of action of clostridial glucosylating toxins: the ABCD model. Trends Microbiol 16, 222–229 (2008).
Chandrasekaran, R. & Lacy, D. B. The role of toxins in Clostridium difficile infection. FEMS Microbiol. Rev. 41, 723–750 (2017).
Qa’Dan, M., Spyres, L. M. & Ballard, J. D. pH-induced conformational changes in Clostridium difficile toxin B. Infect. Immun. 68, 2470–2474 (2000).
Pfeifer, G. et al. Cellular uptake of Clostridium difficile toxin B. Translocation of the N-terminal catalytic domain into the cytosol of eukaryotic cells. J. Biol. Chem. 278, 44535–44541 (2003).
Reineke, J. et al. Autocatalytic cleavage of Clostridium difficile toxin B. Nature 446, 415–419 (2007).
Rupnik, M., Pabst, S., von Eichel-Streiber, C., Urlaub, H. & Soling, H. D. Characterization of the cleavage site and function of resulting cleavage fragments after limited proteolysis of Clostridium difficile toxin B (TcdB) by host cells. Microbiol. (Read.) 151, 199–208 (2005).
Egerer, M., Giesemann, T., Jank, T., Satchell, K. J. & Aktories, K. Auto-catalytic cleavage of Clostridium difficile toxins A and B depends on cysteine protease activity. J. Biol. Chem. 282, 25314–25321 (2007).
Egerer, M. & Satchell, K. J. Inositol hexakisphosphate-induced autoprocessing of large bacterial protein toxins. PLoS Pathog. 6, e1000942 (2010).
Giesemann, T., Egerer, M., Jank, T. & Aktories, K. Processing of Clostridium difficile toxins. J. Med. Microbiol. 57, 690–696 (2008).
Aktories, K., Schwan, C. & Jank, T. Clostridium difficile toxin biology. Annu. Rev. Microbiol. 71, 281–307 (2017).
von Eichel-Streiber, C. & Sauerborn, M. Clostridium difficile toxin A carries a C-terminal repetitive structure homologous to the carbohydrate binding region of streptococcal glycosyltransferases. Gene 96, 107–113 (1990).
Pruitt, R. N. & Lacy, D. B. Toward a structural understanding of Clostridium difficile toxins A and B. Front. Cell. Infect. Mi 2, 28 (2012).
Aminzadeh, A., Larsen, C. E., Boesen, T. & Jorgensen, R. High-resolution structure of native toxin A from Clostridioides difficile. EMBO Rep. 23, e53597 (2021).
Chen, B. et al. Structure and conformational dynamics of Clostridioides difficile toxin A. Life Sci. Alliance 5, e202201383 (2022).
Chen, P. et al. Structure of the full-length Clostridium difficile toxin B. Nat. Struct. Mol. Biol. 26, 712–719 (2019).
Simeon, R. et al. Selection and characterization of ultrahigh potency designed ankyrin repeat protein inhibitors of C. difficile toxin B. PLoS Biol. 17, e3000311 (2019).
Jiang, M. et al. Structural dynamics of receptor recognition and pH-induced dissociation of full-length Clostridioides difficile Toxin B. PLoS Biol. 20, e3001589 (2022).
Luo, J. et al. TFPI is a colonic crypt receptor for TcdB from hypervirulent clade 2 C. difficile. Cell 185, 980–994 (2022).
Zhou, Y. et al. Structural dynamics of the CROPs domain control stability and toxicity of Paeniclostridium sordellii lethal toxin. Nat. Commun. 14, 8426 (2023).
Chen, P. et al. Structural basis for recognition of frizzled proteins by Clostridium difficile toxin B. Science 360, 664–669 (2018).
Lee, H. et al. Recognition of semaphorin proteins by P. sordellii lethal toxin reveals principles of receptor specificity in clostridial toxins. Cell 182, 345–356 (2020).
Chen, P. et al. Structural basis for CSPG4 as a receptor for TcdB and a therapeutic target in Clostridioides difficile infection. Nat. Commun. 12, 3748 (2021).
Tao, L. et al. Sulfated glycosaminoglycans and low-density lipoprotein receptor contribute to Clostridium difficile toxin A entry into cells. Nat. Microbiol. 4, 1760–1769 (2019).
Schottelndreier, D., Langejurgen, A., Lindner, R. & Genth, H. Low density lipoprotein receptor-related protein-1 (LRP1) is involved in the uptake of Clostridioides difficile Toxin A and serves as an internalizing receptor. Front. Cell. Infect. Mi 10, 565465 (2020).
Zhou, Y. et al. Sulfated glycosaminoglycans and low-density lipoprotein receptor mediate the cellular entry of Clostridium novyi alpha-toxin. Cell Res 31, 935–938 (2021).
Zhou, Y. et al. LDLR, LRP1, and Megalin redundantly participate in the uptake of Clostridium novyi alpha-toxin. Commun Biol. 5, 906 (2022).
Schorch, B. et al. LRP1 is a receptor for Clostridium perfringens TpeL toxin indicating a two-receptor model of clostridial glycosylating toxins. Proc. Natl. Acad. Sci. USA 111, 6431–6436 (2014).
LaFrance, M. E. et al. Identification of an epithelial cell receptor responsible for Clostridium difficile TcdB-induced cytotoxicity. Proc. Natl. Acad. Sci. USA 112, 7073–7078 (2015).
Li, X. et al. Paeniclostridium sordellii hemorrhagic toxin targets TMPRSS2 to induce colonic epithelial lesions. Nat. Commun. 13, 4331 (2022).
Bugge, T. H., Antalis, T. M. & Wu, Q. Type II transmembrane serine proteases. J. Biol. Chem. 284, 23177–23181 (2009).
Ma, B., Simala-Grant, J. L. & Taylor, D. E. Fucosylation in prokaryotes and eukaryotes. Glycobiology 16, 158R–184R (2006).
Afar, D. E. et al. Catalytic cleavage of the androgen-regulated TMPRSS2 protease results in its secretion by prostate and prostate cancer epithelia. Cancer Res. 61, 1686–1692 (2001).
Fraser, B. J. et al. Structure and activity of human TMPRSS2 protease implicated in SARS-CoV-2 activation. Nat. Chem. Biol. 18, 963–971 (2022).
Perona, J. J. & Craik, C. S. Evolutionary divergence of substrate specificity within the chymotrypsin-like serine protease fold. J. Biol. Chem. 272, 29987–29990 (1997).
Ho, J. G., Greco, A., Rupnik, M. & Ng, K. K. Crystal structure of receptor-binding C-terminal repeats from Clostridium difficile toxin A. Proc. Natl Acad. Sci. USA 102, 18373–18378 (2005).
Chen, Y. W. et al. TMPRSS2, a serine protease expressed in the prostate on the apical surface of luminal epithelial cells and released into semen in prostasomes, is misregulated in prostate cancer cells. Am. J. Pathol. 176, 2986–2996 (2010).
Shrimp, J. H. et al. An Enzymatic TMPRSS2 Assay for Assessment of Clinical Candidates and Discovery of Inhibitors as Potential Treatment of COVID-19. ACS Pharmacol. Transl. Sci. 3, 997–1007 (2020).
Sonawane, K. D. et al. Structural insights and inhibition mechanism of TMPRSS2 by experimentally known inhibitors Camostat mesylate, Nafamostat and Bromhexine hydrochloride to control SARS-coronavirus-2: A molecular modeling approach. Inform. Med. Unlocked 24, 100597 (2021).
Aktories, K. From signal transduction to protein toxins-a narrative review about milestones on the research route of C. difficile toxins. Naunyn-Schmiedeberg’s Arch. Pharmacol. 396, 173–190 (2023).
Tao, L. et al. Frizzled proteins are colonic epithelial receptors for C. difficile toxin B. Nature 538, 350–355 (2016).
Tian, S. et al. Genome-Wide CRISPR Screen Identifies Semaphorin 6A and 6B as Receptors for Paeniclostridium sordellii Toxin TcsL. Cell Host Microbe 27, 782–792 (2020).
Tian, S. et al. Identification of TFPI as a receptor reveals recombination-driven receptor switching in Clostridioides difficile toxin B variants. Nat. Commun. 13, 6786 (2022).
Pan, Z. et al. Functional analyses of epidemic Clostridioides difficile toxin B variants reveal their divergence in utilizing receptors and inducing pathology. PLoS Pathog. 17, e1009197 (2021).
Forni, D., Sironi, M. & Cagliani, R. Evolutionary history of type II transmembrane serine proteases involved in viral priming. Hum. Genet. 141, 1705–1722 (2022).
Lin, B. et al. Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res 59, 4180–4184 (1999).
Bastus, N. C. et al. Androgen-induced TMPRSS2:ERG fusion in nonmalignant prostate epithelial cells. Cancer Res. 70, 9544–9548 (2010).
Ko, C. J. et al. Androgen-induced TMPRSS2 activates matriptase and promotes extracellular matrix degradation, prostate cancer cell invasion, tumor growth, and metastasis. Cancer Res. 75, 2949–2960 (2015).
Bottcher, E. et al. Proteolytic activation of influenza viruses by serine proteases TMPRSS2 and HAT from human airway epithelium. J. Virol. 80, 9896–9898 (2006).
Shirato, K., Kawase, M. & Matsuyama, S. Middle East respiratory syndrome coronavirus infection mediated by the transmembrane serine protease TMPRSS2. J. Virol. 87, 12552–12561 (2013).
Limburg, H. et al. TMPRSS2 is the major activating protease of influenza A virus in primary human airway cells and influenza B Virus in human type II pneumocytes. J. Virol. 93, e00649-19 (2019).
Hoffmann, M. et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280.e8 (2020).
Shen, E. et al. Subtyping analysis reveals new variants and accelerated evolution of Clostridioides difficile toxin B. Commun. Biol 3, 347 (2020).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: Real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: Algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Stein, N. CHAINSAW: a program for mutating pdb files used as templates in molecular replacement. J. Appl. Crystallogr. 41, 641–643 (2008).
Pettersen, E. F. et al. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. Biol. Crystallogr. 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. Biol. Crystallogr. 66, 213–221 (2010).
Davis, I. W. et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 35, W375–W383 (2007).
Acknowledgements
We would like to thank Dr. Yigong Shi for the guidance and discussion. We also appreciate the technical support from the Biomedical Research Core Facility, High-Performance Computing Center, and Laboratory Animal Resources Center at Westlake University. This study was partially supported by the National Key R&D Program of China (Grant no. 2023YFC2308403 to L.T.), “Pioneer” and “Leading Goose” R&D Program of Zhejiang (Grant no. 2024SSYS0029), the Zhejiang Provincial Natural Science Foundation of China (Grant no. LR20C010001 to L.T.), and the Westlake Center for Genome Editing (Program no. 21200000A992210 to L.T.). L.T. also acknowledges support from the Westlake Laboratory of Life Sciences and Biomedicine and the Westlake Education Foundation.
Author information
Authors and Affiliations
Contributions
L.T. conceived the project and designed the experiments. R.Z., L.H., and J.Z. performed microbiological, cell biological, and biochemical assays. L.H. performed animal experiments. R.Z., L.H., J.Z., X.Zhang, Y.L., and X.Zhan performed protein expression and purification, cryo-EM structure determination, and structural data analysis. R.Z., X.Zhan, and L.T. wrote the manuscript with input from all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Hideaki Tsuge, Rongsheng Jin and Steven Mileto for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, R., He, L., Zhang, J. et al. Molecular basis of TMPRSS2 recognition by Paeniclostridium sordellii hemorrhagic toxin. Nat Commun 15, 1976 (2024). https://doi.org/10.1038/s41467-024-46394-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-46394-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.