Abstract
The microplastic body burden of marine animals is often assumed to reflect levels of environmental contamination, yet variations in feeding ecology and regional trait expression could also affect a species’ risk of contaminant uptake. Here, we explore the global inventory of individual microplastic body burden for invertebrate species inhabiting marine sediments across 16 biogeographic provinces. We show that individual microplastic body burden in benthic invertebrates cannot be fully explained by absolute levels of microplastic contamination in the environment, because interspecific differences in behaviour and feeding ecology strongly determine microplastic uptake. Our analyses also indicate a degree of species-specific particle selectivity; likely associated with feeding biology. Highest microplastic burden occurs in the Yellow and Mediterranean Seas and, contrary to expectation, amongst omnivores, predators, and deposit feeders rather than suspension feeding species. Our findings highlight the inadequacy of microplastic uptake risk assessments based on inventories of environmental contamination alone, and the need to understand how species behaviour and trait expression covary with microplastic contamination.
Similar content being viewed by others
Introduction
Despite ambitious waste management strategies designed to reduce plastic litter1, it is anticipated that the introduction of plastic to the marine environment will continue to rise for decades2, exacerbating any effects that plastic pollution may have on species and ecosystems3,4. Like all particulate matter, the fate of marine microplastic (<5 mm) is to sink to, and accumulate on the seafloor5,6, a habitat that harbours high levels of biodiversity7,8. As these accumulations of microplastics comprise a complex mix of heterogeneous particles with a range of shapes, sizes, colours, polymers, and additives9,10 that match the size spectrum of typical prey items and food parcels11,12, they are bioaccessible to a range of benthic invertebrates13,14. Mean microplastic concentrations in soft sediment habitats can be close to (continental slope, 502 microplastic kg−1), or greatly exceed (hadal trenches, 2782 microplastics kg−1 15) estimated safe limits (540 microplastic particles kg−1 16), particularly in areas that accumulate organic matter17. However, the uptake (here defined as adhesion, entanglement, and/or ingestion) of microplastic by species is unlikely to be a universal function of absolute levels of microplastic contamination or inter-specific differences in body size18,19 because the way in which species interact with the sediment environment is highly dependent on taxonomic position13,20,21, feeding and foraging strategy and individual species behaviours13,20,22,23, all of which can be population dependent and modified by abiotic (nutrient enrichment24; flow25; temperature26) and/or biotic (e.g. predation27) circumstances28. Even closely related species can behave differently29, such that realised levels of microplastic uptake can be highly variable both within and between species30,31,32.
Despite growing knowledge of the ecological effects of microplastic3,33, assessment of the risks posed by exposure and uptake is hindered by major gaps in our understanding of when, how and which species are most likely to interact with microplastic10,34. Resolving species-microplastic interactions is fundamental to setting appropriate contamination thresholds35, designing innovative solutions and predicting the most likely ecological consequences of microplastic contamination, but the relationship between the functional role of species and microplastic contamination at regional to global scales remains unknown. Here, we combine a comprehensive global inventory (55 studies; 244 locations, 412 unique observations; 69.08˚N–73.49˚S, 171.15˚W–170.22˚E) of microplastic body burden in sediment-dwelling marine invertebrates, with taxonomic information, to investigate whether global patterns of microplastic burden are associated with species traits (size, habit, mobility, feeding type, and environmental positioning) and/or differ across 16 biogeographic provinces36,37. Our focus was to establish whether commonly used taxonomic considerations of risk provide consistent and relevant information that will reduce uncertainty in projecting which species are most at risk of microplastic exposure38,39. Given the diversity of feeding modes, we expected that gut morphologies and gut retention times in marine invertebrates40,41, as well as changes in species behaviour that depend on the biotic and/or environmental conditions they experience28,42,43 would be important in determining microplastic burden. We demonstrate that feeding mode (in particular predatory, omnivorous, and deposit-feeding organisms), rather than environmental microplastic loading, coupled with geographical location, determine invertebrate microplastic body burden.
Results
Global inventory of invertebrate microplastic burden
Our analysis reveals that records of microplastic burden are distributed across 16 biogeographical provinces, but that there are substantive gaps in spatial and seasonal coverage of invertebrate microplastic burden across all major oceans, and a global paucity of data beyond shelf depth (Fig. 1). The data showed a strong bias towards the Northern Hemisphere and, in particular, the coastal regions of North America, Europe and Southeast Asia. Remote locations, such as the Pacific, South Atlantic, Indian Ocean and the Poles, were either inadequately constrained or lack information. Nevertheless, taxonomic coverage within the 8353 sampled individuals was reasonable, representing 141 species across 6 phyla (Mollusca, 57.4%, n = 4799; Crustacea, 16%, n = 1338; Echinodermata, 10.5%, n = 880; Cnidaria, 4%, n = 338 Annelida, 9.4%, n = 786; Arthropoda, 2.5%, n = 212; Fig. 2).
(a) The global distribution includes 412 study locations (purple dots) that report the presence of microplastic in marine benthic invertebrates. Detailed panels of the North-East Atlantic and Mediterranean (b) and (c) South-East Asian regions show data spread. Colour shading depicts model100 predictions of microplastic particle distribution (number per km2; key located in China (Panel c)) and boundaries of biogeographical provinces (36,37 ocean boundary lines) are indicated. Maps were drawn using ArcGIS Desktop105 and country boundaries are provided by ESRI using data from Esri; Garmin International, Inc.; U.S. Central Intelligence Agency (The World Factbook); National Geographic Society.
The presence of microplastic was common (microplastic burden >0 in 93% observations) across all phyla, but highly variable (mean particles ind.−1 = 0, n = 29; mean particles ind.−1 = < 1, n = 121; mean particles ind.−1 = 1–294, mean 8.3 ± 1.70; median, 2.95, n = 262) (coefficient of variation: Mollusca, 124.22%; Crustacea, 307.30%; Echinodermata, 277.12%; Cnidaria, 74.99%; Annelida, 90.85%; Arthropoda, 160.27%), indicating that the risk of uptake to microplastic differs between individuals and between taxonomic groups. Within quality control guidelines (see methods and Supplementary Table 1), we found ~2230 microplastic particles, of which 51.4% was measured within Crustacea, followed by the Echinodermata (30%), Mollusca (9.2%), Annelida (6.8%), Arthropoda (1.5%) and Cnidaria (1.2%). The highest burden of microplastic was found in decapod crustaceans, Crangon affinis, collected from the South Yellow Sea, China (294 microplastics ind.−1), and Aristeus antennatus, from the NW Mediterranean Sea (164 microplastics ind.−1). The latter exclusively contained fibrous microplastic particles.
Patterns of invertebrate microplastic burden
To separate any effect of broad habitats and species lifestyles on microplastic body burden, whilst retaining sufficient discriminatory power, we used Spalding’s biogeographical province and Class.
Effects of biogeographical province
Invertebrate microplastic body burden differed with the biogeographic province (L-ratio = 138.359, d.f. 15, p < 0.0001; Fig. 3); individuals collected from the Cold Temperate Northwest Pacific (Yellow Sea [56 locations] and Japan Trench [1 location]) had the highest mean (±s.e.) individual microplastic body burden (25.41 ± 10.71 ind−1), followed by the Mediterranean Sea (8.18 ± 4.02 ind−1, 41 locations, incorporating the Eastern coastline of Spain across to the Turkish Aegean Sea), whilst individuals collected from Northern New Zealand (0.15 ± 0.15 ind−1, 2 locations) and Southern New Zealand (0.24 ± 10.71 ind−1, 14 locations) showed the lowest mean individual microplastic body burden.
Effects of Class
There were consistent differences in individual microplastic body burden between the four classes in our study that included more than 30 observations (L-ratio = 24.957, d.f. = 3, p < 0.0001; Fig. 3). The highest mean (±s.e.) individual microplastic body burden occurred in the Malacostraca (15.44 ± 5.96 ind−1), followed by the Bivalvia (2.88 ± 0.24 ind−1), Gastropoda (2.23 ± 0.41 ind−1), and Polychaeta (1.02 ± 0.17 ind−1).
Furthermore, when analysing the full dataset, save for the Malacostraca, the faunal classes identified to have significantly higher body burdens of plastic were the Asteroidea, Cephalopoda, Echinoidea, Holothuroidea, and Ophiuroidea (Fig. S1) which were also those underrepresented in the literature (<14 observations per Class). Indeed, of the 29 non-symbiont phyla that exist in marine systems44,45, only 5 (Mollusca, Echinodermata, Cnidaria, Crustacea, Annelida) are represented, constituting a significant knowledge gap.
Effects of species traits
We investigated the role of species traits in determining whether the patterns we observe in invertebrate microplastic body burden were constrained by taxon-specific physiological or morphological limitations imposed by phylogenetic history or by the functional role of individuals, irrespective of taxonomic identity. We found that individual microplastic body burden was dependent on feeding mode (F6,358 = 4.41, p < 0.001), but not position within the sediment (F2,358 = 1.19, p = 0.31), mobility (F5,358 = 0.69, p = 0.60), habit (F3,358 = 0.11, p = 0.97), or the wet weight of an organism (F1,358 = 0.59, p = 0.45). The highest mean (±s.e.) individual microplastic body burden occurred in omnivores (20.72 ± 12.42 ind.−1, n = 15) and predators (15.11 ± 5.57 ind.−1, n = 71), followed by herbivores (4.16 ± 1.03 ind.−1, n = 11), grazers (3.72 ± 0.7 ind.−1, n = 12), suspension feeders (2.80 ± 0.23 ind.−1, n = 197), deposit feeders (2.03 ± 0.41 ind.−1, n = 90), and scavengers (1.35 ± 0.31 ind.−1, n = 15) (Fig. 4). However, mean individual microplastic body burden was associated with some variance, often driven by species with disproportionately high microplastic body burdens, in particular, the predators (CV = 310%), omnivores (CV = 232%) and deposit feeders (CV = 194%), relative to the remaining groups (suspension feeders, 117%; scavengers, 89%; herbivores, 82% and grazers, 65.3%). Indeed, the highest observed individual microplastic body burden counts represented a limited number of species (omnivores, the decapod, Oregonia gracilis, from the Yellow Sea, 141 and 137 particles ind.−1; predators, the Crustaceans, Crangon affinis, 294 particles ind.−1 and Romaleon gibbosulum 158 particles ind.−1, the Echinoderms, Ophiura sarsii, 108 particles ind.−1 and Luidia quinarian, 134 particles ind.−1 and, from the Mediterranean off the coast of Barcelona, the Crustacean Aristeus antennatus, 164 particles ind.−1). Removal of these high counts from the analyses reduced mean individual microplastic body burden for the predators (3.26 ± 0.6 s.e. particles ind.−1) and omnivores (2.52 ± 0.76 s.e. particles ind.−1). In contrast, although there were only 15 records and 3 species (the amphipods, Hirondellea dubia, Hirondellea gigas, and Eurythenes gryllus), scavengers were found to have the least individual microplastic body burden (range of 0.9–3.3 particles ind.−1). Deposit feeders show high variance, despite a comparatively low mean (±s.e.) individual microplastic body burden (2.03 ± 0.41 ind.−1). For example, there were 6 species (2 molluscs and 4 crustacea) with no microplastic body burden, but also 5 observations of the Japanese sea cucumber (Apostichopus japonicus) with an individual microplastic body burden ranging from 6.08–24.2 microplastic particles ind.−1 (Fig. 4). Overall, there were consistent effects of feeding traits on individual microplastic body burden, but with varying degrees of confidence across taxonomic and functional groupings that most likely relate to differences in sampling effort.
Relationship between risk factors
Geographic location, Class, and feeding mode were correlated with, and are therefore, potential drivers for, individual microplastic body burden. Geographical location explained the most variance (11%), followed by Class and feeding mode, each explaining ~6% of the data. In addition, there was a significant interaction between feeding mode and province, feeding mode and Class, and province and Class.
Effects of microplastic shape, size and colour
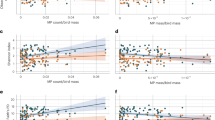
Most observations (n = 412) reported the shape (93%), size (90%), colour (57%) and polymer type (88%) of the recovered microplastic. Our results showed that fibres were the most commonly reported shape (99% of studies), followed by fragments (66%), pellets (32%), films (28%), spheres (15%) and other miscellaneous forms (foams and ‘other’, 5%) (Supplementary Table 2). The size of microplastics ranged from a 0.2 µm fragment (in the polychaete Hediste diversicolor, Tunisia) to a 17 cm fibre (in the decapod Nephrops norvegicus, Irish Sea). The most common colour was blue (28%), followed by clear (20%), black/grey (16%), white (14%), red (11%) and miscellaneous colours (greens, oranges, yellows and ‘other’, range 2–4%). The predominant polymers were cellulose (37%), polyethylene terephthalate (16%), polyethylene (12%), polyamide nylon (8%), polypropylene (7%) and 10 other polymers (range, 0.2–4%) (Supplementary Table 2). There were differences in the mean size ( ± s.e.) of microplastic particles between trophic groups (F5,224 = 2.35, p = 0.042; Fig. 5A), with herbivores retaining the largest microplastic particles (1362.6 ± 123.8 µm) followed by predators (1180.3 ± 160.1 µm), deposit feeders (840.5 ± 73.2 µm) and omnivores (799.3 ± 150.7 µm). There was no strong evidence that the size of microplastic related to individual body size (Pearson’s correlation: wet weight (g), r (226) = −0.05, p = 0.409, n = 214; body size (mm), r (212) = 0.29, p < 0.001, n = 214), indicating that species handle a range of microplastics (Fig. 5a). We also found differences in microplastic shape between trophic groups; predators and deposit feeders primarily retained fibres, whilst suspension feeders retain fibres and fragments (ratio of fibres:fragments; predators, 14.3:1; deposit feeders, 20.5:1; suspension feeders, 2.37:1; Fig. 5b).
(a) The mean (± s.e.) microplastic length for each feeding mode and (b) Principal Component Analysis microplastic shape demonstrating the affinity of feeding modes/Class to shape in marine benthic invertebrates. Overall, there were more fibres in all feeding modes, especially for Malacostracan predators, and a lesser influence of Class. Different colours in (a) are used only for illustrative purposes to indicate different species groups.
The first principal component (PC1) explained 86.6% of the variation in the data and has a strong association with fibres and fragments (Eigenvectors: 0.759 and −0.639, respectively), with PC2 explaining 12% driven predominantly by sphere data. Fragments, whilst found in 66% of observations comprised, on average, 17% ( ± 1.16%) of the individual microplastic body burden. Very few individuals were burdened with spheres (≤4%, ± 0.71%) of microplastics ingested), films (3%, ± 0.38%), pellets (2%, ± 0.15%), foams (0.1%, ± 0.02%), or’other’ shapes (0.01%, ± 0.01%) (Supplementary Table 2).
Discussion
Sea surface microplastic concentrations are known to vary by at least 6 orders of magnitude across the global ocean46, with the frequency of species-microplastic interactions often suggested (e.g.47,48), or explicitly linked to (e.g.47) these environmental levels. Our analyses of the global inventory of marine invertebrate microplastic body burden reveal that whilst geographical location contributed most to the likelihood of uptake for an organism, it only explains 11% of the data variability, with Class and feeding mode each explaining a further 6% of the data. This highlights the importance of local, yet undetermined, environmental correlates and/or the role of organism-sediment interactions in setting and influencing microplastic bioavailability49. Filter feeders are often presented as the functional group most likely to be at risk of microplastic ingestion50,51 due to the high volumes of water they process and the indiscriminate nature of filter feeding52. Such evidence, however, is based on a limited range of species and/or polymer types23,53,54. Further, there is a growing body of evidence to suggest that the sorting and rejection of particles of specific size ranges or polymers by filter-feeding bivalves may skew uptake data towards the preferred particle size for a given species55,56, and experimental designs do not always consider the natural setting or the organisms being tested57,58,59. Our analyses applied to the full global dataset do not support the view that filter feeders are more prone to microplastic uptake than other species, likely due to mechanistic differences between species that alter microplastic capture and retention rates relative to other groups60.
Compared to other groups, omnivores, predators and deposit feeders had much higher body burdens of microplastic. It is reasonable to consider therefore, that the bioaccumulation of microplastic is associated with predatory and omnivorous lifestyles61. Evidence for bioaccumulation of microplastic is not well supported empirically, with very little additional evidence for the translocation of microplastics into tissues; a pre-requisite for bioaccumulation62. Previous laboratory-based work has suggested that lower trophic level benthic organisms are at greater risk from microplastic exposure30, but the higher body burdens in secondary consumers identified here suggest the contrary. Our findings give credence to the view that more subtle functional trait descriptors63 are required to explain the mechanistic processes that determine uptake. Whilst the full risks of accumulating microplastics remain to be determined, elevated microplastic body burden remains an important measure of vulnerability30,31,64. Consequently, whilst changes in environmental microplastic contamination levels will have cascading effects on the microplastic body burden of some species, the relative uptake of microplastic will reflect the functional role and feeding modes of species within a community.
Recommendations for environmental monitoring of marine microplastics often suggest that particles should be measured within defined size categories and be classified according to shape and polymer type as a key component of assessing the risk that they pose to wildlife9,65,66. The relevance of these particle characteristics for risk assessment has been well demonstrated within controlled laboratory ecotoxicology studies (e.g.10,67,68,69). Here, our analyses revealed that the bulk of microplastic particles present within benthic invertebrate species sampled globally were fibres. High numbers of fibres are consistent with the seawater and sediment microplastic data for coastal settings15 but, acknowledging that positive70 or negative71 detection bias cannot be ruled out, these findings do lend further credence to the view that certain lifestyle characteristics can be important in determining uptake, particularly if morphology and shape of a microplastic particle alters the potential to be retained within an individual relative to other shapes through selective feeding or differences in particle handling. An alternative explanation may relate to gut morphology and the structural and functional complexity of the microplastic particle. Species with differentiated or much-folded digestive anatomy, such as crustacean amphipods and decapods18,72,73,74, were strongly associated with an increased body burden of microplastic fibres, indicating that fibrous material becomes entangled and/or concentrated in morphologically complex anatomical features (Fig. 5b)74,75,76,77,78. The gastric mill in crustacea, in particular, can act to shred fibres causing entanglement, leading to higher levels of burden73,79. Retention of microplastic, however, also depends on the ability to excrete microplastics80,81. Gut transit times vary due to the presence and quality of food (e.g. in sea urchins)82, the size of an organism (food takes longer to transit a longer gut)83, as well as the complexity of an organism’s gut morphology84.
There is a weight of evidence from laboratory ecotoxicology studies that interactions with microplastics can impair survival, development, reproduction, growth and feeding3,30,31,85 and alter biogeochemical processes in the sediment86,87. Hence, understanding which, and when, organisms may be at greater risk from microplastic exposure will help with modelling the ecosystem consequences of microplastic contamination into the future. The lack of correlation between individual microplastic body burden and environmental position (demersal, infaunal, and epifaunal) aligns with findings reported elsewhere (reviewed in Bour et al.20), and suggests that more subtle processes, such as, food availability88,89, biofilm formation90,91, and interactions with species (predator-prey49) or the sedimentary habitat, determine microplastic uptake14,92. Few studies (7% of those considered here) reported microplastic body burden alongside environmental contamination levels, so partitioning between organisms and their environment is not possible. Further, the paucity of studies that measure the excretion rates of organisms limits our understanding of net exposure (the number of particles passing through an organism over time) that organisms experience in their natural settings.
This analysis emphasises significant gaps in knowledge about the distribution of microplastic. Completion of a global inventory of microplastic contamination will require the development of more rapid, high throughput methods combined with models that incorporate environmental correlates and important aspects of species behaviour that alter the likelihood of microplastic uptake. Here, we propose a hierarchy for prioritising future research, identifying species groups most at risk from enhanced microplastic body burdens. First, a list of species should be identified for the geographical region of interest, after which taxonomic screening could be used to target those species most at risk, guided by the outcomes of this analysis. Combining these data with faunal behaviour, environmental context, and physiological factors, such as excretion rate, will hasten understanding of the mechanistic processes that determine net uptake in marine benthic invertebrates. Only by adopting a holistic view of ingestion, retention and/or excretion mechanisms that considers individual particle toxicity9, species-specific sensitivity to microplastic and associated contaminants16, and the likelihood of exposure, will progress be made in determining the true extent of bioaccessibility and, ultimately, the risk of microplastics.
Methods
We adopted the PICO method93 to develop a search strategy for our analysis. Briefly, we used the Clarivate Web of Science (https://www.webofknowledge.com/) to source peer-reviewed articles that contained measurements of microplastic body burden using specific search terms (listed in Supplementary Note 1).
Eligibility criteria
To be included in our quantitative synthesis, each study had to meet the following criteria: (1) an empirical study focussing on marine associated and benthic dwelling invertebrate species; (2) the focus was on microplastic exposure; (3) the organisms must be field collected organisms rather than laboratory studies; (4) the study must report particles per individual or particles per gram of wet weight of tissue; (5) evidence of quality assurances such as contamination control and spectroscopic confirmation of plastic presence were required. For more detail on the PICO exercise and our inclusion and exclusion criteria see Supplementary Table 1.
Data curation and manipulation
Due to the wide range of reporting metrics and styles, several data manipulations were necessary for the data recovered from our search, and additional data were curated to complete the dataset, detailed as follows. The taxonomy for all species was aligned to current accepted status using the World Register of Marine Species ‘Match Taxa’ utility (https://www.marinespecies.org/). Sampling locations (latitude/longitude) were taken from the manuscripts and when not provided, the location of the samples was approximated using Google Maps94 utilising the available information (descriptions and maps) in the manuscript. When small numbers of individuals of a species in close proximity were reported, or the reporting of data in the manuscript represented a total population, study sites were pooled and the data averaged.
As the biomass of an individual does not necessarily reflect an organism’s functional biology95,96 (Fig. S2), or correlate with microplastic body burden18,19 (Fig. S3), we express microplastic burden as microplastic particles per individual (MP ind−1) and we treat biomass as a trait. This avoids standardising microplastic burden by whole organism biomass, which assumes all biomass contributes to microplastic uptake or retention, and allows us to test for an overall effect of biomass in our analyses. Individual microplastic body burden was derived from the mean number of reported microplastics for each species record, regardless of the number of individuals reported in each observation (range, 1 – 481 individuals).
The primary data needed for our analyses was the body burden of microplastic particles. In the (micro)plastic literature, ingested particles are generally reported as MP ind−1 or items gram tissue−1 (wet weight). In cases where only items per gram of wet weight was recorded, the authors were contacted for these data and, if not available, the numbers per individual were calculated using the average items per gram and multiplying by the wet weight of the species. Some studies reported MP ind−1for only those organisms that had ingested plastic. As this is a poor representation of the data, these findings were adjusted to MP ind−1 for the total population sampled. Ash-free dry weight or dry weight were converted to wet weight using published conversion factors97, following consultation with authors, or were inferred from the wider literature using, where possible, data from the same geographic region and family level.
All microplastic sizes were converted to micrometers and minimum, maximum and mean values were collected where reported. Where ranges were reported the median value was recorded and where percentages, size bins, or less than/more than values were reported these size data were excluded.
Microplastic colours and polymer types were all converted to the percentage contribution to the total amount of plastic recovered due to the variety of reporting methods. When reporting colours in groups, these were divided up to an equal representation. For instance, when reporting ‘red, blue and green particles made up 30% of the total’ each colour was scored as 10%.
Where data were not presented, or were not provided by authors, data were extracted from figures by A.P. using online software WebPlotDigitizer98.
Trait attribution
Trait categories most likely to influence the likelihood of microplastic ingestion by benthic invertebrate species were selected using the BIOTIC framework produced by The Marine Life Information Network for Britain and Ireland99. Specifically, we used the categories weight (size), environmental position, feeding mechanism, mobility, and habit.
Estimating levels of environmental contamination
Contamination of the sediment was rarely (12/55 studies) measured at the same time as sampling the organisms. We used a global ocean surface microplastic contamination model100 as a predictor of microplastic contamination of the benthic realm. The model is designed for sea surface contamination, but as all our records were taken in shelf sea regions, we assume here that surface contamination is reflective of the contamination levels of the seabed101 as the sinking rate of particles in this model are consistent with observations of microplastic sinking rates102,103,104. The average level of contamination in particles km−2 was calculated for all points falling within 250 km of a record using the van Sebille model100 (1° × 1° resolution) and using ArcGIS Desktop105 to help smooth the interpolated model, although we acknowledge that high levels of spatial heterogeneity can occur106 due to horizontal separation of particles associated with biogeographical context107,108, and particle size class109, density104, and morphology102,110.
We ran linear models to investigate the relationships between the number of microplastics individual−1 and the mean microplastic contamination predicted by the Van Sebille model within a 250 km radius of the sampled organism record, Longhurst Provinces, Spalding’s Ecoregions (using realms and provinces as independent variables), and Class (Fig. S4). The van Sebille model, and Longhurst provinces were not capable of explaining our geographic trends in contamination levels but Spalding’s provinces, realms and Class were. As provinces provided a greater number of geographic areas and still had significant explanatory power these were used alongside Class in our analyses. Other levels of taxonomy were explored but they either did not provide enough explanatory power or the number of data points was insufficient at these lower taxonomic levels to form a rigorous statistical analysis.
Using the Web of Science database, we identified 1519 studies, of which 412 were relevant to our research question. Using a strict set of inclusion and exclusion criteria (see Supplementary Table 1) we identified 55 studies that contained data in a usable format. The data span 16 provinces as defined by Spalding36,37 (Fig. 1, generated using Shapefiles from Flanders Marine Institute111) with 90% of the records located in the Northern Hemisphere, predominantly across Europe and Southeast Asia.
Data analyses
We focussed our analysis on Classes with a minimum of 30 observations: Bivalvia (n = 192), Malacostraca (n = 69), Gastropoda (n = 54) and Polychaeta (n = 31). MP ind−1 were grouped based on Spalding’s classification of biogeographical provinces37 to allow regional assessment of MP ind−1 112. To determine the relationship of MP ind−1 Class−1 between provinces we used a two-way ANOVA with Class (4 classes) and Province (15 provinces) as categorical explanatory variables. As the graphical model validation procedure (residuals vs fitted values and QQ-plot to assess the homogeneity of variances and normality) indicated that the statistical assumption of homogeneity of variance was not met, we continued with a generalised least-squares (GLS) estimation procedure that incorporates a variance-covariance term (using varIdent for categorical variables) to model the variance structure113. To determine the optimal structure in terms of random components we used restricted maximum-likelihood (REML) estimation and compared the model without a variance-covariate term to alternate models, including either Class or Province as variance-covariates using AIC and validation of model residual patterns. The optimal fixed-effects structure was then determined by backward selection using a likelihood ratio test obtained by maximum-likelihood (ML) estimation114. All analyses were performed using the nlme package115 in the R statistical programming environment (v 4.1.2, R Core Team116).
To understand whether biological traits affect microplastic body burden, we used a linear model, and the entire dataset, with wet weight, environmental position, feeding mode, mobility, and habit as the explanatory variables and MP individual−1 as the independent variable. Insignificant variables were removed by backward selection and and the comparison of AIC.
The relative contribution of our significant variables was determined by calculating the proportion of data explained by each variable (scaling the sum of squares by 1 and dividing by their sum). Tukeys HSD tests were performed using the agricolae package117 in R.
We used Pearson’s correlation (r) to assess whether organism size (mm) or wet weight (g) influenced the size or number of microplastics observation−1. Principle component analyses were carried out in PRIMER (version 6.3.13)118.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The research data supporting this publication are openly available from Harvard Dataverse at: https://doi.org/10.7910/DVN/E57LOA. The data for the Van Sebille 2015 model can be found at: https://figshare.com/collections/data_of_Van_Sebille_et_al_2015_ERL_paper/5764184. Ocean boundaries (Spalding’s Provinces) used in Fig. 1 are freely available at: https://www.worldwildlife.org/publications/marine-ecoregions-of-the-world-a-bioregionalization-of-coastal-and-shelf-areas. Longhurst provinces used as a geographical variable in the initial analysis are freely available from: https://www.marineregions.org/gazetteer.php?p=details&id=22538. The world country shapefiles used in Fig. 1 are available from ESRI at: https://hub.arcgis.com/datasets/esri::world-countries-generalized/about and available for use under the ESRI Master License Agreement. Taxonomy for all species was verified and curated using the World Register of Marine Species match taxa function available at: https://www.marinespecies.org/aphia.php?p=match. Biological trait categories were modified using those provided by the Marine Life Information Network (MarLIN) Biological Traits Information Catalogue (BIOTIC) available at: https://www.marlin.ac.uk/biotic/resources.php. Latitudes and Longitudes when not specifically mentioned in the individual study were approximated using Google Maps.
References
Lau, W. W. Y. et al. Evaluating scenarios toward zero plastic pollution. Science 369, 1455–1461 (2020).
Borrelle, S. B. et al. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 369, 1515–1518 (2020).
Galloway, T. S., Cole, M. & Lewis, C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 1, 0116 (2017).
Koelmans, A. A. et al. Risks of Plastic Debris: Unravelling Fact, Opinion, Perception, and Belief. Environ. Sci. Technol. 51, 11513–11519 (2017).
Koelmans, A. A., Kooi, M., Lavender Law, K. & van Sebille, E. All is not lost: deriving a top-down mass budget of plastic at sea. Environ. Res. Lett. 12, 114028 (2017).
Ling, S. D., Sinclair, M., Levi, C. J., Reeves, S. E. & Edgar, G. J. Ubiquity of microplastics in coastal seafloor sediments. Mar. Pollut. Bull. 121, 104–110 (2017).
Grassle, J. F. & Maciolek, N. J. Deep-Sea Species Richness: Regional and Local Diversity Estimates from Quantitative Bottom Samples. Am. Nat. 139, 313–341 (1992).
Coleman, N., Gason, A. S. H. & Poore, G. C. B. High species richness in the shallow marine waters of south-east Australia. Mar. Ecol. Prog. Ser. 154, 17–26 (1997).
Bucci, K. & Rochman, C. M. Microplastics: a multidimensional contaminant requires a multidimensional framework for assessing risk. Microplast. Nanoplast. 2, 7 (2022).
Koelmans, A. A. et al. Risk assessment of microplastic particles. Nat. Rev. Mater. 7, 138–152 (2022).
Gray, A. D. & Weinstein, J. E. Size- and shape-dependent effects of microplastic particles on adult daggerblade grass shrimp (Palaemonetes pugio). Environ. Toxicol. Chem. 36, 3074–3080 (2017).
Xu, J. et al. Unpalatable Plastic: Efficient Taste Discrimination of Microplastics in Planktonic Copepods. Environ. Sci. Technol. 56, 6455–6465 (2022).
Pagter, E., Nash, R., Frias, J. & Kavanagh, F. Assessing microplastic distribution within infaunal benthic communities in a coastal embayment. Sci. Total Environ. 791, 148278 (2021).
Coppock, R. L. et al. Benthic fauna contribute to microplastic sequestration in coastal sediments. J. Hazard. Mater. 415, 125583 (2021).
Harris, P. T., Maes, T., Raubenheimer, K. & Walsh, J. P. A marine plastic cloud - Global mass balance assessment of oceanic plastic pollution. Continental Shelf Res. 255, 104947 (2023).
Everaert, G. et al. Risk assessment of microplastics in the ocean: Modelling approach and first conclusions. Environ. Pollut. 242, 1930–1938 (2018).
Glud, R. N. et al. Hadal trenches are dynamic hotspots for early diagenesis in the deep sea. Commun. Earth Environ. 2, 21 (2021).
Welden, N. A. C. & Cowie, P. R. Environment and gut morphology influence microplastic retention in langoustine, Nephrops norvegicus. Environ. Pollut. 214, 859–865 (2016).
Ferreira, G. V. B. et al. Influencing factors for microplastic intake in abundant deep-sea lanternfishes (Myctophidae). Sci. Total Environ. 867, 161478 (2023).
Bour, A., Avio, C. G., Gorbi, S., Regoli, F. & Hylland, K. Presence of microplastics in benthic and epibenthic organisms: Influence of habitat, feeding mode and trophic level. Environ. Pollut. 243, 1217–1225 (2018).
Setälä, O., Fleming-Lehtinen, V. & Lehtiniemi, M. Ingestion and transfer of microplastics in the planktonic food web. Environ. Pollut. 185, 77–83 (2014).
Graham, E. R. & Thompson, J. T. Deposit- and suspension-feeding sea cucumbers (Echinodermata) ingest plastic fragments. J. Exp. Mar. Biol. Ecol. 368, 22–29 (2009).
Porter, A. et al. Uptake of microplastics by marine worms depends on feeding mode and particle shape but not exposure time. Sci. Total Environ. 857, 159287 (2023).
Lopez, G. R. & Levinton, J. S. Ecology of Deposit-Feeding Animals in Marine Sediments. Q. Rev. Biol. 62, 235–260 (1987).
Solan, M. & Kennedy, R. Observation and quantification of in situ animal-sediment relations using time-lapse sediment profile imagery (t-SPI). Mar. Ecol. Prog. Ser. 228, 179–191 (2002).
Ouellette, D. et al. Effects of temperature on in vitro sediment reworking processes by a gallery biodiffusor, the polychaete Neanthes virens. Mar. Ecol. Prog. Ser. 266, 185–193 (2004).
Maire, O. et al. Indirect effects of non-lethal predation on bivalve activity and sediment reworking. J. Exp. Mar. Biol. Ecol. 395, 30–36 (2010).
Wohlgemuth, D., Solan, M. & Godbold, J. A. Species contributions to ecosystem process and function can be population dependent and modified by biotic and abiotic setting. Proc. R. Soc. B: Biol. Sci. 284, 20162805 (2017).
Cassidy, C., Grange, L. J., Garcia, C., Bolam, S. G. & Godbold, J. A. Species interactions and environmental context affect intraspecific behavioural trait variation and ecosystem function. Proc. R. Soc. B: Biol. Sci. 287, 20192143 (2020).
Berlino, M., Mangano, M. C., De Vittor, C. & Sarà, G. Effects of microplastics on the functional traits of aquatic benthic organisms: A global-scale meta-analysis. Environ. Pollut. 285, 117174 (2021).
Mason, V. G., Skov, M. W., Hiddink, J. G. & Walton, M. Microplastics alter multiple biological processes of marine benthic fauna. Sci. Total Environ. 845, 157362 (2022).
Savoca, M. S., McInturf, A. G. & Hazen, E. L. Plastic ingestion by marine fish is widespread and increasing. Glob. Change Biol. 27, 2188–2199 (2021).
Bowley, J., Baker-Austin, C., Porter, A., Hartnell, R. & Lewis, C. Oceanic Hitchhikers – Assessing Pathogen Risks From Marine Microplastic. Trend Microbiol https://doi.org/10.1016/j.tim.2020.06.011 (2021).
Burns, E. E. & Boxall, A. B. A. Microplastics in the aquatic environment: Evidence for or against adverse impacts and major knowledge gaps. Environ. Toxicol. Chem. 0 https://doi.org/10.1002/etc.4268 (2018).
Mitrano, D. M. & Wohlleben, W. Microplastic regulation should be more precise to incentivize both innovation and environmental safety. Nat. Commun. 11, 5324 (2020).
Spalding, M. D., Agostini, V. N., Rice, J. & Grant, S. M. Pelagic provinces of the world: A biogeographic classification of the world’s surface pelagic waters. Ocean Coast. Manag. 60, 19–30 (2012).
Spalding, M. D. et al. Marine Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas. BioScience 57, 573–583 (2007).
Morgado, V., Palma, C. & Bettencourt da Silva, R. J. N. Bottom-Up Evaluation of the Uncertainty of the Quantification of Microplastics Contamination in Sediment Samples. Environ. Sci. Technol. 56, 11080–11090 (2022).
Naeem, S. Advancing realism in biodiversity research. Trends Ecol. Evol. 23, 414–416 (2008).
Riisgård, H. U. & Larsen, P. S. Particle Capture Mechanisms in Suspension-Feeding Invertebrates. Marine Ecology Progress Series https://doi.org/10.3354/meps08755 (2010).
Jumars, P. A., Dorgan, K. M. & Lindsay, S. M. Diet of Worms Emended: An Update of Polychaete Feeding Guilds. Annu. Rev. Mar. Sci. 7, 497–520 (2015).
Albert, C. H. et al. Intraspecific functional variability: extent, structure and sources of variation. J. Ecol. 98, 604–613 (2010).
Cardinale, B. J. et al. Biodiversity loss and its impact on humanity. Nature 486, 59–67 (2012).
Snelgrove, P. V. R. Getting to the Bottom of Marine Biodiversity: Sedimentary Habitats: Ocean bottoms are the most widespread habitat on Earth and support high biodiversity and key ecosystem services. BioScience 49, 129–138 (1999).
Ray, G. C. & Grassle, J. F. Marine Biological Diversity Program. BioScience 41, 453–457 (1991).
Eriksen, M. et al. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 9, e111913 (2014).
Clark, J. R. et al. Marine microplastic debris: a targeted plan for understanding and quantifying interactions with marine life. Front. Ecol. Environ. 14, 317–324 (2016).
Scott, N. et al. Particle characteristics of microplastics contaminating the mussel Mytilus edulis and their surrounding environments. Mar. Pollut. Bull. 146, 125–133 (2019).
D’Avignon, G., Hsu, S. S. H., Gregory-Eaves, I. & Ricciardi, A. Feeding behavior and species interactions increase the bioavailability of microplastics to benthic food webs. Sci. Total Environ. 896, 165261 (2023).
Lim, H. S., Fraser, A. & Knights, A. M. Spatial arrangement of biogenic reefs alters boundary layer characteristics to increase risk of microplastic bioaccumulation. Environ. Res. Lett. 15, 064024 (2020).
Desforges, J.-P. W., Galbraith, M. & Ross, P. S. Ingestion of Microplastics by Zooplankton in the Northeast Pacific Ocean. Arch. Environ. Contamin. Toxicol. 69, 320–330 (2015).
Walkinshaw, C., Lindeque, P. K., Thompson, R., Tolhurst, T. & Cole, M. Microplastics and seafood: lower trophic organisms at highest risk of contamination. Ecotoxicol. Environ. Saf. 190, 110066 (2020).
Setälä, O., Norkko, J. & Lehtiniemi, M. Feeding type affects microplastic ingestion in a coastal invertebrate community. Mar. Pollut. Bull. 102, 95–101 (2016).
Savage, G., Porter, A. & Simpson, S. D. Uptake of microplastics by the snakelocks anemone (Anemonia viridis) is commonplace across environmental conditions. Sci. Total Environ. 836, 155144 (2022).
Ward, J. E. et al. Selective Ingestion and Egestion of Plastic Particles by the Blue Mussel (Mytilus edulis) and Eastern Oyster (Crassostrea virginica): Implications for Using Bivalves as Bioindicators of Microplastic Pollution. Environ. Sci. Technol. 53, 8776–8784 (2019).
Mladinich, K., Holohan, B. A., Shumway, S. E., Brown, K. & Ward, J. E. Determining the Properties that Govern Selective Ingestion and Egestion of Microplastics by the Blue Mussel (Mytilus edulis) and Eastern Oyster (Crassostrea virginica). Environ. Sci. Technol. 56, 15770–15779 (2022).
Zinkann, A.-C., Wooller, M. J., O’Brien, D. & Iken, K. Does feeding type matter? Contribution of organic matter sources to benthic invertebrates on the Arctic Chukchi Sea shelf. Food Webs 29, e00205 (2021).
Stead, R. A. & Thompson, R. J. The Influence of an Intermittent Food Supply on the Feeding Behaviour of Yoldia Hyperborea (Bivalvia: Nuculanidae). J. Exp. Marine Biol. Ecol. https://doi.org/10.1016/j.jembe.2005.11.001 (2006).
Koch, C. N., Cooper, L., Grebmeier, J. M., Frey, K. E. & Brown, T. D. Ice Algae Resource Utilization by Benthic Macro- And Megafaunal Communities on the Pacific Arctic Shelf Determined Through Lipid Biomarker Analysis. Mar. Ecol. Progress Series https://doi.org/10.3354/meps13476 (2020).
Vecchi, S., Bianchi, J., Scalici, M., Fabroni, F. & Tomassetti, P. Field evidence for microplastic interactions in marine benthic invertebrates. Sci. Rep. 11, 20900 (2021).
Miller, M. E., Hamann, M. & Kroon, F. J. Bioaccumulation and biomagnification of microplastics in marine organisms: A review and meta-analysis of current data. PLoS One 15, e0240792 (2020).
Gouin, T. Toward an Improved Understanding of the Ingestion and Trophic Transfer of Microplastic Particles: Critical Review and Implications for Future Research. Environ. Toxicol. Chem. 39, 1119–1137 (2020).
Hale, R., Mavrogordato, M. N., Tolhurst, T. J. & Solan, M. Characterizations of how species mediate ecosystem properties require more comprehensive functional effect descriptors. Sci. Rep. 4, 6463 (2014).
Doyle, D., Sundh, H. & Almroth, B. C. Microplastic exposure in aquatic invertebrates can cause significant negative effects compared to natural particles - A meta-analysis. Environ. Pollut. 315, 120434 (2022).
Kooi, M. & Koelmans, A. A. Simplifying Microplastic via Continuous Probability Distributions for Size, Shape, and Density. Environ. Sci. Technol. Lett. 6, 551–557 (2019).
Coffin, S., Weisberg, S. B., Rochman, C., Kooi, M. & Koelmans, A. A. Risk characterization of microplastics in San Francisco Bay, California. Microplast. Nanoplast. 2, 19 (2022).
Qiao, R. et al. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 236, 124334 (2019).
Choi, J. S., Hong, S. H. & Park, J.-W. Evaluation of microplastic toxicity in accordance with different sizes and exposure times in the marine copepod Tigriopus japonicus. Mar. Environ. Res. 153, 104838 (2020).
Thornton Hampton, L. M. et al. Characterizing microplastic hazards: which concentration metrics and particle characteristics are most informative for understanding toxicity in aquatic organisms? Microplast. Nanoplast. 2, 20 (2022).
Lindeque, P. K. et al. Are we underestimating microplastic abundance in the marine environment? A comparison of microplastic capture with nets of different mesh-size. Environ. Pollut. 265, 114721 (2020).
KeChi-Okafor, C. et al. Prevalence and characterisation of microfibres along the Kenyan and Tanzanian coast. Front. Ecol Evol. 11 https://doi.org/10.3389/fevo.2023.1020919 (2023).
Schmitz, E. H. & Scherrey, P. M. Digestive anatomy of Halella azteca (Crustacea, Amphipoda). J. Morphol. 175, 91–100 (1983).
Watts, A. J. R. et al. Uptake and Retention of Microplastics by the Shore Crab Carcinus maenas. Environ. Sci. Technol. 48, 8823–8830 (2014).
Joyce, H. et al. Plastics, prawns, and patterns: Microplastic loadings in Nephrops norvegicus and surrounding habitat in the North East Atlantic. Sci. Total Environ. 826, 154036 (2022).
Murray, F. & Cowie, P. R. Plastic contamination in the decapod crustacean Nephrops norvegicus (Linnaeus, 1758). Mar. Pollut. Bull. 62, 1207–1217 (2011).
Welden, N. A. C. & Cowie, P. R. Long-term microplastic retention causes reduced body condition in the langoustine, Nephrops norvegicus. Environ. Poll. https://doi.org/10.1016/j.envpol.2016.08.020 (2016).
Hara, J., Frias, J. & Nash, R. Quantification of microplastic ingestion by the decapod crustacean Nephrops norvegicus from Irish waters. Mar. Pollut. Bull. 152, 110905 (2020).
Carreras-Colom, E. et al. Anthropogenic pollutants in Nephrops norvegicus (Linnaeus, 1758) from the NW Mediterranean Sea: Uptake assessment and potential impact on health. Environ. Pollut. 314, 120230 (2022).
Piarulli, S. et al. Do different habits affect microplastics contents in organisms? A trait-based analysis on salt marsh species. Mar. Pollut. Bull. 153, 110983 (2020).
Van Colen, C., Moereels, L., Vanhove, B., Vrielinck, H. & Moens, T. The biological plastic pump: Evidence from a local case study using blue mussel and infaunal benthic communities. Environ. Pollut. 274, 115825 (2021).
Ezgeta-Balić, D. et al. Competitive feeding interactions between native Ostrea edulis and non-native Crassostrea gigas with implications of introducing C. gigas into commercial aquaculture in the eastern Adriatic Sea. Mar. Environ. Res. 160, 105051 (2020).
Lawrence, J. M., Lawrence, A. L. & Watts, S. A. in Developments in Aquaculture and Fisheries Science Vol. 37 (ed J. M. Lawrence) 135–158 (Elsevier, 2007).
Craig, C. A., Fox, D. W., Zhai, L. & Walters, L. J. In-situ microplastic egestion efficiency of the eastern oyster Crassostrea virginica. Mar. Pollut. Bull. 178, 113653 (2022).
Feder, H. M. & Pearson, T. H. The benthic ecology of Loch Linnhe and Loch Eil, a sea-loch system on the west coast of Scotland. V. Biology of the dominant soft-bottom epifauna and their interaction with the infauna. J. Exp. Mar. Biol. Ecol. 116, 99–134 (1988).
Cole, M., Lindeque, P., Fileman, E., Halsband, C. & Galloway, T. S. The Impact of Polystyrene Microplastics on Feeding, Function and Fecundity in the Marine Copepod Calanus helgolandicus. Environ. Sci. Technol. 49, 1130–1137 (2015).
Ladewig, S. M., Coco, G., Hope, J. A., Vieillard, A. M. & Thrush, S. F. Real-world impacts of microplastic pollution on seafloor ecosystem function. Sci. Total Environ. 858, 160114 (2023).
Huang, Y. et al. Effect of microplastics on ecosystem functioning: Microbial nitrogen removal mediated by benthic invertebrates. Sci. Total Environ. 754, 142133 (2021).
Hodgson, D. J., Bréchon, A. L. & Thompson, R. C. Ingestion and fragmentation of plastic carrier bags by the amphipod Orchestia gammarellus: Effects of plastic type and fouling load. Mar. Pollut. Bull. 127, 154–159 (2018).
Porter, A., Smith, K. E. & Lewis, C. The sea urchin Paracentrotus lividus as a bioeroder of plastic. Sci. Total Environ. 693, 133621 (2019).
Savoca, M. S., Wohlfeil, M. E., Ebeler, S. E. & Nevitt, G. A. Marine plastic debris emits a keystone infochemical for olfactory foraging seabirds. Sci. Adv. 2 (2016).
Botterell, Z. L. R. et al. Bioavailability of Microplastics to Marine Zooplankton: Effect of Shape and Infochemicals. Environ. Sci. Technol. 54, 12024–12033 (2020).
Fan, S. et al. Biological effects on the migration and transformation of microplastics in the marine environment. Mar. Environ. Res. 185, 105875 (2023).
Higgins, J. P. & Green, S. Cochrane handbook for systematic reviews of interventions. (2008).
Google. Google Maps https://www.google.com/maps (2021).
Self, R. & Jumars, P. Cross-phyletic patterns of particle selection by deposit feeders. J. Mar. Res. 46, 119–143 (1988).
Bos, R. P., Zhao, S., Sutton, T. T. & Frank, T. M. Microplastic ingestion by deep-pelagic crustaceans and fishes. Limnol. Oceanogr. 68, 1595–1610 (2023).
Ricciardi, A. & Bourget, E. Weight-to-Weight Conversion Factors for Marine Benthic Macroinvertebrates. Marine Ecology Progress Series https://doi.org/10.3354/meps171245 (1998).
Rohatgi, A. Webplotdigitizer Version 4.6 [on-line]., https://automeris.io/WebPlotDigitizer (2022).
Tyler-Walters, H. et al. Marine Life Information Network: Biology and Sensitivity Key Information Review Database [on-line]., www.marlin.ac.uk (2023).
van Sebille, E. et al. A global inventory of small floating plastic debris. Environ. Res. Lett. 10, 124006 (2015).
Nooteboom, P. D., Bijl, P. K., van Sebille, E., von der Heydt, A. S. & Dijkstra, H. A. Transport Bias by Ocean Currents in Sedimentary Microplankton Assemblages: Implications for Paleoceanographic Reconstructions. Paleoceanogr. Paleoclimatol. 34, 1178–1194 (2019).
Porter, A., Lyons, B. P., Galloway, T. S. & Lewis, C. Role of Marine Snows in Microplastic Fate and Bioavailability. Environ. Sci. Technol. 52, 7111–7119 (2018).
Cole, M. et al. Microplastics Alter the Properties and Sinking Rates of Zooplankton Faecal Pellets. Environ. Sci. Technol. 50, 3239–3246 (2016).
Mountford, A. S. & Morales Maqueda, M. A. Eulerian Modeling of the Three-Dimensional Distribution of Seven Popular Microplastic Types in the Global Ocean. J. Geophys. Res.: Oceans 124, 8558–8573 (2019).
ArcGIS Desktop v. 10.8 (Environmental Systems Research Institute, Redlands, CA, 2020).
Lorenz, C. et al. Spatial distribution of microplastics in sediments and surface waters of the southern North Sea. Environ. Pollut. 252, 1719–1729 (2019).
Sanchez-Vidal, A., Thompson, R. C., Canals, M. & de Haan, W. P. The imprint of microfibres in southern European deep seas. PLOS ONE 13, e0207033 (2018).
Jones, J. S. et al. Plastic contamination of a Galapagos Island (Ecuador) and the relative risks to native marine species. Sci. Total Environ. 789, 147704 (2021).
Cózar, A. et al. Plastic debris in the open ocean. Proc. Natl Acad. Sci. 111, 10239–10244 (2014).
Van Melkebeke, M., Janssen, C. & De Meester, S. Characteristics and Sinking Behavior of Typical Microplastics Including the Potential Effect of Biofouling: Implications for Remediation. Environ. Sci. Technol. 54, 8668–8680 (2020).
Flanders Marine Institute. (2009).
Reed, A. J., Godbold, J. A., Grange, L. J. & Solan, M. Growth of marine ectotherms is regionally constrained and asymmetric with latitude. Glob. Ecol. Biogeogr. 30, 578–589 (2021).
Pinheiro, J. C. & Bates, D. M. Mixed-Effects Models in S and S-Plus. 1 edn, (Springer New York, 2000).
Zuur, A. F., Ieno, E. N., Walker, N. J., Saveliev, A. A. & Smith, G. M. Mixed effects models and extensions in ecology with R. Vol. 574 (Springer, 2009).
Pinheiro, J. C., Bates, D. M. & R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1−162. https://CRAN.R-project.org/package=nlme (2023).
R Core Team. R: A Language and Environment for Statistical Computing. https://www.R-project.org/ (2021).
de Mendiburu, F. & Yaseen, M. agricolae: Statistical Procedures for Agricultural Research. https://cran.r-project.org/package=agricolae (2020).
Clarke, K. R. & Gorley, R. N. Primer V6: User Manual - Tutorial. (Plymouth Marine Laboratory, 2006).
Acknowledgements
The authors would like to thank our colleagues at the Centre for Environment, Fisheries and Aquaculture Science (CEFAS) for their counsel in the initial conception of this work and to M. Curtis, P. McIlwaine and S. Ware in particular for their assistance with collating species weights missing from publications. We also want to thank the Galloway and Lewis lab group: Lara, Clara, Alice, Kat, Paul, Jake, Jen, Steve, Steph, Daisy, Francisca, and Emily for their support. The authors also want to thank all those who supplied additional information from their own research needed to complete the original dataset and in particular T. Souster (BAS), T. Palmer (Texas A&M), and Å.I. Wilhelmsen (NHM Oslo) for filling in data gaps with their expert knowledge. AP, TG, CL, JAG, and MS would like to acknowledge support from NERC grant NE/S003975/1.
Author information
Authors and Affiliations
Contributions
A.P.: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization. J.A.G.: Methodology, Validation, Formal analysis, Writing – review & editing, Visualization, Funding acquisition. G.S.: Methodology, Validation, Data curation, Writing – review & editing. C.N.L.: Conceptualization, Validation, Writing – Review & Editing, Supervision, Funding acquisition. M.S.: Methodology, Validation, Data curation, Writing – review & editing, Funding acquisition. T.G.: Resources, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Porter, A., Godbold, J.A., Lewis, C.N. et al. Microplastic burden in marine benthic invertebrates depends on species traits and feeding ecology within biogeographical provinces. Nat Commun 14, 8023 (2023). https://doi.org/10.1038/s41467-023-43788-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-43788-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.