Abstract
Metabolism, the biological processing of energy and materials, scales predictably with temperature and body size. Temperature effects on metabolism are normally studied via acute exposures, which overlooks the capacity for organisms to moderate their metabolism following chronic exposure to warming. Here, we conduct respirometry assays in situ and after transplanting salmonid fish among different streams to disentangle the effects of chronic and acute thermal exposure. We find a clear temperature dependence of metabolism for the transplants, but not the in-situ assays, indicating that chronic exposure to warming can attenuate salmonid thermal sensitivity. A bioenergetic model accurately captures the presence of fish in warmer streams when accounting for chronic exposure, whereas it incorrectly predicts their local extinction with warming when incorporating the acute temperature dependence of metabolism. This highlights the need to incorporate the potential for thermal acclimation or adaptation when forecasting the consequences of global warming on ecosystems.
Similar content being viewed by others
Introduction
Metabolic rate is commonly described as the transformation of energy or materials within an organism over time1. Metabolic rate varies within2 and among species3 with the two most important variables thought to be temperature and individual body size4. The relationship linking temperature, body size, and metabolic rate has been explained by the Metabolic Theory of Ecology (MTE), with important implications for multiple levels of biological organisation from the individual or cellular level to entire ecosystems1,5,6.
Temperature is particularly relevant for ectotherms such as fish, whose body temperature is largely regulated by that of their environment7. The limited dispersal of freshwater fish within river networks makes them particularly susceptible to global change8, highlighting the need to understand their responses to warming for better conservation and management. The thermal sensitivity of ectothermic metabolic rate has been widely studied, and it generally rises with temperature over the range an organism normally experiences9,10. According to MTE, the slope of the relationship between metabolic rate and temperature (termed the activation energy, \({E}_{A}\)) is approximately 0.65 eV1, although it can vary between 0.2 and 1.2 eV4. The slope of the relationship between metabolic rate and body mass (termed the allometric exponent, b) has long been assumed to approximate 0.75 across all organisms1,11,12, however, some studies have questioned the universality of this value, finding large variation among taxa13,14.
The majority of previous studies focused on metabolic theory and respirometry assays have been conducted in the laboratory, using oxygen consumption rate as a proxy for aerobic metabolic rates15,16. Here, organisms are normally transported to the laboratory where they are exposed to acute temperature changes over short time scales (hours to days). However, this might not adequately represent the metabolic rates of individuals experiencing chronic temperature exposure (years to multiple generations), since adaptive responses including thermal acclimation and adaptation might modulate the metabolic process. Here, acclimation occurs through plastic changes such as alteration of phenotypes as a function of the environment with unchanged genotypes, which is often a short-term response within the lifetime of an individual17, whilst adaptation occurs through evolutionary changes such as alteration of genetic variation, which is often a long-term response across multiple generations18. Accordingly, previous studies have revealed plasticity or evolution in metabolic rates of fish19,20,21 and aquatic invertebrates22. To the best of our knowledge, however, no studies have been conducted in the wild to examine the effects of chronic exposure to warming on the physiological responses of native populations (i.e. experiencing elevated temperature over long time scales, encompassing many generations). Therefore, a lack of field experiments and empirical data means we still have limited knowledge about the extent to which adaptive responses to warming might modulate metabolism in natural environments.
Metabolic theory has been incorporated into models to predict how global change will affect food webs23,24 or carbon cycling25,26. However, these ignore the potential for organisms to adjust their physiological response to warming through phenotypic or evolutionary changes. If adaptive responses to warming can alleviate the energetic demands of organisms (e.g. by downregulating their metabolic rate), then modelling studies that do not account for this are likely to overestimate the impacts of long-term warming. Studies quantifying the effect of chronic exposure to higher temperatures on the thermal sensitivity of metabolic rates are therefore urgently needed to improve our ability to forecast the effects of global warming on ecosystems.
To address this, we measured the metabolism of a widespread cold-water fish species – the brown trout (Salmo trutta Linnaeus, 1758) – across multiple streams in the same catchment (Hengill, SW Iceland; Fig. 1). Due to geothermal activity, the streams vary in their mean annual temperature by 3–20 °C, but are otherwise alike in their physicochemical properties, making this a large-scale natural warming experiment. We conducted respirometry assays in situ and after transplanting fish among streams with contrasting temperatures, allowing us to disentangle the effects of chronic and acute thermal exposure (Fig. 1b, c). Additionally, we characterized the metabolism of brown trout and Atlantic salmon (Salmo salar Linnaeus, 1758) in situ in three additional locations (UK, Spain, and NE Iceland, spanning temperatures of 6–20 °C) to test the generality of our findings across the full continental-scale latitudinal range of these ubiquitous salmonid species (Fig. 1a). Lastly, we used a bioenergetic population dynamical model27 to assess the food-web implications of altered thermal sensitivity of fish metabolism for the community biomass of algae, invertebrates, and fish. Our first hypothesis was that metabolic rates increase with both temperature and body mass. Our second hypothesis was that populations experiencing chronic temperature exposure will be less thermally sensitive than those experiencing acute temperature exposure due to adaptive responses. Our third hypothesis was that any reduction in thermal sensitivity of metabolic rate will support a higher-than-expected biomass of fish in warmer environments.
a Locations of the study sites, incorporating five assay contexts: SW Iceland_CH, SW Iceland_AC, NE Iceland_CH, UK_CH, and Spain_CH (subscripts indicate whether chronic [CH] or acute [AC] temperature exposures were investigated). b Graphical representation of transplant (i.e. acute exposure) and (c) in situ (i.e. chronic exposure) fish respiration assays performed in the Hengill geothermal catchment. Streams are labelled with the same code used in previous studies65 and the solid arrows indicate water flow direction. In both assays, the stream code on the fish icon indicates the river where the fish was caught, and the dashed arrow denotes the river where respirometry assays were conducted. Note that fish from IS1, IS5, and IS12 were not “transplanted” to their own streams. The geographical delimitation for countries in (a) has been obtained through Natural Earth (www.naturalearthdata.com) under public domain. The fish silhouette in (b) and (c) was adapted from an image of Salmo trutta (by Carlos Cano-Barbacil) downloaded from PhyloPic (https://www.phylopic.org/) under CC0 1.0 Universal Public Domain Dedication.
In this work, we find that chronic exposure to warming can attenuate salmonid thermal sensitivity and we show how a bionergetic model can capture the presence of fish in warmer streams when accounting for chronic exposure.
Results
Routine metabolic rates (i.e. when individuals exhibit normal activity; see Methods) were quantified for a total of 511 individual fish (none of which were ever reused) in field respirometry assays. This included 83 brown trout in SW Iceland_AC, 90 brown trout in SW Iceland_CH, 188 brown trout in UK_CH, 93 brown trout and 22 Atlantic salmon in Spain_CH, and 35 Atlantic salmon in NE Iceland_CH, (subscripts indicate whether chronic [CH] or acute [AC] temperature exposures were investigated; see Fig. 1, Table S1, and Methods for study site descriptions).
Temperature-dependence of metabolism
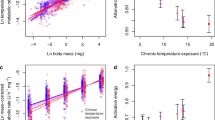
Metabolic rate only increased significantly with temperature in the acute thermal exposure (transplant) assay in SW Iceland, with an activation energy of 0.361 ± 0.160 eV (mean ± 95% CI; Table 1, Fig. 2a). There was no significant effect of temperature on metabolic rate in the chronic thermal exposure (in situ) assay in SW Iceland (0.054 ± 0.399 eV; Table 1, Fig. 2b), nor in any of the other locations (Table 1, Fig. 3a–d). This only agrees with our first hypothesis for the acute exposure assay (i.e. confidence intervals of the activation energy do not include zero), and not the chronic exposure assay (i.e. confidence intervals include zero and thus thermal sensitivity is attenuated). Note that the activation energy of the chronic exposure assay was lower than that of the acute exposure assay, but the confidence intervals overlapped, indicating no clear support for our second hypothesis. Values of activation energies differed from the expected value of 0.65 eV from MTE (including 95% CI) for trout in SW Iceland_CH, SW Iceland_AC, and UK_CH. Note that supplementary analysis indicated that the source stream from which the fish were collected in the acute thermal exposure assays had no significant effect on metabolic rate (Table S3).
Effects of (a, b) temperature and (c, d) mass on the metabolic rate of brown trout following (a, c) acute exposure (SW Iceland_AC) and (b, d) chronic exposure (SW Iceland_CH) assays. Linear regressions: (a) ln(IM)=-0.551 + 0.361 TA, F1,77 = 20.61, p < 0.001, r2 = 0.201; (b) \({{{{\mathrm{ln}}}}}\left({I}_{M}\right)=0.124\) + \(0.054{T}_{A}\), F1,73 = 0.075, p = 0.785, r2 = −0.013; (c) \({{{{\mathrm{ln}}}}}\left({I}_{T}\right)=-0.551\) + \(0.628{{{{\mathrm{ln}}}}}M\), F1,77 = 63.94, p < 0.001, r2 = 0.447; (d) \({{{{\mathrm{ln}}}}}\left({I}_{T}\right)=0.124\) + \(0.591{{{{\mathrm{ln}}}}}M\), F1,73 = 79.27, p < 0.001, r2 = 0.514. Source data are provided as a source data file.
Effects of (a–d) temperature and (e–h) mass on the metabolic rate of brown trout and Atlantic salmon following chronic exposure assays in (a, e) UK, (b, c, f, g) Spain, and (d, h) NE Iceland. Linear regressions: (a) \({{{{\mathrm{ln}}}}}\left({I}_{M}\right)=-0.037\)-\(0.443{T}_{A}\), F1,155 = 0.870, p = 0.352, r2 = −0.001; (b) \({{{{\mathrm{ln}}}}}\left({I}_{M}\right)=0.094+\)0.15\(0{T}_{A}\), F1,77 = 0.180, p = 0.673, r2 = −0.011; (c) \({{{{\mathrm{ln}}}}}\left({I}_{M}\right)=0.337\)-0.015\(\,{T}_{A}\), F1,17 < 0.001, p = 0.986, r2 = −0.059; (d) \({{{{\mathrm{ln}}}}}\left({I}_{M}\right)=-2.612\) + \(0.062{T}_{A}\), F1,32 = 0.023, p = 0.882, r2 = −0.031; (e) \({{{{\mathrm{ln}}}}}\left({I}_{T}\right)=-0.037\) + \(0.668{{{{\mathrm{ln}}}}}M\), F1,155 = 16.48, p < 0.001, r2 = 0.090; (f) \({{{{\mathrm{ln}}}}}\left({I}_{T}\right)=0.094\) + \(0.567{{{{\mathrm{ln}}}}}M\), F1,77 = 53.42, p < 0.001, r2 = 0.402; (g): \({{{{\mathrm{ln}}}}}\left({I}_{T}\right)=0.337\) + \(0.511{{{{\mathrm{ln}}}}}M\), F1,17 = 8.648, p = 0.009, r2 = 0.298; (h) \({{{{\mathrm{ln}}}}}\left({I}_{T}\right)=-2.612\) + \(1.644{{{{\mathrm{ln}}}}}M\), F1,32 = 25.68, p < 0.001, r2 = 0.428. Source data are provided as a source data file.
Size-dependence of metabolism
In support of our first hypothesis, there was a significant log-linear increase in metabolic rate with body mass for all assay contexts (Table 1), regardless of species and whether the thermal exposure was chronic or acute. The allometric exponents obtained for assays on brown trout in the acute and chronic exposures in SW Iceland were 0.628 ± 0.158 (mean ± 95% CI; Fig. 2c) and 0.591 ± 0.133 (Fig. 2d), respectively. For the remaining locations with brown trout presence, scaling exponents ranged from 0.567 ± 0.156 in Spain_CH (Fig. 3f) to 0.668 ± 0.326 in UK_CH (Fig. 3e). For Atlantic salmon, scaling exponents ranged from 0.511 ± 0.391 in Spain_CH (Fig. 3g) to 1.644 ± 0.693 in NE Iceland_CH (Fig. 3h). Values of allometric exponents differed from the “universally expected” value of 0.75 from MTE (including 95% CI) for brown trout in SW Iceland_CH and Spain_CH, and for Atlantic salmon in NE Iceland_CH.
Food-web implications
The bioenergetic model parameterised with values from our previous work27 (i.e. optimised for chronic exposure to the natural system and thus assuming Ex3 = 0.054) explained 32%, 84%, and 97% of the variation across streams in the empirical biomass of diatoms, invertebrates, and fish, respectively (Fig. 4a). Altering only the parameter associated with thermal sensitivity of fish metabolism in the bioenergetic model to the value obtained in the SW Iceland_AC (transplant) assays (Ex3 = 0.361) dramatically changed the predictions, whereby fish were instead predicted to exist in colder streams and go extinct as temperature increased (Fig. 4b). This supports our third hypothesis that a reduction in thermal sensitivity of fish due to chronic exposure would result in a higher than expected biomass of fish in warmer streams. The higher biomass of fish in the colder streams predicted from the acute exposure assays also exerted stronger top-down control on invertebrates, leading to an increase in algal biomass, which was reversed in the warmer streams without the fish.
a Model predictions (lines) were obtained using the parameter values from our previous work27, i.e. optimised for chronic exposure to the natural system and thus assuming the same thermal sensitivity of fish metabolic rate in the chronic temperature exposure (in situ) assays in SW Iceland. Empirical biomasses (circles, triangles and squares) of the three major trophic groups in the Hengill streams are also shown, along with mean temperatures during the two-week sampling period27. b Model predictions obtained using the thermal sensitivity of fish metabolic rate in the acute temperature exposure (transplant) assays in SW Iceland. Source data are provided as a source data file.
Discussion
We found a clear temperature dependence of metabolism for the transplant, but not the in-situ assays, suggesting that chronic exposure to warmer environments can attenuate the thermal sensitivity of metabolism in brown trout. This dramatic physiological change suggests that simplistic extrapolations from theory or laboratory studies that do not account for adaptive responses may be of questionable validity when forecasting the consequences of global warming in wild ecosystems.
Studies performed in the laboratory with fish exposed to different experimental temperatures have found activation energies between 0.2 and 1.2 eV4,28. We only found mean values of \({E}_{A}\) within this range in our transplant assays (SW Iceland_AC), suggesting that thermal sensitivity may only emerge for brown trout following acute exposure to new temperatures. Remarkably, we did not find any significant relationships between temperature and metabolic rate for any of the assay contexts where we measured fish in situ (i.e. chronic temperature exposure), with values of \({E}_{A}\) ≤ 0.15 eV. In laboratory experiments, fish are usually caught in the wild or from hatcheries and transported to experimental facilities, with the stress of movement from their home environment to artificial conditions one possible explanation for the difference to our results. Additionally, the use of environmental temperatures rather than acute exposures to new thermal regimes suggests that the longer salmonids spend in a warmer environment, the more capable they are of downregulating their metabolism.
The observed attenuation of thermal sensitivity in the chronic exposure assays has major implications for models using a universal temperature dependence of metabolic rate when forecasting the long-term impacts of warming on ecosystems. MTE is based on the laws of thermodynamics1, and assumes that factors such as thermal acclimation or adaptation can only alter the intercept and not the slope of the relationship between metabolism and temperature29. However, some studies have shown that fish populations from warmer environments can display metabolic rates below expectations30, suggesting that adaptive responses can lower the activation energy20. The comparative lowering of respiration rates in warmer environments is referred to as metabolic compensation and temperature-independence of metabolism has been demonstrated for different aquatic organisms such as fish31 or copepods32, among others. At the same time, numerous previous studies have shown the temperature-dependence of metabolism in several aquatic organism10,33 and therefore it is essential to carry out research such as our study to analyse in depth the thermal sensitivity of organism in warmer environments.
Our study differs from previous research in one key aspect: we measured metabolic rates in situ, utilising natural temperature gradients to examine the effects of chronic exposure to a given temperature on metabolic rate. Populations of salmonid fish in the Hengill system are characterised by a high percentage of stationary individuals with very low dispersal beyond their home stream, indicating they will potentially have experienced the same thermal regime over many generations34. We are aware of one other study using in-situ respirometry across a wide temperature gradient20, which found low values of \({E}_{A}\) for western mosquitofish, offering some support for adaptive responses reducing the metabolic cost of warming. However, there was still a significant relationship between temperature and metabolic rate, which may be related to the mosquitofish being an invasive population in the study system, potentially limiting the scope for thermal adaptation over longer time scales21. In-situ respirometry in aquatic systems has otherwise only been used to quantify metabolic rates for sharks35, deep-sea demersal fish36, corals37, and salmonids38, but not across a natural temperature gradient. More in-situ respirometry studies are thus needed and should where possible include population genetics and transcriptomics to determine the relative extent of evolutionary adaptation versus phenotypic plasticity in moderating metabolic responses to warming39,40.
We observed a positive relationship between metabolic rate and body mass in all our assays with values of b ranging from 0.5 to 1.7. The latter value is much higher than typically reported41,42, and indicates that larger fish use more oxygen per unit mass than smaller fish, in contrast to the more typical sublinear allometric scaling of metabolic rate. There is still plenty of debate in the literature about whether the theoretical value of b should approximate two-thirds, three-quarters, or linear scaling13, however, highlighting the inherent variability that can be found. For example, steeper allometric scaling of fish (b = 0.89) has been reported from a meta-analysis of 25 studies43. Nevertheless, there was a significant positive scaling of metabolic rates with body mass throughout our results, regardless of species or geographic location, supporting the idea that metabolism consistently exhibits a positive relationship with body mass13,44.
Our model results accurately captured the long-term response of brown trout to warming across different streams in the Hengill catchment when considering the effects of chronic exposure to warmer environments on metabolism, with a greater biomass of fish as stream temperature increased. This pattern is in contrast to our model predictions based on the acute thermal exposure assays and the general expectation that warming will result in declining body size45 and loss of apex predators46, highlighting how a single parameter can completely reverse the expectations of community responses to warming. The surprising success of brown trout in the warmer streams (given it is largely a cold-water species) is most likely driven by increased productivity underpinned by greater nutrient supply as temperature increases27, helping to meet the greater metabolic demands of a warmer environment. Moreover, trout feed selectively on more energetically valuable prey and benefit from an increased trophic transfer efficiency in the warmer streams34. The fact that trout may experience long-term adaptation to stream temperatures over multiple generations in the system highlights the potential for salmonids to adjust their physiology to compensate for warming impacts over the decadal timescales relevant to future global climate change.
There are of course limitations to the approach presented here. Whilst our modelling shows the potential for parameters based on acute versus chronic exposure assays to qualitatively change the effects of temperature on food web dynamics, the quantitative changes in community biomass are rather extreme (i.e. several orders of magnitude in Fig. 4). This may largely be driven by the fact that all other parameters are unlikely to remain equal in a warming scenario. For instance, if global warming alters the temperature dependence of fish metabolic rate (the only parameter we changed here), it should also change the temperature dependence of their feeding rates and indeed the biological rates of lower trophic levels. Previous studies have shown that adaptive responses to warming could influence invertebrate predator–prey interactions and population dynamics47. This highlights the importance of quantifying the effects of chronic exposure to warmer environments on the thermal response of biological rates for organisms spanning multiple trophic levels if we are to accurately parameterise predictive models of future warming scenarios on food web dynamics.
Our results emphasise the importance of measuring metabolic rates in situ for a more comprehensive understanding of thermal sensitivity in field conditions48. The next key step is to incorporate intermittent flow respirometry in future in-situ studies to quantify basal and maximum metabolic rate (and thus aerobic scope), which would reduce the variability associated with estimates of routine metabolic rate30,49. Future studies should evaluate whether there are contrasting adaptive responses depending on the target organism or trophic group, given the different generation times and pace of life for organisms throughout the food web. Our results may be more relevant for high latitude ecosystems where organisms often have scope for increased performance, with potentially different adaptive responses for tropical species close to their thermal limits. Studies are also needed to determine whether adaptation or acclimation can keep pace with, or their extent is sufficient to mitigate, the effects of global climate change. Incorporating thermal acclimation and adaption into predictive models may help to account for some of the ecological surprises in response to warming that have been reported in previous studies50,51 and help to avoid overestimating the long-term effects of warming on ecosystems.
Methods
All procedures were performed in accordance with the relevant guidelines and regulations of each country. Icelandic fieldwork was performed in collaboration with the Marine and Freshwater Research Institute under their permits and regulations. Biological field sampling permits were requested in Spain from the regional governments of the study area and the Picos de Europa National Park, and the corresponding authorisation was received. All procedures in the UK were carried out by licenced personnel under a UK Home Office A(SP)A licence (PPL 30/3277).
Study area
The Hengill geothermal catchment of SW Iceland in May 2018 (transplant assays) and August 2022 (in situ assays; see Fig. 1) was our focal field system (more detailed descriptions can be found in22,34,52,53). Headwater streams in the system differ in mean annual temperature from 3–20 °C due to geothermally warmed groundwater, but are otherwise alike in their physical and chemical characteristics27. Temperature differences between streams are consistent throughout entire years27, and over at least a 20-year period of research in Hengill54, increasing the likelihood that trout (the only fish in the catchment) experience long-term adaptation to streams over multiple generations. Trout populations are composed of a high percentage of stationary (93%; low mobility and dispersal linked to the home range) and low percentage of mobile (7%; high mobility) individuals (based on a mark-recapture study in the Hengill catchment34), increasing the possibility for adaptive responses to warming over multiple generations. Thus, this model systems allows us to embed short-term manipulative assays within a long-term temperature gradient55.
Two types of assays were conducted within the Hengill geothermal streams: (1) transplant respirometry (SW Iceland_AC): fish were collected from cold (IS12 with a mean ± standard deviation annual temperature of 7.8 ± 4.2 °C) and warm streams (IS1 = 11.3 ± 4.0 °C and IS5 = 13.8 ± 1.6 °C), and their metabolism was measured in five different streams (i.e. transplant of fish between rivers; see Fig. 1b and Assay procedure section for more information) to examine the effects of acute thermal exposure; and (2) in situ respirometry (SW Iceland_CH): metabolism was measured in the same nine streams where the fish were caught (i.e. no transplant of fish between streams; see Fig. 1c) to examine the effects of chronic temperature exposure. Due to logistical limitations, the number of streams in the transplant assay was lower than the in-situ assay, but the temperature gradient and number of fish measured was similar across assays (Table S1).
Performing both assays in non-geothermal catchments is complicated by smaller temperature gradients and the logistical difficulty and stress to the fish of implementing transplant assays in rivers that are very far from each other. However, to test the generality of the chronic temperature exposure effects, we conducted in situ assays in three additional locations across the natural latitudinal range of brown trout and its widespread close congener, the Atlantic salmon: (1) UK_CH, consisting of two carriers of the River Frome (East Bourton Boundary Stream and Woodsford North Stream), sampled in August 2021; (2) Spain_CH, consisting of 10 rivers spread evenly across the Deva and Pas catchments, sampled in July 2021; and (3) NE Iceland_CH, consisting of four rivers (Hafralónsá, Hofsá, Selá, and Vesturdalsá) sampled in June 2018 (Fig. 1a).
Assay procedure
Fish were captured by electrofishing in all study sites and the methodology for field respirometry was consistent. Assay chambers consisted of 7.2 L round (32 cm diameter), airtight, and transparent plastic containers (LocknLock brand), which were submerged in a 50 L container of river water, filtered through a 250 µm sieve (Fig. S2). One individual fish was placed in each chamber and the lid was sealed underwater, ensuring there were no air bubbles in the chamber. Chambers were secured in shallow water for approximately 1.5-3 hours. Each time assays were run, one chamber contained only filtered river water (i.e. no fish) to act as a control for background photosynthesis and/or respiration of micro-organisms. A miniDOT logger (Precision Measurements Engineering “PME”) was inserted into each chamber to measure dissolved oxygen concentrations and water temperature every minute. At the end of each assay, fish were weighed and measured (fork length) and released into the same river from which they were captured. Weights of all fish individuals from Hengill 2018 and 20 individuals from NE_Iceland were estimated according to length-weight relationships obtained from empirical data collected at those locations (see Fig. S1).
Quantifying metabolic rates
Fish exhibited some activity during the assays since movement is necessary to maintain their position in the water, thus we consider the decline in dissolved oxygen consumption rate as a proxy for routine metabolic rate here15,56,57. To reduce potential effects of stress associated with collection and handling of fish, the first 30 minutes of recorded oxygen data in each assay were excluded from further analysis. The maximum duration of the recorded data was also standardised to 120 minutes, resulting in a 90-minute assay measurement period (after excluding the first 30 minutes). Note that the maximum duration of the recorded data was >90 & ≤100 minutes for 1% of assays, >100 & ≤110 minutes for 2% of assays, and >110 & <120 minutes for 8% of assays. The ‘auto_rate’ function in the ‘respR’ package v2.0.258 of R v4.1.359 was used to calculate oxygen depletion rates through a combination of rolling regression and Kernel density estimation (KDE) algorithms58. This procedure identifies the most linear portion of the data (representative of routine metabolic rates) through rolling linear regressions of 30-minute time frames. Metabolic rates were only retained for further analysis if the r2 value for the regression was greater than 0.860, which was the case for 87% of the data. Background respiration rates were calculated as the slope of the linear regression through the entire 90-minute period of the control chamber and subtracted from the corresponding fish metabolic rates for that assay block. Positive oxygen depletion rates after background correction were excluded from further analysis (which was the case in just one assay). Per volume rates (mg O2 m−1 L−1) were converted into whole organism rates (mg O2 h−1) using the effective volume of the chamber, estimated as total volume minus the volumes of the miniDOT and the fish (assuming a density of 1,000 kg m−3)61.
Statistical analysis
All statistical analyses were conducted in R v4.1.359. In accordance with MTE1, metabolic rate, I (mg O2 h−1), depends on body mass and temperature as:
where \({I}_{0}\) is the intercept, \(M\) is the wet weight of fish (g), \(b\) is an allometric exponent, \({E}_{A}\) is the activation energy (eV), and \({T}_{A}\) is a standardised Arrhenius temperature:
where T is the temperature of the chamber (\(K\)), \({T}_{0}\) is a normalisation constant set to the average temperature (\(K\)) of all the chambers for each case study, and \(k\) is the Boltzman constant (8. 617 × 10−5 eV K−1). The average temperature for each chamber was calculated over the same time period selected by the ‘auto_rate’ function to obtain the metabolic rate. \({I}_{0}\) varies depending on the type of organism5,62, whilst b and \({E}_{A}\) are often argued to centre around values of 0.75 and 0.65 eV, respectively1. We performed a multiple linear regression (‘lm’ function in the ‘stats’ package v4.1.3 of R) on the natural logarithmic transformation of Eq. 1 for each assay context to explore the main effects of temperature and body mass on the metabolic rate. Subsequently, metabolic rates were mass-corrected by dividing by \({M}^{b}\) (denoted as IM) and temperature-corrected by dividing by \({e}^{{E}_{A}{T}_{A}}\) (denoted as IT) to visualise the independent effects of temperature and body mass on metabolic rate, respectively. Moreover, we performed a supplementary analysis for the acute assays, including an extra categorical variable in Eq. 1 for the main effect of the source stream (S) that the fish were collected from (3 levels: IS1, IS12, and IS5).
Bioenergetic model
In our previous work27, we built a bioenergetics model to describe the effects of temperature on the biomass of diatoms, invertebrates, and fish, estimating parameter values by maximum likelihood optimisation based on the observed biomass of the three groups in the Hengill system. Here, we summarise the model and its application to this study, with full details in our previous work27. The model is:
Here, B1, B2, and B3 denote the biomass of diatoms, invertebrates, and fish, respectively (mg m−2); r is the maximum mass-specific growth rate of diatoms (day−1); K is the carrying capacity (mg m−2); xi is the mass-specific metabolic rate of trophic group i (day−1); yi represents the attack rate of trophic group i (m2 mg−1 day−1); e2 = 0.45 is the assimilation efficiency when invertebrates consume diatoms63; and e3 = 0.85 is the assimilation efficiency when fish consume invertebrates27.
The equilibrium biomasses in the absence of fish are:
and the equilibrium biomasses in the presence of fish are:
Whether the fish is present or absent is determined by \({\lambda }_{{{{{\mathrm{3,1}}}}}\&2}={e}_{3}{y}_{3}{B}_{2}^{C}-{x}_{3}\). If \({B}_{2}^{C} > {x}_{3}/{e}_{3}{y}_{3}\), i.e. \({B}_{2}^{C} > {B}_{2}^{D}\), then \({\lambda }_{{{{{\mathrm{3,1}}}}}\&2} > 0\) and fish can invade the equilibrium coexistence state of diatoms and invertebrates. Conversely, if \({B}_{2}^{C} < {B}_{2}^{D}\), then \({\lambda }_{{{{{\mathrm{3,1}}}}}\&2} < 0\) and fish are absent.
To avoid overfitting, we reduced the number of parameters by letting G2 = x2/y2, G3 = x3/y3, H2 = y2/r, and H3 = y2/y3. Then the equilibrium biomasses in the absence of fish are:
and the equilibrium biomasses in the presence of fish are:
Meanwhile, \({\lambda }_{{{{{\mathrm{3,1}}}}}\&2}={y}_{3}({e}_{3}{B}_{2}^{C}-{G}_{3})\). Considering y3 is always positive, the presence of fish is determined by \({\lambda }_{{{{{\mathrm{3,1}}}}}\&2}^{{\prime} }={e}_{3}{B}_{2}^{C}-{G}_{3}\).
The temperature dependences of the parameters are:
The values of AG2, AG3, AH2, AH3, AK, CG2, CG3, CH2, CH3, and CK were estimated in our previous work27 using the maximum likelihood optimisation. The estimated values are listed in Table S2. Substituting these estimates into Eqs. 8 and 9, we can simulate the equilibrium biomass of the three groups.
In the Hengill system, we can consider fish to be chronically exposed to the temperature of each stream, and thus the activation energy of metabolic rate should be identical to the chronic temperature assays conducted here, i.e. 0.054 (Table 1). Note that the bioenergetic model does not directly use the parameter value of Ex3, but rather a combination of Ex3 and Ey3 (since G3 = x3/y3 and CG3 = Ex3 – Ey3). Thus, we cannot isolate Ex3 to verify its exact value from the optimisation against the empirical biomasses in our previous work27, and so must assume that it is 0.054. We can then examine how a change in the activation energy of fish metabolism from the value in the chronic to the acute temperature exposure assays (i.e. from 0.054 to 0.361) alters the biomass of fish and the lower trophic level groups. That is, we increase CG3 by 0.361 – 0.054 = 0.307. The remaining parameters in the model were maintained at the same values as in our previous work27.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data generated in this study64 have been deposited with the University of Essex Research Data Repository at http://researchdata.essex.ac.uk/189/. Source data are provided with this paper.
Code availability
Code used to assess the data and generate the figures and tables64 have been deposited with the University of Essex Research Data Repository at http://researchdata.essex.ac.uk/189/.
References
Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M. & West, G. B. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (2004).
Burton, T., Killen, S. S., Armstrong, J. D. & Metcalfe, N. B. What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proc. R. Soc. B: Biol. Sci. 278, 3465–3473 (2011).
White, C. R. & Kearney, M. R. Determinants of inter-specific variation in basal metabolic rate. J. Comp. Physiol. B 183, 1–26 (2013).
Gillooly, J. F., Brown, J. H., West, G. B., Savage, V. M. & Charnov, E. L. Effects of size and temperature on metabolic rate. Science (1979) 293, 2248–2251 (2001).
Schramski, J. R., Dell, A. I., Grady, J. M., Sibly, R. M. & Brown, J. H. Metabolic theory predicts whole-ecosystem properties. Proc. Natl Acad. Sci. USA 112, 2617–2622 (2015).
Woodward, G. et al. The effects of climatic fluctuations and extreme events on running water ecosystems. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 371, 20150274 (2016).
Brett, J. R. Energetic Responses of Salmon to Temperature. A Study of Some Thermal Relations in the Physiology and Freshwater Ecology of Sockeye Salmon (Oncorhynchus nerkd). Am. Zool. 11, 99–113 (1971).
Ficke, A. D., Myrick, C. A. & Hansen, L. J. Potential impacts of global climate change on freshwater fisheries. Rev. Fish. Biol. Fish. 17, 581–613 (2007).
Di Santo, V. & Bennett, W. A. Effect of rapid temperature change on resting routine metabolic rates of two benthic elasmobranchs. Fish. Physiol. Biochem 37, 929–934 (2011).
Rangel, R. E. & Johnson, D. W. Metabolic responses to temperature in a sedentary reef fish, the bluebanded goby (Lythrypnus dalli, Gilbert). J. Exp. Mar. Biol. Ecol. 501, 83–89 (2018).
Kleiber, M. Body size and metabolism. Hilgardia 6, 315–353 (1932).
Savage, V. M. et al. The predominance of quarter-power scaling in biology. Funct. Ecol. 18, 257–282 (2004).
White, C. R., Cassey, P. & Blackburn, T. M. Allometric exponents do not support a universal metabolic allometry. Ecology 88, 315–323 (2007).
Bokma, F. Evidence against universal metabolic allometry. Funct. Ecol. 18, 184–187 (2004).
Cech, J. J. & Brauner, C. J. Techniques in Whole Animal Respiratory Physiology. in Encyclopedia of Fish Physiology vol. 2 846–853 (Elsevier Inc., 2011).
Clark, T. D., Sandblom, E. & Jutfelt, F. Aerobic scope measurements of fishes in an era of climate change: Respirometry, relevance and recommendations. J. Exp. Biol. 216, 2771–2782 (2013).
Horowitz, M. Heat acclimation: Phenotypic plasticity and cues to the underlying molecular mechanisms. J. Therm. Biol. 26, 357–363 (2001).
Bautista, N. M. & Crespel, A. Within- and Trans-Generational Environmental Adaptation to Climate Change: Perspectives and New Challenges. Front Mar. Sci. 8, 1337 (2021).
Donelson, J. M., Munday, P. L., McCormick, M. I. & Nilsson, G. E. Acclimation to predicted ocean warming through developmental plasticity in a tropical reef fish. Glob. Chang Biol. 17, 1712–1719 (2011).
Moffett, E. R., Fryxell, D. C., Palkovacs, E. P., Kinnison, M. T. & Simon, K. S. Local adaptation reduces the metabolic cost of environmental warming. Ecology 99, 2318–2326 (2018).
Jutfelt, F. Metabolic adaptation to warm water in fish. Funct. Ecol. 34, 1138–1141 (2020).
Kordas, R. L., Pawar, S., Kontopoulos, D. G., Woodward, G. & O’Gorman, E. J. Metabolic plasticity can amplify ecosystem responses to global warming. Nat. Commun. 13, 1–8 (2022).
Woodward, G. et al. Using Food Webs and Metabolic Theory to Monitor, Model, and Manage Atlantic Salmon—A Keystone Species Under Threat. Front Ecol. Evol. 9, 912 (2021).
Petchey, O. L., Brose, U. & Rall, B. C. Predicting the effects of temperature on food web connectance. Philos. Trans. R. Soc. B: Biol. Sci. 365, 2081–2091 (2010).
Yvon-Durocher, G., Allen, A. P., Montoya, J. M., Trimmer, M. & Woodward, G. The temperature dependence of the carbon cycle in aquatic ecosystems. Advances in Ecological Research vol. 43 (Academic Press, 2010).
Yvon-Durocher, G., Jones, J. I., Trimmer, M., Woodward, G. & Montoya, J. M. Warming alters the metabolic balance of ecosystems. Philos. Trans. R. Soc. B: Biol. Sci. 365, 2117–2126 (2010).
O’Gorman, E. J. et al. Unexpected changes in community size structure in a natural warming experiment. Nat. Clim. Chang 7, 659–663 (2017).
Dell, A. I., Pawar, S. & Savage, V. M. Systematic variation in the temperature dependence of physiological and ecological traits. Proc. Natl Acad. Sci. USA 108, 10591–10596 (2011).
Gillooly, J. F. et al. Response to Clarke and Fraser: effects of temperature on metabolic rate. Funct. Ecol. 20, 400–404 (2006).
Pilakouta, N. et al. Multigenerational exposure to elevated temperatures leads to a reduction in standard metabolic rate in the wild. Funct. Ecol. 34, 1205–1214 (2020).
Eme, J., Bennett, W. A., Eme, J. & Bennett, W. A. Acute temperature quotient responses of fishes reflect their divergent thermal habitats in the Banda Sea, Sulawesi, Indonesia. Aust. J. Zool. 57, 357–362 (2009).
Scheffler, M. L., Barreto, F. S. & Mueller, C. A. Rapid metabolic compensation in response to temperature change in the intertidal copepod, Tigriopus californicus. Comp. Biochem Physiol. A Mol. Integr. Physiol. 230, 131–137 (2019).
Réveillon, T., Rota, T., Chauvet, É., Lecerf, A. & Sentis, A. Repeatable inter-individual variation in the thermal sensitivity of metabolic rate. Oikos 128, 1633–1640 (2019).
O’Gorman, E. J. et al. Temperature effects on fish production across a natural thermal gradient. Glob. Chang Biol. 22, 3206–3220 (2016).
Byrnes, E. E., Lear, K. O., Morgan, D. L. & Gleiss, A. C. Respirometer in a box: development and use of a portable field respirometer for estimating oxygen consumption of large‐bodied fishes. J. Fish. Biol. 96, 1045–1050 (2020).
Drazen, J. C. & Yeh, J. Respiration of four species of deep-sea demersal fishes measured in situ in the eastern North Pacific. Deep Sea Res 1 Oceanogr. Res Pap. 60, 1–6 (2012).
Camp, E. F. et al. The “Flexi-Chamber”: A Novel Cost-Effective In Situ Respirometry Chamber for Coral Physiological Measurements. PLoS One 10, e0138800 (2015).
Rasmussen, J. B., Robinson, M. D., Hontela, A. & Heath, D. D. Metabolic traits of westslope cutthroat trout, introduced rainbow trout and their hybrids in an ecotonal hybrid zone along an elevation gradient. Biol. J. Linn. Soc. 105, 56–72 (2012).
Logan, M. L. & Cox, C. L. Genetic Constraints, Transcriptome Plasticity, and the Evolutionary Response to Climate Change. Front Genet 11, 1088 (2020).
DeBiasse, M. B. & Kelly, M. W. Plastic and evolved responses to global change: What can we learn from comparative transcriptomics? in Journal of Heredity vol. 107 71–81 (Oxford University Press, 2016).
Glazier, D. S. Beyond the ‘3/4-power law’: variation in the intra- and interspecific scaling of metabolic rate in animals. Biol. Rev. 80, 611 (2005).
Glazier, D. S. Activity alters how temperature influences intraspecific metabolic scaling: testing the metabolic-level boundaries hypothesis. J. Comp. Physiol. B 190, 445–454 (2020).
Jerde, C. L. et al. Strong Evidence for an Intraspecific Metabolic Scaling Coefficient Near 0.89 in Fish. Front Physiol. 10, 1166 (2019).
Hudson, L. N., Isaac, N. J. B. & Reuman, D. C. The relationship between body mass and field metabolic rate among individual birds and mammals. J. Anim. Ecol. 82, 1009–1020 (2013).
Daufresne, M., Lengfellner, K. & Sommer, U. Global warming benefits the small in aquatic ecosystems. Proc. Natl Acad. Sci. USA 106, 12788–12793 (2009).
Petchey, O. L., McPhearson, P. T., Casey, T. M. & Morin, P. J. Environmental warming alters food-web structure and ecosystem function. Nature 402, 69–72 (1999).
Sohlström, E. H. et al. Thermal acclimation increases the stability of a predator–prey interaction in warmer environments. Glob. Chang Biol. 27, 3765–3778 (2021).
Treberg, J. R., Killen, S. S., MacCormack, T. J., Lamarre, S. G. & Enders, E. C. Estimates of metabolic rate and major constituents of metabolic demand in fishes under field conditions: Methods, proxies, and new perspectives. Comp. Biochem Physiol. A Mol. Integr. Physiol. 202, 10–22 (2016).
Svendsen, M. B. S., Bushnell, P. G. & Steffensen, J. F. Design and setup of intermittent-flow respirometry system for aquatic organisms. J. Fish. Biol. 88, 26–50 (2016).
Fordham, D. A. Mesocosms Reveal Ecological Surprises from Climate Change. PLoS Biol. 13, e1002323 (2015).
Dodds, W. K. et al. Surprises and Insights from Long-Term Aquatic Data Sets and Experiments. Bioscience 62, 709–721 (2012).
Arnason, B., Theodorsson, P., Björnsson, S. & Saemundsson, K. Hengill, a high temperature thermal area in Iceland. Bull. Volcanologique 33, 245–259 (1969).
Friberg, N. et al. Relationships between structure and function in streams contrasting in temperature. Freshw. Biol. 54, 2051–2068 (2009).
Ólafsson, J. S., Ingimundardóttir, G. V., Hansen, I. & Sigurðardóttir, S. G. Smádýralíf í afrennslisvatni frá háhitasvæðunum við Kröflu, Ölkelduháls og í Miðdal í Henglinum. Macroinvertebrate assemblages in effluent water from the high temperature geothermal areas of Krafla, Ölkelduháls and Miðdalur in Hengill, Iceland].[Report in Icelandic with English summary]. Report no. VMST/10019. Institute of Freshwater Fisheries (2010).
O’Gorman, E. J. et al. Climate change and geothermal ecosystems: Natural laboratories, sentinel systems, and future refugia. Glob. Chang Biol. 20, 3291–3299 (2014).
Makarieva, A. M. et al. Mean mass-specific metabolic rates are strikingly similar across life’s major domains: Evidence for life’s metabolic optimum. Proc. Natl Acad. Sci. USA 105, 16994–16999 (2008).
Ikeda, T. Routine metabolic rates of pelagic marine fishes and cephalopods as a function of body mass, habitat temperature and habitat depth. J. Exp. Mar. Biol. Ecol. 480, 74–86 (2016).
Harianto, J., Carey, N. & Byrne, M. respR—An R package for the manipulation and analysis of respirometry data. Methods Ecol. Evol. 10, 912–920 (2019).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. (2022).
Schaal, P. et al. Links between host genetics, metabolism, gut microbiome and amoebic gill disease (AGD) in Atlantic salmon. Anim. Microbiome 4, 1–17 (2022).
Rosewarne, P. J., Wilson, J. M. & Svendsen, J. C. Measuring maximum and standard metabolic rates using intermittent-flow respirometry: a student laboratory investigation of aerobic metabolic scope and environmental hypoxia in aquatic breathers. J. Fish. Biol. 88, 265–283 (2016).
Ohlberger, J., Mehner, T., Staaks, G. & Hölker, F. Intraspecific temperature dependence of the scaling of metabolic rate with body mass in fishes and its ecological implications. Oikos 121, 245–251 (2012).
Yodzis, P. & Innes, S. Body size and consumer-resource dynamics. Am. Naturalist 139, 1151–1175 (1992).
O’Gorman, E. Chronic exposure to environmental temperature attenuates the thermal sensitivity of salmonids. [Dataset]. Colchester, University of Essex. https://doi.org/10.5526/ERDR-00000189 (2023).
Woodward, G. et al. Sentinel systems on the razor’s edge: effects of warming on Arctic geothermal stream ecosystems. Glob. Chang Biol. 16, 1979–1991 (2010).
Acknowledgements
This study was part of the R&D project WATERLANDS, code PID2019-107085RB-I00, funded by MCIN/AEI/10.13039/501100011033 (J.B “PI”), the R&D project RIFFLE PID2020-114427RJ-I00 funded by MCIN/AEI/10.13039/501100011033 (F.J.P. “PI”, A.M.G.F. “Team researcher”), and NERC (NE/L011840/1, E.J.O.G. “PI” and NE/M020843/1, G.W. “PI”, E.J.O.G. “Co-Investigator”). Alexia María González-Ferreras acknowledges the financial support from the Government of Cantabria through the Fénix Programme. G.W., E.J.O.G., O.F.M. and R.L. acknowledge the financial support received from Six Rivers Iceland. We thank everyone involved in the field data collection.
Author information
Authors and Affiliations
Contributions
A.M.G.F., E.J.O.G., G.W., J.B., and F.J.P. were responsible for funding. E.J.O.G., A.M.G.F., G.W., and J.B. designed the research work. A.M.G.F., J.B., P.S.A.B., J.H., H.K., R.L., O.F.M., F.J.P., G.E.T., G.W., L.Z. and E.J.O.G. participated in field sampling design and field data collection. A.M.G.F., E.J.O.G and L.Z. analysed the data. A.M.G.F. wrote the first draft with a key contribution from E.J.O.G.. A.M.G.F., J.B., P.S.A.B., J.H., H.K., R.L., O.F.M., F.J.P., G.E.T., G.W., L.Z. and E.J.O.G. revised and contributed to the final draft of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interest.
Peer review
Peer review information
Nature Communications thanks James Junker and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
González-Ferreras, A.M., Barquín, J., Blyth, P.S.A. et al. Chronic exposure to environmental temperature attenuates the thermal sensitivity of salmonids. Nat Commun 14, 8309 (2023). https://doi.org/10.1038/s41467-023-43478-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-43478-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.