Abstract
The evolutionary transition to self-compatibility facilitates polyploid speciation. In Arabidopsis relatives, the self-incompatibility system is characterized by epigenetic dominance modifiers, among which small RNAs suppress the expression of a recessive SCR/SP11 haplogroup. Although the contribution of dominance to polyploid self-compatibility is speculated, little functional evidence has been reported. Here we employ transgenic techniques to the allotetraploid plant A. kamchatica. We find that when the dominant SCR-B is repaired by removing a transposable element insertion, self-incompatibility is restored. This suggests that SCR was responsible for the evolution of self-compatibility. By contrast, the reconstruction of recessive SCR-D cannot restore self-incompatibility. These data indicate that the insertion in SCR-B conferred dominant self-compatibility to A. kamchatica. Dominant self-compatibility supports the prediction that dominant mutations increasing selfing rate can pass through Haldane’s sieve against recessive mutations. The dominance regulation between subgenomes inherited from progenitors contrasts with previous studies on novel epigenetic mutations at polyploidization termed genome shock.
Similar content being viewed by others
Introduction
Polyploid species originating from genome duplication are found in animals, fungi, and plants and they are estimated to share approximately 30% of plant species1,2,3. The typical traits of natural and crop polyploid species include large cell size, distinct distribution range with environmental robustness, and self-fertilization2,3,4. A pioneer of the study of polyploidy, Ledyard Stebbins, pointed out the association between polyploidy and self-fertilization5,6,7. Frequent evolutionary transitions from self-incompatibility to self-compatibility of polyploid species have been attributed to the advantage of reproductive assurance in overcoming minority cytotype exclusion and reduced inbreeding depression4,7,8,9,10,11,12,13,14,15. However, Stebbins also argued that polyploid species may suffer from a “retarded” phenotypic evolution because of the masking effect5,6. Theoretical studies have suggested that additional alleles or duplicated copies may confer a masking effect on recessive and additive mutations because of redundancy, and therefore a mutation in each duplicated gene may need to be accumulated in allopolyploid species for phenotypic evolution such as the evolution of self-compatibility3,4,16,17. Dominance relationships can reconcile these two aspects of polyploidy by alleviating the masking effect2,11,18,19. Haldane suggested that dominant or partially dominant mutations contribute more to adaptive evolution than recessive mutations, which is referred to as Haldane’s sieve20. The principle can be valid for both allelic interactions in diploid species and epistatic interactions between homeologous copies in polyploid species because allotetraploid species are effectively fixed heterozygotes of subgenomes derived from different species. Theoretical studies have shown that mutations that increase the selfing rate would be dominant to pass through the Haldane’s sieve21.
The role of epigenetics in polyploid evolution has long been discussed3,4,22. In the synthetic allopolyploid plants of many species, genome-wide instability in gene expression and methylation has been reported and termed as “genome shock“3,4,22,23. It has been debated whether novel genetic and epigenetic variants contributed to adaptive evolution of polyploids or were deleterious3,24,25. Besides genome-wide studies on small RNA (sRNA) of allopolyploid species26,27, the dominance relationship of self-incompatibility in Brassicaceae regulated by sRNA has also been studied in polyploid species2,11,19. Self-incompatibility in Brassicaceae including Arabidopsis and Brassica is characterized by the dominance relationship in pollen (hereafter, male dominance) through sRNAs that function as “dominance modifiers”28,29,30,31,32. In this self-recognition self-incompatibility system, SRK (S-locus receptor kinase) on the female stigma and SCR/SP11 (S-locus cysteine-rich protein/S-locus protein 11) on the pollen bind together when both of them are derived from the same S-haplogroup and then inhibit the growth of self-pollen tubes through the downstream signaling pathway33,34,35. A large number of highly divergent S-haplogroups of the female and male specificity genes (SRK and SCR/SP11, respectively) segregate trans-specifically at the S-locus11. The S-haplogroups typically show dominance-recessiveness relationships, i.e., when Sx is dominant to Sy, only Sx-specificity is functional and increases mating success by enabling mating with non-self individuals that possess Sy while preventing self-fertilization caused by Sx36,37,38. Both male and female dominance (dominance in stigma) are known in Brassicaceae36,37,38, however, male dominance is more frequent, i.e., two S-haplogroups often show dominance in pollen but no dominance (called co-dominance) in stigma36. The molecular mechanism of male dominance has been revealed by transgenic experiments in diploid species33. sRNAs from a dominant S-haplogroup at the S-locus regulate the tissue-specific methylation of the SCR gene of a recessive S-haplogroup, thereby suppressing its expression and function28,30,31,32. In natural allotetraploid species in Brassicaceae, sequencing and crossing experiments suggested the relevance of dominance in self-compatibility2,11,19. In the crop allotetraploid species Brassica napus, loss-of-function mutations were detected in the dominant haplotypes of SRK or SCR39,40. In Arabidopsis suecica, a loss-of-function mutation in the SCR-A and mir867 sRNA that has a homology to the recessive SCR02 were inherited from A. thaliana41. In Capsella bursa-pastoris, the B subgenome has loss-of-function mutations in SCR as well as mirS3 that has a potential binding site in the A subgenome, although its relevance in SCR dominance regulation is unclear due to the lack of full-length SCR sequence in the A genome42. Dominance in a hybrid between different species and in synthetic allopolyploid plants was also studied43. Although these data suggest the importance of dominance in the allopolyploid species2,11,18,19, functional evidence using the transgenic technique has been awaited to test the relevance of dominance.
Arabidopsis kamchatica (2n = 4x = 32) is a model allopolyploid species with a broad distribution range including locality with heavy metal, and the transgenic technique using floral dip was established44,45,46,47. It is a natural self-compatible and allotetraploid species derived from multiple individuals of two progenitor species, A. halleri and A. lyrata44,48,49. Both progenitor species are predominantly self-incompatible, although self-compatible individuals of A. lyrata have been reported49,50,51,52. A. kamchatica has two S-loci derived from each progenitor species and shows disomic inheritance18. S-haplogroups A, B, and C segregate at the S-locus derived from A. halleri, and haplogroups D and E from A. lyrata18. The genotyping of 49 individuals covering most of the distribution range identified the following combinations of S-haplogroups: AD (47%, Takashima accession as a representative in this study, abbreviated as Tak), BD (34%, Potter accession, Pot), BE (8%, Okhotsk accession, Okh), individuals with only a single S-haplogroup amplified (6%) and CD (5%)18. Despite the self-compatibility of A. kamchatica, full-length sequences with no obvious gene-disruptive mutations were found in SRK-A, SRK-B, SRK-D, and SRK-E18. Furthermore, crossing experiments showed functional female self-incompatibility in individuals with full-length SRK-A, SRK-B, and SRK-D18. These data showed that these SRK haplogroups as well as downstream female signaling genes retained functionality even though all of these individuals are self-compatible18. This suggests that the self-compatibility of A. kamchatica was caused by gene-disruptive mutations of the male self-incompatibility gene SCR or other genes necessary for self-incompatibility in pollen, although further transgenic evidence is required to show which gene was responsible for the evolution of self-compatibility53. Thus far, no experimental evidence on the existence of dominance either in pollen or in stigma has been reported among the three major S-haplogroup combinations of A. kamchatica (AD, BD, BE), although S-haplogroup C is shown to be most recessive both in male and female self-incompatibility in diploid species37. The crossing experiments described above also showed that S-haplogroups B and D exhibit no dominance (that is, co-dominant) in the pistil of A. kamchatica18, although it is possible that male dominance exists between B and D haplogroups because many pairs of S-haplogroups showed dominance only in pollen and co-dominance in stigma36.
Functional experiments such as transgenic technique and genome editing are important to identify the genes responsible for the evolution of self-compatibility because sequence analysis alone may be confounded by mutations that are difficult to be detected such as those in regulatory regions resulting in expression loss and by secondary degrading mutations in other genes in the self-incompatibility system after the loss of self-incompatibility11. In A. thaliana, the standard accession Col-0 had a gene-disruptive mutation both in SCR and SRK54 but several accessions including Wei-1 have a full-length SRK55. By the transgenic introduction of a repaired SCR, A. thaliana Wei-1 restored self-incompatibility53. Such transgenic restoration by introducing a single gene shows that the mutation in the gene is responsible for self-compatibility and that all other genes for self-incompatibility are functional. The self-incompatibility system of Brassicaceae involves many genes other than SCR and SRK, and therefore self-compatibility may be attributed to so-called modifier genes such as M-locus protein kinase gene that are not linked to S-locus35, or to an unknown locus in the self-compatible populations of A. lyrata50,56. Because the sRNAs regulating male dominance are located at the S-locus28,30, the self-compatibility mutation must be located at the S-locus for dominance to be linked.
In this study, we experimentally examined the male dominance relationships using the self-incompatible diploid species. Based on the sequence analysis of SCR and sRNA sequences in A. kamchatica, we experimentally restored the SCR sequences of S-haplogroups B and D by transgenic experiments. Using them, we tested whether the male self-incompatibility specificity gene SCR was responsible for the evolution of self-compatibility and whether restoration of the dominant but not the recessive S-haplogroup can confer self-incompatibility.
Results
Dominance relationship between S-haplogroups in pollen
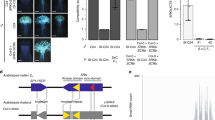
By exploiting the trans-specific sharing of the S-haplogroups18, we examined the dominance relationship and functional sequences using the diploid self-incompatible A. halleri. Crossing experiments showed that in the pollen, the S-haplogroup B was dominant over D (noted as B > D), that is, the pollen of heterozygote BD was rejected only by the pistil with B specificity, but not by that with D specificity (Fig. 1a, Supplementary Fig. 1a). Similarly, the S-haplogroup A was dominant over D (A > D) (Fig. 1a, and Supplementary Fig. 1a). In addition, S-haplogroups A and B were co-dominant (A = B) (Supplementary Fig. 1a). Together with a previous report showing S-haplogroup D (=Ah12) > E (=Ah02)37, our data revealed the dominance relationship in pollen, as A = B > D > E. These experiments suggest that S-haplogroups A and B are dominant, whereas D and E are recessive in A. kamchatica.
a Representative pictures of the crossings to investigate the dominance hierarchy of the S-haplogroups A, B, and D. Stigma of Arabidopsis halleri with the haplogroups A (top left), B (bottom left), and D (top and bottom right) crossed with pollen grains derived from heterozygote of AD or BD. Yellow arrows indicate a bundle of pollen tubes. Scale bar = 0.25 mm. At least six observations showed similar results for each crossing (sample numbers in Supplementary Fig. 1a). See Supplementary Table 8 for the genotypes of A. halleri individuals. b Gene structure of SCR-B. Blue boxes: exons; blue lines: intron and intergenic regions; red triangle: insertion in AkSCR-B. c The expression level of SCR genes in the anther cDNA of A. halleri and A. kamchatica bearing different S-haplogroups. A. kamchatica accessions from Takashima, Japan (Tak; haplogroups A and D); Murodo, Japan (Mur; haplogroups A and D); Taiwan (Tw; haplogroups A and D); and Potter, Alaska (Pot; haplogroups B and D). ACTIN was amplified as a quantitative control. d Expression of AkAmirS2, AkBmirS2, and AkBmirS3 precursor genes in the anther cDNA of A. kamchatica from Potter (Pot; haplogroups B and D), Okhotsk (Okh; haplogroups B and E), and Takashima (Tak, haplogroups A and D). +: Reverse transcription with reverse transcriptase; −: reverse transcription without reverse transcriptase as the negative control because primers amplifying the precursor genes do not flank an intron. e Sequence alignment of AkBmirS2 of dominant S-haplogroup B and its target site at the promoter of recessive SCR-D obtained from A. halleri. AkBmirS2 sRNA sequences are highly homologous to their target site (22 of 24 nucleotides were identical). X: mismatches.
Gene-disruptive mutations in SCR genes
The S-locus harbors highly divergent sequences of SRK and SCR and sRNAs33. We previously found that female self-incompatibility is functional in some A. kamchatica accessions having full-length alleles of SRK-A, B, and D18. Thus, self-compatibility may have evolved through the loss-of-function of SCR. We used the transcriptome sequencing approach to isolate SCR sequences from A. kamchatica and A. halleri because SCR sequences are too short and highly polymorphic to be isolated using polymerase chain reaction (PCR). Then, we confirmed the linkage to S-locus by checking co-segregation with the corresponding SRK haplogroup (Supplementary Table 1). The repressed expression in A. halleri corresponded to the dominance relationships in pollen as described in the previous section (Supplementary Fig. 1b). We searched for loss-of-function mutations in the dominant S-haplogroups of A. kamchatica. AkSCR-B had a 3,960-bp insertion with homology to a transposable element of the Mutator family, AtMU13, at the intron region in all tested A. kamchatica accessions bearing haplogroup B (Fig. 1b). The expression of AkSCR-B was lost in both accessions with the BD and BE haplogroups (Fig. 1c, Supplementary Fig. 1c), strongly suggesting that it is nonfunctional. Among AkSCR-A accessions, Taiwan accessions had a frameshift by G to AC substitution, whereas all the other tested accessions had a frameshift by insertion of a 7-bp repeat (Supplementary Fig. 2). Next, we examined the recessive S-haplogroups. In agreement with the dominance relationship in A. halleri, AkSCR-E was not expressed in the BE haplogroup, despite the absence of a large indel or obvious loss-of-function mutation (Supplementary Fig. 3). Southern blotting analysis and read mapping of high-throughput genomic sequence data of A. kamchatica bearing S-haplogroup D to bacterial artificial chromosome (BAC) sequences of S-haplogroup Ah12 (=AkD) suggested a deletion in the chromosomal region encompassing AkSCR-D (Supplementary Fig. 4), consistent with the lack of AkSCR-D expression (Fig. 1c). Due to the lack of SCR-D sequence in A. kamchatica, that of A. halleri is used in the following analyses.
Isolation of small RNAs and their precursor genes
To examine the involvement of sRNA in A. kamchatica self-compatibility, we searched for S-located sRNAs that show homology to the genomic region near the two recessive SCRs, SCR-D and SCR-E. First, we found the precursor genes of mirS2 and mirS3 sRNAs from each of the two dominant S-haplogroups B and A (Supplementary Figs. 5–8, named AkBmirS2, AkBmirS3, AkAmirS2, AkAmirS3 precursor genes). We confirmed that the former three of them co-segregated with SCR sequences at the S-locus (Supplementary Table 1). Second, sRNA high-throughput sequencing was conducted to detect sRNA processed from the precursors. Mapping sRNA reads to the AkBmirS2 precursor gene from the BD (Potter) and BE (Okhotsk) accessions revealed the presence of a 24-nt sRNA in anthers (Supplementary Fig. 6a), and the plant miRNA-target scoring system supported its binding to the promoter of the recessive SCR-D (Fig. 1e and Supplementary Fig. 6c). Similarly, mapping sRNA reads to the AkBmirS3 precursor gene revealed a 24-nt sRNA that targets the intron of SCR-E (Supplementary Figs. 7, 8). These findings support the hypothesis that mirS2 and mirS3 sRNAs are derived from S-haplogroup B precursors and can target SCR-D and SCR-E, respectively. The 24-nt sRNA of AkAmirS3 was found in the AD (Takashima) accession (Supplementary Fig. 8b), but not that of AkAmirS2 (Supplementary Fig. 6b), which was not detected despite the expression of its precursor gene AkAmirS2 (Fig. 1d). This may indicate a secondary decay of the precursor processing of AkAmirS2 and provide a unique control material for subsequent experiments.
Evolutionary restoration of self-incompatibility by repairing a mutation in the dominant S-haplogroup
In natural populations, the S-haplogroup B was combined with both the recessive haplogroups D and E. The sRNA data suggested that the 3,960-bp insertion in AkSCR-B represents a self-compatible mutation that is dominant over the S-haplogroups D and E. These data suggested that self-incompatibility should be restored when the function of the dominant SCR is restored, but not when that of the recessive SCR is restored. Therefore, a restored AkSCR-B of Potter accession without the 3960-bp insertion was generated. The restored AkSCR-B, under the control of its native promoter, was transformed into Potter accessions (Supplementary Fig. 9a). AkSCR-B expression at the anthers was detected in four independent transgenic lines. Self-incompatibility reaction upon self-pollination was detected, except for the line with a low expression (Pot_SCRB_4) (Fig. 2a–f and Supplementary Table 2). Moreover, they produced significantly shorter siliques with fewer seeds per silique than did the wild-type Potter accession (Fig. 2g–i). Further crossing experiments confirmed the viability of the pollen and pistils of these transgenic lines (Supplementary Fig. 9b, c, and Supplementary Tables 3 and 4). These results indicate that self-incompatibility was restored by repairing the single loss-of-function mutation in AkSCR-B.
Self-pollination assays of a wild-type (WT) Potter, b transgenic Potter with restored AkSCR-B (Pot_SCRB_1), c Pot_SCRB_2, d Pot_SCRB_3, and e Pot_SCRB_4. At least five observations showed similar results for each (sample numbers in Supplementary Table 2). Yellow arrows indicate a bundle of pollen tubes. Scale bar = 0.25 mm. f Expression of SCR-B in the anther cDNAs of A. halleri bearing haplogroup B (Ahal-B), WT Potter, and four transgenic Potter lines (mean ± S.D., neach line = 3). g Siliques of WT Potter and four transgenic Potter lines. Scale bar = 10 mm. h Comparison of silique length from self-pollination of WT Potter and four transgenic Potter lines. Silique lengths were significantly reduced in the transgenic lines compared to the WT, except for Pot_SCRB_4 (one-way ANOVA and Tukey HSD test, neach line = 5). i Comparison of the number of seeds per silique from self-pollination of WT Potter and four transgenic Potter lines. The number of seeds per silique was significantly fewer in the transgenic Potter lines than in the WT, except for Pot_SCRB_4 (one-way ANOVA and Tukey HSD test, neach line = 5, see Supplementary Table 7 for exact P-values). Boxplots h and i show center line: median; box limits: upper and lower quartiles; whiskers: within 1.5 times the interquartile range; dots: data points. Source data underlying f, h and i are provided as a Source data file.
No restoration of self-incompatibility by repairing a mutation in the recessive S-haplogroup
In contrast, four transgenic Potter lines transformed with AhSCR-D under the control of its native promoter neither expressed SCR-D nor recover self-incompatible reactions upon self-pollination (Fig. 3a, b and Supplementary Table 5). No significant differences in the silique length or number of seeds per silique between the transgenic lines and the wild-type Potter accession were observed (Fig. 3c, d). This result in the recessive S-haplogroup contrasts with that in the dominant one; however, it does not rule out the possibility that the AhSCR-D genomic fragment is nonfunctional because of the lack of some regulatory elements.
a SCR-D expression in the anther cDNAs of Arabidopsis halleri bearing haplogroup D (Ahal-D), WT Potter, and four transgenic Potter lines (Pot_SCRD). ACTIN was amplified as a quantitative control. b Siliques of WT Potter and transgenic Potter lines. Scale bar = 10 mm. c Comparison of silique length from self-pollination of WT Potter and transgenic Potter lines. The silique length of transgenic lines was not significantly different from that of the WT. d Comparison of the number of seeds per silique from self-pollination of WT Potter and transgenic Potter lines. The length of silique (c) or the number of seeds per silique (d) in the transgenic lines was not significantly different from that in the WT (one-way ANOVA and Tukey HSD test, neach line = 5, see Supplementary Table 7 for exact P-values). Boxplots c and d show center line: median; box limits: upper and lower quartiles; whiskers: within 1.5 times the interquartile range; dots: data points. Source data underlying c and d are provided as a Source data file.
To confirm that the AhSCR-D construct is adequate to confer self-incompatibility, it was introduced into the Takashima accession. Consistent with the lack of the 24-nt mirS2 sRNA in this accession, the expression of SCR-D was detected in all seven transgenic lines with a variable expression level. Self-incompatibility reaction was fully or partially observed in all the lines (Supplementary Table 6). Significantly shorter siliques and fewer seeds compared to those in the wild type were observed except for the silique length of the transgenic line Tak_SCRD_9, which showed the weakest SCR-D expression (Fig. 4a–d and Supplementary Table 7). Lines with a higher expression level such as Tak_SCRD_8 and Tak_SCRD_13 produced nearly no seeds (Fig. 4a–d). This experiment showed that the AhSCR-D genomic fragment is adequate to confer self-incompatibility in this accession. Furthermore, when the AhSCR-D promoter was introduced into the self-compatible A. thaliana, it was active in the stamen (Supplementary Fig. 10), indicating that the cloned promoter region is sufficient for expression.
a Expression of SCR-D in the anther cDNAs of Arabidopsis halleri bearing haplogroup D (Ahal-D), WT Takashima accession (WT), and seven transgenic Takashima lines (Tak_SCRD). ACTIN was amplified as a quantitative control. b Siliques derived from the WT Takashima accession and transgenic lines. Scale bar = 10 mm. c Comparison of silique length from self-pollination of WT and transgenic lines. Silique lengths were significantly reduced in the transgenic lines compared with those in the WT, except for in Tak_SCRD_9 (one-way ANOVA and Tukey HSD test, neach line = 5). d Comparison of the number of seeds per silique from self-pollination of WT and transgenic lines. The number of seeds per silique was significantly fewer in the transgenic lines than in the WT (one-way ANOVA and Tukey HSD test, neach line = 5, see Supplementary Table 7 for exact P-values). Boxplots c and d show center line: median; box limits: upper and lower quartiles; whiskers: within 1.5 times the interquartile range; dots: data points. Source data underlying c and d are provided as a Source data file.
Discussion
In this study, we first found the dominance of S-haplogroups A/B over D in pollen, and identified SCR sequences and sRNA. Transgenic plants with repaired SCR of the dominant S-haplogroup B restored the self-incompatibility. Similar to a previous transgenic study of the diploid natural species A. thaliana53, our results indicate that the mutation in the male specificity gene at the S-locus was responsible for self-compatibility also in A. kamchatica, supporting the adaptive spread of male self-compatible mutations predicted by the theory of sexual asymmetry12. Furthermore, our transgenic experiment showed that the self-compatible mutation was located at the S-locus, which encompasses the sRNAs responsible for male dominance. By contrast, the self-incompatibility was not restored when the recessive S-haplogroup D was repaired in the same accession, although this construct turned out functional in another accession that lacked the mirS2 sRNA. Together, the data suggest that a single loss-of-function mutation in the self-incompatibility specificity gene SCR-B conferred dominant self-compatibility.
The high frequency of loss-of-function of SCR or SRK, which can be induced by various mutations, such as frameshift and transposable insertion, can explain the prevalence and rapid evolution of self-compatibility in both polyploids and diploids. We suggest that the male dominance relationship plays a role in the evolution of self-compatibility in many natural and domesticated polyploid species2. In Brassica napus39,40 or the BE haplogroup of A. kamchatica, recessive S-haplogroups did not harbor obvious loss-of-function mutations, consistent with the current functional importance of suppression. In A. suecica41, Capsella bursa-pastoris42,43, and individuals with S-haplogroups D and C of A. kamchatica18, recessive S-haplogroups also had loss-of-function mutations. These mutations may represent secondary decay because self-incompatibility genes are not constrained after becoming self-compatible53,55. Alternatively, the mutations may have been segregated or fixed in diploid progenitor species, considering that the transition to self-compatibility is among the most frequent evolutionary transitions in angiosperms7,11,49,51. Female dominance can also contribute to the self-compatibility of allopolyploid species. In any case, dominance can allow a single mutation to confer self-compatibility in allopolyploid species, indicating that the existence of duplicated loci did not necessarily “retard” evolution6. In addition to duplicated S-loci in allopolyploid species, where experimental study is facilitated by fixed heterozygosity, we propose that dominance played a role in heterozygous alleles in diploid species and in each S-locus of allotetraploid species transiently during the evolution of self-compatibility until becoming homozygous. The relevance in diploid species is supported by the prevalence of dominant S-haplogroups having self-compatible mutations11. These findings support the theoretical prediction of Haldane’s sieve regarding the prevalence of dominant mutations increasing selfing rate11,20,21.
Our study highlighted the importance of experimental evidence in addition to sequencing for clarifying complex dominance mechanisms in the Brassicaceae self-incompatibility. The co-dominance of S-haplogroups B and D was observed in pistils18, and a previous report pointed out that S-haplogroup D belongs to the most dominant “phylogenetic class“51 based on phylogenetic analysis of SRK sequences32. However, our crossing experiment showed the male dominance of B over D. Furthermore, the evolutionary reversal experiment of SCR-B showed that the mutation in SCR was responsible for the evolution of self-compatibility, not that in other known or unknown genes such as an S-unlinked modifier gene recently reported from a self-compatible population of A. lyrata56. In this study, we focused on the transgenic experiments of SCR but did not manipulate sRNA. The epigenetic regulation of SCR expression by sRNA was also supported by transgenic experiments in diploid self-incompatible species28,30,31,32, and therefore we also suggest the importance of epigenetic regulation in the dominance of the allopolyploid A. kamchatica.
The S-locus inherited from A. halleri and another inherited from A. lyrata were combined in A. kamchatica18. Our study suggested that the S-haplogroup located on one of the two subgenomes was dominant over another S-haplogroup located on another subgenome. Analogous to the epigenetic regulation by sRNAs in diploid heterozygotes, our data lead to a hypothesis of epigenetic regulation between subgenomes. Many previous studies on the epigenetics of polyploid species focused on the contribution of stochastic epigenetic mutations in synthetic polyploids, which were considered to yield novel variations3,4,22,23,24,25. Our findings suggest that epigenetic interactions between inherited and combined subgenomes provide a mechanism for facilitating the evolution of a typical trait of polyploidy, i.e., self-compatibility. Arabidopsis kamchatica and other polyploid species with transgenic technique57,58 will be valuable to assess the role of stochastic and inherited epigenetic regulations in other adaptive traits.
Methods
Plant materials
Populations of A. halleri and A. kamchatica used in this study are previously reported18,48 and listed in Supplementary Table 8. Arabidopsis kamchatica (formerly called Arabidopsis lyrata subsp. kamchatica) is distributed across a broad latitudinal range in East Asia and North America, and can grow in soils with a broad range of the concentration of heavy metals44,46,47,59. The plants were grown in a plant chamber under long-day conditions (16 h light/8 h dark cycle, 22 °C). After the production of a few adult leaves, they were moved to a 4 °C chamber (8 h light/16 h dark cycle) for 4–6 weeks for vernalization to induce flowering.
Isolation of SCR genes
Total RNAs were extracted from the anthers of 20 flower buds (stage 12)60 each of A. halleri bearing haplogroups A, B, or D using an RNeasy Plant Mini kit (Qiagen, Hilden, Germany). Complementary DNA (cDNA) was synthesized using the SMARTer kit (TaKaRa Bio USA Inc., San Jose, CA, USA) and submitted for high-throughput sequencing using the Ion Torrent PGM sequencer (Life Technologies, Carlsbad, CA, USA) at the Functional Genomic Center Zurich (Zurich, Switzerland). RNA-seq data were de novo assembled by Trinity61 (default parameters). A total of 15 known SCR amino acid sequences were used as queries in the TBLASTN homology search against the assembled data as a database (Supplementary Fig. 1d). AkSCR-E was isolated using the amino acid sequence of AhSCR2932, which is phylogenetically close to SCR-E18, as a query in the TBLASTN homology searches against the assembled high-throughput genomic sequence data of A. kamchatica from Okhotsk that has S-haplogroups B and E (assembled by SOAPdenovo261 with k-mer = 57 as predicted by KmerGenie62). From the TBLASTN results, potential contigs were selected by manually screening for the presence of the eight conserved cysteine residues in SCR. Next, candidates that were detected in more than one A. halleri bearing different haplogroups were eliminated because SCR genes of different S-haplogroups must be highly polymorphic among each other. Primers were designed around the stop codons of the candidates for genotyping by PCR using ExTaq polymerase (TaKaRa Bio Inc., Shiga, Japan) (Supplementary Table 9).
SCR co-segregates with SRK of the same haplogroup, as they are tightly linked at the S-locus. Using the specific primers that amplify putative SCR-A, SCR-B, SCR-E, AkSRK-A, AkSRK-B, and AkSRK-E genes, segregation of SCR-A, SCR-B, and SCR-E were checked in an F2 population (94 individuals) generated by crossing A. kamchatica bearing S-haplogroups A and D and A. kamchatica bearing S-haplogroups B and E (Supplementary Table 1). Additionally, SCR-D isolated from A. halleri could not be amplified in A. kamchatica. Therefore, the segregation pattern of SCR-D was examined in F2 populations (32 individuals) generated by crossing the A. halleri bearing haplogroup D with A. halleri bearing haplogroup B or A (Supplementary Table 10).
Isolation of full-length SCR genes
Genomic DNA was extracted from the leaf tissues of A. halleri and A. kamchatica using a DNeasy Plant Mini Kit (Qiagen). Primers were designed using SCR genes based on the assembled RNA-seq data to isolate full-length SCR genes of A. halleri and A. kamchatica (Supplementary Tables 8 and 9). Mutations in the AkSCR genes were detected by comparing the SCR sequences of A. halleri and A. kamchatica in Geneious 6.1.763. Insertion in AkSCR-B was isolated following the instructions of the GenomeWalker™ Universal Kit User Manual (TaKaRa Bio USA Inc., San Jose, CA, USA). The promoter regions of SCR genes were also isolated using the same method. DNA samples of A. halleri from various populations were genotyped to identify the A. halleri bearing haplogroup E for the isolation of full-length AhSCR-E.
Expression of SCR genes
Total RNA was extracted from the anthers at stage 12 of A. halleri and wild and transgenic A. kamchatica using an RNeasy Plant Mini kit (Qiagen). cDNAs were synthesized from 500 ng of each of the total RNA using a High-Capacity RNA-to-cDNA™ Kit (Applied Biosystems, Foster City, CA, USA). Primers flanking the intron of SCR genes were used for reverse transcription polymerase chain reaction (RT-PCR) and a reverse transcription quantitative PCR (RT-qPCR) (Supplementary Table 9). The latter was performed using PowerUp SYBR Green Master Mix (A25742) in StepOnePlus Real-Time PCR System of Applied Biosystems. Gel pictures were produced using Gel Doc XR+ Gel Documentation System with Image Lab Software ver. 4.1 (Bio-Rad, Hercules, CA, USA) and ImageJ ver. 2.15.0. ACTIN or EF1α (ELONGATION FACTOR 1α) was amplified as the quantitative control.
Probe preparation for southern blot
A 317-bp probe designed at the second exon and 3′ UTR of SCR-D was cloned into the pCR™4-TOPO® vector using the TOPO® TA Cloning® kit (Thermo Fisher Scientific, Waltham, MA, USA) (Supplementary Table 9). Using the vector as a template, the probe was labeled with DIG-11-dUTP via PCR according to the manufacturer’s instruction of the PCR DIG Probe Synthesis Kit (Roche Applied Science, Indianapolis, IN, USA). Labeling was confirmed using gel electrophoresis, as the labeled probes have higher molecular weight than unlabeled probes.
Southern blotting analysis
Genomic DNA was extracted from the leaf tissues of A. halleri bearing haplogroup D and three A. kamchatica accessions—Murodo, Takashima, and Potter accessions using DNeasy Plant Maxi Kit (Qiagen, Hilden, Germany). A total of 10 µg of each of the accessions was digested with XbaI (New England Biolabs, Beverly, MA, USA) at 37 °C overnight. Digested DNA, together with DIG-labeled DNA Molecular Weight Marker VII (Roche Applied Science), was separated using 1% agarose gel (at 100 V for 1 h). Blotting analysis and hybridization were performed according to the DIG Application Manual for Filter Hybridization (Roche Applied Science). Capillary transfer of DNA to a positively charged nylon membrane (Roche Applied Science) was performed overnight. DNA was then fixed to the blot via UV crosslinking. The blots were hybridized at 40 °C in 10 ml of hybridization buffer at a concentration of 6 μl of the labeled probes per ml of hybridization buffer. Membranes were washed and chromogenic detections were performed using anti-DIG-alkaline phosphatase and NBT/BCIP (DIG-High Prime DNA Labeling and Detection Starter Kit I, Roche Applied Science) according to the manufacturer’s instructions.
Dominance hierarchy of S-haplogroups A, B, and D
A. halleri individuals bearing haplogroup A, B, or D were crossed among each other to generate A. halleri bearing haplogroups A and B (AB), A. halleri bearing haplogroups A and D (AD), and A. halleri bearing haplogroups B and D (BD). To test the dominance relationship between A and D in pollen, the pollen grains of A. halleri individuals with AD were applied to the stigmas of A. halleri bearing haplogroup A and that bearing haplogroup D. Similarly, to test the dominance relationship between B and D in pollen, the pollen grains of A. halleri individuals with BD were crossed to stigmas bearing haplogroup B and those bearing haplogroup D. To test the dominance relationship between A and B, in addition to stigmas, the pollen grains of A. halleri individuals with AB were crossed to the stigmas bearing haplogroup A, B, as well as D, the last of which was performed to show that the pollen grains were viable in this co-dominant case. Pollen tube growth was examined using aniline blue staining18. S-specificity of A. halleri used in this experiment was previously confirmed via interspecific crossings with A. kamchatica18. The expression of SCR-A, SCR-B, and SCR-D were also examined using these genotypes.
Isolation of sRNA precursors, detection of sRNA, and mapping
The sRNA precursor of A. lyrata bearing haplogroup Sb has been previously isolated31. Primers were designed based on this precursor to isolate sRNA precursors in A. kamchatica (Supplementary Table 9). Both the precursor sequences amplified from A. kamchatica bearing haplogroup A and A. kamchatica bearing haplogroup B showed high sequence homology with the mirS2 family, thus designated as AkAmirS2 and AkBmirS2, respectively. The segregation patterns of AkAmirS2 and AkBmirS2 were examined in an F2 population (94 individuals) generated by crossing A. kamchatica bearing haplogroups A and D and A. kamchatica bearing haplogroups B and E. AkAmirS2 and AkBmirS2 were perfectly linked to S-haplogroups A and B, respectively (Supplementary Table 1). The secondary structures of the precursor genes were predicted by Geneious 6.1.763.
Total RNA was extracted from the anthers of 10 flower buds (stages 9–11) from each of these accessions: A. kamchatica from Potter and Okhotsk and Takashima using a mirVana™ miRNA Isolation Kit (Ambion, Austin, TX, USA). To check the expression of sRNA precursors, total RNA was treated with DNase (DNA-free™ DNA Removal Kit, Ambion) and reverse-transcribed to cDNA by High-Capacity RNA-to-cDNA™ Kit (Applied Biosystems). Primers amplifying AkAmirS2 and AkBmirS2 were used for RT-PCR (Supplementary Table 9). ACTIN was amplified as the quantitative control.
Total RNA before DNase treatment was submitted for small RNA sequencing by Illumina HiSeq 2500 (Illumina, San Diego, CA, USA) at the Functional Genomics Center Zürich. Adaptors were trimmed, and reads shorter than 20 bp were filtered out before being mapped to sRNA precursors to examine the presence of AkAmirS2 and AkBmirS2. Reads were mapped to sRNA precursors using Bowtie 2.3.2 (command: bowtie2 --no-unal -x [file name of reference fasta] -U [file name of fastq])64 and viewed in the Integrative Genomics Viewer (IGV) 2.3.3565. To isolate the sRNA targeting SCR-E, known sRNA precursor genes (mirS1, mirS2, mirS4, mirS5, mir867, mir1887, and mir4239)32 were used as query in a homology search against the de novo assembled sequence data of Okhotsk and Takashima, assembled by SOAPdenovo266 with k-mer value of 85 as predicted by KmerGenie62. High-throughput sRNA reads of Okhotsk anthers were mapped to the potential precursors to detect the presence of 24-nt sRNA using Bowtie 2.3.264 and viewed in the IGV 2.3.3565 as described above. A 24-nt sRNA showing high sequence homology, with a target site at the intron region of AkSCR-E, was identified from the mirS3 precursor gene, and thus designated as AkBmirS3. AkBmirS3 co-segregates with SCR-B (Supplementary Tables 1 and 9). The presence of 24-nt mirS3 targeting AkSCR-E was also detected in Takashima, thus designated as AkAmirS3. BWA 0.7.12 (command: bwa mem -t8 [file name of reference fasta] [file name of fastq])67 and IGV 2.3.3565 were similarly used for the mapping and the visualization of genomic sequences (SAMD00045705, SAMD00089932, SAMD00089933)49,68 on the BAC sequences of the S-locus region of S-haplogroup Ah12 (Genbank KJ772374.1)32.
Cloning and transformation of restored AkSCR-B and AhSCR-D
The full-length sequences, including the promoter and coding sequences of AkSCR-B of Potter accession, were restored via a series of PCR amplification using high-fidelity PrimeSTAR GXL DNA polymerase (TaKaRa Bio Inc.) (Supplementary Table 9). First, two different fragments eliminating the insertion were amplified (AkSCR-B_1°). Then, 100× diluted PCR products (AkSCR-B_1°) were used as the templates for the second round of PCR to amplify the full-length restored AkSCR-B (AkSCR-B_2°). Since SCR-D has been deleted in A. kamchatica, SCR-D of A. halleri was cloned for transformation into A. kamchatica. A 3092-bp region of AhSCR-D, including 648-bp of promoter regions, two exons, an intron, and 85-bp after stop codon, was amplified by high-fidelity PrimeSTAR GXL DNA polymerase (TaKaRa Bio Inc.) (Supplementary Table 9). Then, 3′ A-overhangs were added to the restored sequences and cloned into pCR®8/GW/TOPO® (Thermo Fisher Scientific) according to the manufacturer’s instructions. The sequences were inserted into the destination vector pFAST-G0169 via LR recombination using LR Clonase® II Enzyme mix (Thermo Fisher Scientific). The vectors were then transformed into Agrobacterium tumefaciens GV3101 via electroporation. Constructs generated were transformed into the Potter accession bearing haplogroups B and D and the Takashima accession bearing haplogroups A and D using Agrobacterium-mediated floral dip transformation45.
Genotyping and phenotyping of transformed A. kamchatica
Transformed seeds were selected by observation under a fluorescence stereomicroscope with a GFP filter (Olympus SZX12, Japan). The DNA was extracted from the leaf tissues of T1 plants, and insertion of generated constructs was confirmed via PCR using primers designed from the vectors and respective inserted SCR genes (Supplementary Table 9). Pollination assays were performed to test whether the male components of the transformed A. kamchatica are functional. The anthers of transgenic lines were removed from the flower buds at early stage 13 (before anthesis) and visualized under a stereomicroscope to ensure that there is no pollen on the stigmas. They were then manually pollinated with self-pollen. Stigmas were harvested 24 h after pollination, and pollen tube growth was examined using aniline blue staining18. Silique length and the number of seeds per silique of the wild type (WT) and transgenic plants were measured. Statistical significance was assessed by the Bonferroni-corrected Mann–Whitney U test and one-way ANOVA and Tukey HSD test in R. In addition to self-pollination, crosses between transformed pollen and WT pistil and between WT pollen and transformed pistil were checked to ensure the viability of the pollen and pistil, respectively.
Cloning of the AhSCR-D promoter and GUS staining
The 648-bp of the promoter region of AhSCR-D was amplified and cloned into the pCRTM8/GW/TOPO® vector using a TA Cloning Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. The promoter was inserted upstream of the β-Glucuronidase (GUS) reporter gene in the binary vector pGWB3 by LR recombination using LR Clonase® II Enzyme mix (Thermo Fisher Scientific). The vectors were then transformed into Agrobacterium tumefaciens GV3101 via electroporation. The construct was introduced into A. thaliana using the floral dip transformation method. Transformants were selected on 50 µl/ml kanamycin selection plates. Flower buds were collected and immersed in ice-chilled 90% acetone for 20 min. Samples were vacuum-filtrated for 15 min with GUS staining solution containing 1 mM X-Gluc, 50 mM sodium phosphate buffer, 0.1% Triton X-100, 2 mM potassium ferrocyanide, and 2 mM potassium ferricyanide. Samples were incubated overnight at 37 °C and treated with Herr clearing solution before observation under microscope70.
Statistics and reproducibility
Statistical analyses were conducted using R (4.2.2). Exact P-values are shown in Supplementary Table 7. The results of all RT-PCR gel images were verified by two more independent experiments.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The sequence data are available in the INSDC databases under accession code, BioProject: PRJDB7179, DRA accession ID: DRA007456 and GenBank accession numbers: OQ852734-OQ852742. The source data are provided in the source data file. Source data are provided with this paper.
References
Van de Peer, Y., Mizrachi, E. & Marchal, K. The evolutionary significance of polyploidy. Nat. Rev. Genet. 18, 411–424 (2017).
Akagi, T., Jung, K., Masuda, K. & Shimizu, K. K. Polyploidy before and after domestication of crop species. Curr. Opin. Plant Biol. 69, 102255 (2022).
Shimizu, K. K. Robustness and generalist niche of polyploid species: genome shock or gradual evolution? Curr. Opin. Plant Biol. 69, 102292 (2022).
Comai, L. The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 6, 836–846 (2005).
Soltis, D. E., Visger, C. J. & Soltis, P. S. The polyploidy revolution then…and now: Stebbins revisited. Am. J. Bot. 101, 1057–1078 (2014).
Stebbins, G. L. Chromosomal Evolution in Higher Plants (Edward Arnold Ltd., London., 1971).
Stebbins, G. L. Variation and Evolution in Plants (Columbia University Press, 1950).
Barringer, B. C. Polyploidy and self-fertilization in flowering plants. Am. J. Bot. 94, 1527–1533 (2007).
Mable, B. K. Polyploidy and self-compatibility: is there an association? New Phytol. 162, 803–811 (2004).
Robertson, K., Goldberg, E. E. & Igić, B. Comparative evidence for the correlated evolution of polyploidy and self-compatibility in Solanaceae. Evolution 65, 139–155 (2011).
Shimizu, K. K. & Tsuchimatsu, T. Evolution of selfing: recurrent patterns in molecular adaptation. Annu. Rev. Ecol. Evol. Syst. 46, 593–622 (2015).
Tsuchimatsu, T. & Shimizu, K. K. Effects of pollen availability and the mutation bias on the fixation of mutations disabling the male specificity of self-incompatibility. J. Evol. Biol. 26, 2221–2232 (2013).
Darwin, C. The Effects of Cross and Self Fertilisation in the Vegetable Kingdom (John Murray, 1876).
Levin, D. A. Minority cytotype exclusion in local plant populations. Taxon 24, 35–43 (1975).
Clo, J. & Kolář, F. Inbreeding depression in polyploid species: a meta-analysis. Biol. Lett. 18, 20220477 (2022).
Otto, S. P. The evolutionary consequences of polyploidy. Cell 131, 452–462 (2007).
Soltis, P. S. & Soltis, D. E. The role of genetic and genomic attributes in the success of polyploids. Proc. Natl Acad. Sci. USA 97, 7051–7057 (2000).
Tsuchimatsu, T., Kaiser, P., Yew, C.-L., Bachelier, J. B. & Shimizu, K. K. Recent loss of self-incompatibility by degradation of the male component in allotetraploid Arabidopsis kamchatica. PLoS Genet. 8, e1002838 (2012).
Novikova, P. Y., Kolesnikova, U. K. & Scott, A. D. Ancestral self-compatibility facilitates the establishment of allopolyploids in Brassicaceae. Plant Reprod. 36, 125–138 (2022).
Haldane, J. B. S. A mathematical theory of natural and artificial selection, part V: selection and mutation. Math. Proc. Camb. Philos. Soc. 23, 838–844 (1927).
Glémin, S. & Ronfort, J. Adaptation and maladaptation in selfing and outcrossing species: new mutations versus standing variation. Evolution 67, 225–240 (2013).
Bird, K. A., VanBuren, R., Puzey, J. R. & Edger, P. P. The causes and consequences of subgenome dominance in hybrids and recent polyploids. New Phytol. 220, 87–93 (2018).
Qiu, T., Liu, Z. & Liu, B. The effects of hybridization and genome doubling in plant evolution via allopolyploidy. Mol. Biol. Rep. 47, 5549–5558 (2020).
Gordon, S. P. et al. Gradual polyploid genome evolution revealed by pan-genomic analysis of Brachypodium hybridum and its diploid progenitors. Nat. Commun. 11, 3670 (2020).
Burns, R. et al. Gradual evolution of allopolyploidy in Arabidopsis suecica. Nat. Ecol. Evol. 5, 1367–1381 (2021).
Bente, H., Foerster, A. M., Lettner, N. & Scheid, O. M. Polyploidy-associated paramutation in Arabidopsis is determined by small RNAs, temperature, and allele structure. PLoS Genet. 17, e1009444 (2021).
Ng, D. W. K., Lu, J. & Chen, Z. J. Big roles for small RNAs in polyploidy, hybrid vigor, and hybrid incompatibility. Curr. Opin. Plant Biol. 15, 154–161 (2012).
Tarutani, Y. et al. Trans-acting small RNA determines dominance relationships in Brassica self-incompatibility. Nature 466, 983–986 (2010).
Fisher, R. A. The possible modification of the response of the wild type to recurrent mutations. Am. Nat. 62, 115–126 (1928).
Yasuda, S., Kobayashi, R., Ito, T., Wada, Y. & Takayama, S. Homology-based interactions between small RNAs and their targets control dominance hierarchy of male determinant alleles of self-incompatibility in Arabidopsis lyrata. Int. J. Mol. Sci. 22, 6990 (2021).
Yasuda, S. et al. A complex dominance hierarchy is controlled by polymorphism of small RNAs and their targets. Nat. Plants 3, 16206 (2016).
Durand, E. et al. Dominance hierarchy arising from the evolution of a complex small RNA regulatory network. Science 346, 1200–1205 (2014).
Fujii, S., Kubo, K. I. & Takayama, S. Non-self- and self-recognition models in plant self-incompatibility. Nat. Plants 2, 1–9 (2016).
Iwano, M. et al. Calcium signalling mediates self-incompatibility response in the Brassicaceae. Nat. Plants 1, 1–9 (2015).
Murase, K. et al. A membrane-anchored protein kinase involved in Brassica self-incompatibility signaling. Science 303, 1516–1519 (2004).
Hatakeyama, K., Watanabe, M., Takasaki, T., Ojima, K. & Hinata, K. Dominance relationships between S-alleles in self-incompatible Brassica campestris L. Heredity 80, 241–247 (1998).
Llaurens, V. et al. Does frequency-dependent selection with complex dominance interactions accurately predict allelic frequencies at the self-incompatibility locus in Arabidopsis halleri? Evolution 62, 2545–2557 (2008).
Prigoda, N. L., Nassuth, A. & Mable, B. K. Phenotypic and genotypic expression of self-incompatibility haplotypes in Arabidopsis lyrata suggests unique origin of alleles in different dominance classes. Mol. Biol. Evol. 22, 1609–1620 (2005).
Okamoto, S. et al. Self-compatibility in Brassica napus is caused by independent mutations in S-locus genes. Plant J. 50, 391–400 (2007).
Gao, C. et al. Helitron-like transposons contributed to the mating system transition from out-crossing to self-fertilizing in polyploid Brassica napus L. Sci. Rep. 6, 33785 (2016).
Novikova, P. Y. et al. Genome sequencing reveals the origin of the allotetraploid Arabidopsis suecica. Mol. Biol. Evol. 34, 957–968 (2017).
Bachmann, J. A. et al. On the origin of the widespread self-compatible allotetraploid Capsella bursa-pastoris (Brassicaceae). Heredity 127, 124–134 (2021).
Nasrallah, J. B., Liu, P., Sherman-Broyles, S., Schmidt, R. & Nasrallah, M. E. Epigenetic mechanisms for breakdown of self-incompatibility in interspecific hybrids. Genetics 175, 1965–1973 (2007).
Shimizu, K., Fujii, S., Marhold, K., Watanabe, K. & Kudoh, H. Arabidopsis kamchatica (Fisch. ex DC.) K. Shimizu & Kudoh and A. kamchatica subsp. kawasakiana (Makino) K. Shimizu & Kudoh, new combinations. Acta Phytotaxon. Geobot. 56, 163–172 (2005).
Yew, C.-L., Kakui, H. & Shimizu, K. K. Agrobacterium-mediated floral dip transformation of the model polyploid species Arabidopsis kamchatica. J. Plant Res. 131, 349–358 (2018).
Paape, T. et al. Conserved but attenuated parental gene expression in allopolyploids: constitutive zinc hyperaccumulation in the allotetraploid Arabidopsis kamchatica. Mol. Biol. Evol. 33, 2781–2800 (2016).
Akiyama, R. et al. Seasonal pigment fluctuation in diploid and polyploid Arabidopsis revealed by machine learning-based phenotyping method PlantServation. Nat. Commun. 14, 5792 (2023).
Shimizu-Inatsugi, R. et al. The allopolyploid Arabidopsis kamchatica originated from multiple individuals of Arabidopsis lyrata and Arabidopsis halleri. Mol. Ecol. 18, 4024–4048 (2009).
Paape, T. et al. Patterns of polymorphism and selection in the subgenomes of the allopolyploid Arabidopsis kamchatica. Nat. Commun. 9, 3909 (2018).
Mable, B. K. et al. What causes mating system shifts in plants? Arabidopsis lyrata as a case study. Heredity. 118, 52–63 (2017).
Kolesnikova, U. K. et al. Transition to self-compatibility associated with dominant S-allele in a diploid Siberian progenitor of allotetraploid Arabidopsis kamchatica revealed by Arabidopsis lyrata genomes. Mol. Biol. Evol. 40, msad122 (2023).
Akama, S., Shimizu-Inatsugi, R., Shimizu, K. K. & Sese, J. Genome-wide quantification of homeolog expression ratio revealed nonstochastic gene regulation in synthetic allopolyploid Arabidopsis. Nucleic Acids Res. 42, e46 (2014).
Tsuchimatsu, T. et al. Evolution of self-compatibility in Arabidopsis by a mutation in the male specificity gene. Nature 464, 1342–1346 (2010).
Kusaba, M. et al. Self-incompatibility in the genus Arabidopsis: characterization of the S locus in the outcrossing A. lyrata and its autogamous relative A. thaliana. Plant Cell 13, 627–643 (2001).
Shimizu, K. K., Shimizu-Inatsugi, R., Tsuchimatsu, T. & Purugganan, M. D. Independent origins of self-compatibility in Arabidopsis thaliana. Mol. Ecol. 17, 704–714 (2008).
Li, Y., Mamonova, E., Köhler, N., van Kleunen, M. & Stift, M. Breakdown of self-incompatibility due to genetic interaction between a specific S-allele and an unlinked modifier. Nat. Commun. 14, 3420 (2023).
Przetakiewicz, A., Karaś, A., Orczyk, W. & Nadolska-Orczyk, A. Agrobacterium-mediated transformation of polyploid cereals. The efficiency of selection and transgene expression in wheat. Cell. Mol. Biol. Lett. 9, 903–917 (2004).
Shan, S. et al. Application of CRISPR/Cas9 to Tragopogon (Asteraceae), an evolutionary model for the study of polyploidy. Mol. Ecol. Resour. 18, 1427–1443 (2018).
Paape, T. et al. Experimental and field data support range expansion in an allopolyploid Arabidopsis owing to parental legacy of heavy metal hyperaccumulation. Front. Genet. 11, 1162 (2020).
Smyth, D. R., Bowman, J. L. & Meyerowitz, E. M. Early flower development in Arabidopsis. Plant Cell 2, 755–767 (1990).
Grabherr, M. G. et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 29, 644–652 (2011).
Chikhi, R. & Medvedev, P. Informed and automated k-mer size selection for genome assembly. Bioinformatics 30, 31–37 (2014).
Kearse, M. et al. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649 (2012).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, 1–10 (2009).
Robinson, J. T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26 (2011). 2011 291.
Luo, R. et al. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience 1, 18 (2012).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Novikova, P. Y. et al. Sequencing of the genus Arabidopsis identifies a complex history of nonbifurcating speciation and abundant trans-specific polymorphism. Nat. Genet. 48, 1077–1082 (2016).
Shimada, T. L., Shimada, T. & Hara-Nishimura, I. A rapid and non-destructive screenable marker, FAST, for identifying transformed seeds of Arabidopsis thaliana. Plant J. 61, 519–528 (2010).
Herr, J. M. A new clearing‐squash technique for the study of ovule development in angiosperms. Am. J. Bot. 58, 785–790 (1971).
Acknowledgements
We thank Ueli Grossniklaus, Andreas Wagner, Barbara Mable, Vincent Castric, Yuko Wada, and Risa Kobayashi for contributing to the discussion and the Functional Genomics Center Zurich for providing technical support. This study was supported by the Swiss National Science Foundation 310030_212551, 31003A_182318, 31003A_159767, 31003A_140917 to KKS, 310030_212674 to R.S.-I.; the Japan Science and Technology Agency CREST (grant number JPMJCR16O3), Japan; SystemsX.ch SXPHX0-124233; UZH Global Strategy and Partnerships Funding Scheme of the University of Zurich to KKS; the University Research Priority Program of Evolution in Action of University of Zurich to KKS and RSI; the MEXT JSPS KAKENHI (grant numbers 16H06469, 16K21727, 22H02316 to KKS; 16H06467 and 17H05833 to TT; International Leading Research: 22K21352 to TT and KKS; 22H05172 and 22H05179 to KKS and MW; 21H02162 to MW; 21H04711 and 21H05030 to ST); and an EMBO long-term fellowship and a JSPS postdoctoral fellowship for research abroad to TT.
Author information
Authors and Affiliations
Contributions
C-L.Y., T.T., and K.K.S. conceived and designed the study; C.-L.Y., H.K., and T.O. performed the experiments and generated the data supported by R.S.-I., K.S., M.W., and K.K.S.; C-L.Y. analyzed the data with help from T.T., M.H., S.Y., S.T, and K.K.S.; C.-L.Y. and K.K.S. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Daphne Goring, Yijing Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yew, CL., Tsuchimatsu, T., Shimizu-Inatsugi, R. et al. Dominance in self-compatibility between subgenomes of allopolyploid Arabidopsis kamchatica shown by transgenic restoration of self-incompatibility. Nat Commun 14, 7618 (2023). https://doi.org/10.1038/s41467-023-43275-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-43275-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.