Abstract
The conserved SR-like protein Npl3 promotes splicing of diverse pre-mRNAs. However, the RNA sequence(s) recognized by the RNA Recognition Motifs (RRM1 & RRM2) of Npl3 during the splicing reaction remain elusive. Here, we developed a split-iCRAC approach in yeast to uncover the consensus sequence bound to each RRM. High-resolution NMR structures show that RRM2 recognizes a 5´-GNGG-3´ motif leading to an unusual mille-feuille topology. These structures also reveal how RRM1 preferentially interacts with a CC-dinucleotide upstream of this motif, and how the inter-RRM linker and the region C-terminal to RRM2 contribute to cooperative RNA-binding. Structure-guided functional studies show that Npl3 genetically interacts with U2 snRNP specific factors and we provide evidence that Npl3 melts U2 snRNA stem-loop I, a prerequisite for U2/U6 duplex formation within the catalytic center of the Bact spliceosomal complex. Thus, our findings suggest an unanticipated RNA chaperoning role for Npl3 during spliceosome active site formation.
Similar content being viewed by others
Introduction
Serine and Arginine-rich (SR) proteins belong to a family of proteins best known for their function in pre-mRNA splicing regulation, as well as multiple steps of gene expression from transcription to translation1,2. SR proteins typically contain one or two N-terminal RNA-recognition motifs (RRM) followed by a C-terminal arginine-serine-rich (RS) domain3. In humans, 12 proteins belong to the SR protein family named SRSF1 to SRSF123. They are found in all metazoans in which they play a role in constitutive and alternative splicing regulation of most genes4. In contrast, only around 4% of the genes of the budding yeast Saccharomyces cerevisiae contain introns and a few among them are alternatively spliced5,6. Consequently, only three SR-like proteins were identified in budding yeast (Gbp2, Hrb1, and Npl3)7,8. Of the three proteins, only Npl3 can promote splicing of intron-containing genes9. Npl3 was proposed to facilitate the splicing reaction by promoting the co-transcriptional recruitment of the spliceosome on chromatin through interactions via U1 and possibly U2 snRNPs9 and with the Rad6 complex that adds a mono-ubiquitin to the histone H2B10. Recently, Npl3 was also shown to be involved in the late steps of yeast spliceosome assembly by stimulating Prp28 helicase activity when Npl3 is phosphorylated. It was proposed that Npl3 may be the functional counterpart of the metazoan Prp28 N-terminal region that is absent in the yeast counterpart11. Finally, Npl3 was also shown to be required for the proper execution of the meiotic cell cycle by promoting splicing of introns containing non-consensus splice sites12. Npl3 has been implicated to function in multiple processes of gene expression including mRNA transcription elongation and termination13,14,15,16,17, mRNA export under stress7,8,18,19 and translation20,21. Npl3 was also reported to maintain genome chromatin stability by preventing R-loops formation22, contributing to telomere maintenance23 and promoting double-strand DNA break repair24. Npl3 shares many functions with other metazoan SR proteins, and therefore serves as an ideal model to understand the evolution of SR proteins.
Npl3 is composed of two consecutive RRMs separated by a flexible eight amino acids linker that are followed by a C-terminal RS domain containing an Arg-Gly-Gly (RGG) repeat25,26. The first canonical RRM is followed by a second pseudo-RRM27,28. Although Npl3 binding to RNA is important for its functions, its mode of RNA-recognition remains elusive. The structures of the two RRMs of Npl3 were previously determined in their free form and showed that both RRMs do not interact29,30. Npl3 was shown to bind preferentially to UG-rich RNA sequences using primarily its pseudo-RRM (RRM2)29, and a structural model of both RRMs bound to RNA indicates that the RRM1 could bind to a CA motif31.
Here, we develop split-iCRAC, a combination of CRAC16 and iCLIP technologies32, to identify the RNA sequences bound by yeast Npl3 in vivo. Using NMR spectroscopy, we determine the structures of both RRMs bound to a representative RNA consensus sequence obtained with the split-iCRAC approach. Our analyses reveal that RRM1 binds preferentially upstream of the RRM2 binding site. Both domains recognize a distinct RNA motif: 5’-NCCN-3’ and 5’-GNGGN-3’, respectively (N is for A, C, G or U) with the interdomain linker contributing to the RNA binding. Structure-guided studies reveal that mutations within RRM1, but not RRM2, negatively impact Npl3 function. However, Npl3 RRM2 has a specific effect on the splicing reaction, that is mediated through an unanticipated interaction of the protein with the U2 snRNA. We show that this interaction destabilizes the U2 snRNA stem-loop I, thus suggesting an RNA chaperoning role for Npl3 during the formation of the spliceosome active site.
Results
Identification of a consensus RNA motif recognized by Npl3 using iCRAC
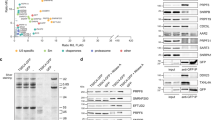
Npl3 preferentially binds to U/G rich sequences in vitro29 and in vivo16. However, the precise RNA motif(s) recognized by the two RRMs has remained elusive. To uncover the recognition motif of Npl3 in vivo, we performed a Crosslinking and Analysis of cDNA (CRAC) experiment with two key modifications16. First, we introduced an HRV-3C protease cleavage site directly after the sequence of RRM2 to distinguish RNAs that interact with the RRMs from those that are bound to the RGG/RS domain (Fig. 1A). A similar strategy was used to analyze exosome targets33. Second, we used the individual-nucleotide resolution Cross-Linking and Immunoprecipitation (iCLIP) strategy to prepare the cDNA library and obtain single nucleotide resolution of the crosslinking sites32. We termed this approach split-iCRAC. After HRV-3C cleavage, most of the RNA bound by Npl3 was detected with the RRMs (Fig. 1B), and a smaller fraction of RNA was detected bound to the RGG/RS domain. iCRAC libraries were then prepared with RNA isolated from the full-length protein (FL) and the RRM1/2 or RGG/RS domains. After mapping the sequencing reads to the S. cerevisiae genome, unique cDNA sequences from all replicates per sample were merged and used for cluster definition. De novo motif search was done using the HOMER software34 with sequences containing ±5 nucleotides around the identified clusters. Among all identified consensus motifs (Supplementary Fig. 1A), only three were present in both FL and RRM1/2 and absent in the negative control (same experiment without the expression of tagged protein in cells) (Supplementary Fig. 1B) leading to the determination of three consensus sequences, two for the FL protein and one for RRM1/2 (Fig. 1C). The three motifs have very similar sequences suggesting that the RGG/RS domain does not contribute to the specificity of RNA recognition. Nevertheless, we noticed a small preference of the RGG/RS domain in isolation for the GCGUAUAUC motif, which suggests that the domain could, in this context, preferentially contact this sequence (Supplementary Fig. 1B). Finally, the identified RNA bound by the RGG/RS domain are U-rich, which most likely reflects the higher efficiency of UV crosslinking to uracil over other nucleotides35.
A Schematic representation of Npl3 domain composition. The two RRMs are followed by an RS domain that is rich in RGG repeats. Amino acids at the border of each domain are numbered. The HRV-3C cleavage site inserted between the RRMs and the RGG/RS domain is shown. B Autoradiography of 32P labeled RNA after migration of the crosslinked complex on an SDS-PAGE gel. A negative control without exposure to UV shows no RNA band at the size of Npl3. Upon crosslinking with UV, the band becomes sensitive to RNase treatment. The stars represent unspecific bands that may come from the phosphorylation reaction (e.g. T4 PNK). The membrane blotting shows that after treatment of the samples with HRV-3C protease, the RNAs bound to both RRMs are separated from the RNAs bound to the RGG/RS domain. Four replicates of the full-length protein and RRM1/2 domain, three replicates of the RGG/RS and one negative control sample were Illumina sequenced. Source data are provided as a Source Data file. C Enriched motifs identified by split-iCRAC with Npl3 full length (top 2) and RRM1/2 using HOMER de novo motif finding on split-iCRAC derived clusters ±5 nucleotides. D Overlay of 1H-15N HSQC spectra recorded during the NMR titration of 15N labeled Npl3 RRM1/2 with increasing amount of unlabeled 5´-AUCCAGUGGAA-3´ RNA containing the bipartite motif identified by split-iCRAC (free form of the protein in blue, protein:RNA ratios of 1:0.3 and 1:1 in orange and red, respectively). Key residues of RRM1, RRM2 and the inter-domain linker for which a shift is observed upon RNA binding are indicated by an arrow labeled with pink, cyan and green colors, respectively. E ITC measurement performed in duplicate with Npl3 RRM1/2 and the AUCCAGUGGAA RNA.
Mode of interaction of Npl3 RRM1/2 with RNA
The split-iCRAC approach identified 5’-WCCAGWGGA-3’ (where W is U or A) as the consensus sequence interacting with RRM1/2 and the full-length Npl3 (Fig. 1C). To validate these findings, we monitored the binding of a recombinant Npl3 containing the two RRMs connected by their natural linker (RRM1/2, amino acids 114–282) to 5´-AUCCAGUGGAA-3´ RNA using NMR spectroscopy. Upon titration of the RRM1/2 construct by the RNA, several NMR chemical shift perturbations were observed for both RRM1 and RRM2 amide protons in the fast to intermediate exchange regimes (Fig. 1D). Saturation was reached at a 1:1 RNA:protein ratio indicating that one molecule of RNA was bound by both RRMs. The average correlation time measured for the complex was ~13 ns (Supplementary Fig. 2), which corresponds to a size of about 22 kDa36,37. These data are consistent with molecular weights of 19 and 3 kDa for RRM1/2 and the RNA, respectively, and indicate that the two RRMs tumble with RNA as a single unit. A dissociation constant (Kd) of 0.5 µM was determined with ITC for this complex (Fig. 1E). The identified consensus sequence contains a GG dinucleotide (Fig. 1C), which has been previously reported to be the common binding site of most known pseudo-RRMs, including Npl3 RRM238 since residues involved in the recognition of this dinucleotide are conserved in pseudo-RRMs (Fig. 2A). Moreover, chemical shift perturbations observed with the isolated RRM2 of Npl3 and a sequence corresponding to the 3´ end of the RNA (5´-AGUGGAC-3´) (Fig. 2B) were very similar to those observed in the context of RRM1/2 bound to the longer RNA (Supplementary Fig. 3). A Kd of 2.2 µM was determined with ITC for this complex (Fig. 2C). Taken together, this indicates that Npl3 RRM2 interacts with the 3’ extremity of the RNA, using a mode of RNA recognition common to all pseudo-RRMs38.
A Schematic representation of Npl3 domain composition. The sequence of Npl3 RRM2 is shown and aligned with the one of the RRM2 of human SRSF1. Residues that are important for SRSF1 RRM2 binding to RNA are colored in red. B Overlay of 1H-15N HSQC spectra recorded during the NMR titration of 15N labeled Npl3 RRM2 with increasing amount of unlabeled 5´-AGUGGAC-3´ RNA. The titration was performed at 40 °C in the RRM2 NMR buffer. The peaks corresponding to the free and RNA-bound protein states (RNA:protein ratios of 0.3:1 and 1:1) are colored in blue, orange, and red, respectively. Black arrows indicate the most prominent chemical shift perturbations observed upon RNA binding. C ITC measurement performed in duplicate with Npl3 RRM2 and the AGUGGAC RNA. D Representation of the combined chemical shift perturbations of Npl3 RRM2 amide residues upon binding to the 5´-AGUGGAC-3´ RNA at a ratio of 1:1 as a function of residue numbers. The corresponding secondary structure elements are represented at the top of the graph. The highest chemical shift perturbations annotated in B are indicated. Source data are provided as a Source Data file.
Structure of Npl3 RRM1 bound to RNA
To elucidate the RNA-binding specificity of Npl3 RRM1 independently from RRM2, we performed NMR titrations of the isolated RRM1 with several 6mer ssDNA containing stretches of A, C, G or T as well as a 8mer polyU RNA (Supplementary Fig. 4A). Chemical shift perturbations were only detected with the polyC sequence indicating a strong preference of RRM1 for this nucleobase. We then used a modified version of the scaffold independent analysis39 with ssDNA containing CX or XC motif (X is for A, C, G or T) flanked by degenerated sequences (Fig. 3A and Supplementary Fig. 4B). We could then identify by NMR spectroscopy the motifs bound by Npl3 RRM1 with the highest affinity using the mean chemical shift perturbations observed upon ssDNA binding. As shown in Fig. 3A, a clear preference for a CC dinucleotide over the other sequences was observed. Interestingly, the sequences selected with the split-iCRAC experiment were enriched in cytosines upstream of the motif bound by RRM2 (Fig. 1C), strongly suggesting that it corresponds to the RRM1 binding site. This result was rather unexpected, as Npl3 was never reported to bind preferentially to cytosines. Therefore, we investigated the interaction of the domain with the split-iCRAC derived RNA sequence 5´-AUCCAA-3´. A Kd value of about 16.2 µM was determined with ITC for this interaction (Fig. 3B). TOCSY experiments revealed that both cytosines are bound by different protein pockets (Fig. 3C) since two different chemical shifts were observed in their bound forms. Chemical shift perturbations of RRM1 bound to this short RNA (Fig. 3D, E) were very similar to those observed with RRM1/2 bound to the larger split-iCRAC RNA sequence (Supplementary Fig. 3), indicating the functional relevance of using this small complex to characterize the mode of RNA recognition of Npl3 RRM1.
A Modified Scaffold independent analysis performed by titrating Npl3 RRM1 with 6mer ssDNAs. N is for any nucleotide (A, T, C or G). The normalized CSP represents the sum of combined chemical shift perturbations of non-overlapping peaks upon binding of the ssDNA to the RRM1 at a 1:1 ratio. The value was then normalized to the one obtained with the 5´-NNCCNN-3´ ssDNA. Source data are provided as a Source Data file. B ITC measurement performed in duplicate with Npl3 RRM1 and the AUCCAA RNA. C Overlay of TOCSY spectra recorded with unlabeled RNA in the absence (in blue) and in the presence of Npl3 RRM1 at a 1:1 ratio (in red). Arrows represent the movement of the H5-H6 cross peaks for U2, C3, and C4 in different directions upon protein binding. D Overlay of 1H-15N HSQC spectra recorded during the NMR titration of 15N labeled Npl3 RRM1 with increasing amount of unlabeled 5´-AUCCAA-3´ RNA. The titration was performed at 40 °C in the RRM1 NMR buffer. The peaks corresponding to the free and RNA-bound protein states (RNA:protein ratio of 1:1) are colored in blue and red, respectively. Black arrows indicate the most prominent chemical shift perturbations observed upon RNA binding. E Representation of the combined chemical shift perturbations of Npl3 RRM1 amide residues upon binding to the 5´-AUCCAA-3´ RNA at a ratio of 1:1 as a function of residue number. The corresponding secondary structure elements are represented at the top of the graph. The highest chemical shift perturbations annotated in D are indicated. The largest shift is observed for Lys194 with a value of 1.36 ppm. Source data are provided as a Source Data file.
We then determined the solution structure of RRM1 bound to 5´-AUCCAA-3´ using 2475 NOE-derived distance restraints including 135 intermolecular ones. We obtained a precise structure with an RMSD of 0.41 Å (Fig. 4A, Supplementary Table 1). The RNA is lying on the surface of the RRM β-sheet with all nucleotides adopting an “anti” conformation and a C2´ endo sugar pucker conformation (Fig. 4B). Intermolecular contacts were observed between the U2, C3, C4, A5 and residues from the β-sheet and C-terminal extremity. U2 and A5 are not sequence-specifically recognized by the domain but provide binding affinity via their stacking on Arg130 side chain and C4 base, respectively. The C3 and C4 are sequence-specifically recognized by Npl3 RRM1. The C3 base stacks on Phe128 aromatic ring located on the β1-strand and forms two H-bonds between the amino and carbonyl groups of the base and the main chains of Tyr192 and Lys194, respectively (Fig. 4C). This latter H-bond is well supported by the fact that the amide of Lys194 experiences the largest chemical shift perturbation (1.36 ppm) upon binding to RNA (Fig. 3E). In addition, Lys194 from the C-terminus lies on top of the C3 base and its Lys194 amino group forms an H-bond with the 2’OH of C3. The C4 base stacks on Phe162 and is recognized by two H-bonds involving its O2 and N3 atoms and the side chain of Arg126. Finally, the aromatic ring of Phe160 contacts the riboses of both cytosines contributing to the binding affinity (Fig. 4C). Based on these structural data, we conclude that Npl3 RRM1 recognizes a 5´-NCCN-3´ motif.
A Overlay of the 10 lowest-energy structures superimposed on the backbone of the structured parts of the protein and heavy atoms of RNA. The protein backbone is shown in gray and heavy atoms are shown in orange (P atoms), yellow (C atoms of RNA), red (O atoms) and blue (N atoms). The RRM (residues 120–198) and the ordered region of RNA (C3, C4, A5) are shown. B The solution structure of the complex is shown in ribbon (protein backbone) and stick (RNA) representation. Protein side-chains or backbone involved in RNA interactions are shown as sticks. C atoms of the protein are in green. C Details of the RNA recognition by Npl3 RRM1. H-bonds are in magenta.
Structure of Npl3 RRM1/2 bound to RNA
The affinity of RRM1/2 for RNA (Kd = 0.5 µM) was significantly higher than each isolated RRM (Kd values of 16.2 and 2.2 µM, respectively) (Figs. 1E, 2C and 3B) suggesting a cooperative mode of interaction of the two domains with RNA and/or additional contacts mediated by the inter-domain linker. The binding of RRM1 to the 5´-NCCN-3´ motif indicated an interaction of RRM1 with RNA upstream of the RRM2 binding site on the split-iCRAC defined sequence. To investigate whether the orientation of the two RRMs was important for the binding efficiency of Npl3 to RNA, we performed an NMR titration of RRM1/2 with the 5´-AUGGAGUCCAA-3´ RNA containing inverted binding motifs (RRM2 binding site at the 5´-end and RRM1 binding motif at the 3´-end). As illustrated in Supplementary Fig. 5A, smaller CSPs were consistently observed at saturation (1:1 protein:RNA ratio) with this RNA compared to the 5´-AUCCAGUGGAA-3´ RNA, showing that the binding of Npl3 RRM1/2 to this RNA is apparently weaker than with the split-iCRAC derived sequence. Accordingly, the affinity measured for this complex by ITC indicates a Kd of 1.2 µM (Supplementary Fig. 5B) which is almost 3 times weaker than with the 5´-AUCCAGUGGAA-3´ RNA (Fig. 1E). Although both RRMs are bound to this RNA with RRM2 binding upstream and RRM1 binding downstream, these data suggest that for optimal RNA binding, RRM1 and RRM2 should bind their respective sequence upstream and downstream, respectively.
Next, we investigated the mode of RNA recognition of both Npl3 RRMs. Although many intermolecular NOEs between Npl3 RRM1/2 and 5´-AUCCAGUGGAA-3´ could be observed, the quality of the NMR data was always better in the complexes with isolated RRMs. However, the similarity of the chemical shift perturbations observed upon RNA binding for the isolated RRMs and the RRM1/2 complex (Supplementary Fig. 3) and the presence of similar inter-NOE patterns for the complex formed with isolated domains and the RRM1/2 protein (Supplementary Fig. 6), indicated that the mode of interaction of Npl3 RRMs was identical in both cases. In addition, most intermolecular NOEs found in the complexes with the isolated RRMs were also observed with RRM1/2-RNA complex confirming the same mode of interaction of the domains with RNA in both contexts. Therefore, to calculate the structure of the RRM1/2 complex, we used the same intermolecular NOEs as in each single RRM complex although some of them were too broad to be observed with the larger Npl3 RRM1/2 complex. We used this strategy only when the intermolecular contacts were confirmed by similar chemical shift perturbations. Additionally, due to an unfavorable exchange condition, we could not detect any intermolecular NOEs between G9 imino and RRM2 in any complexes. However, in our preliminary structures, the position of the G9 is very similar to the equivalent guanine in SRSF1 RRM2-GGA complex but less precisely defined due to the missing intermolecular constraints38. In order to more precisely position this base, we used in our structure calculations the same restraints for G9 H1 and residues of Npl3 RRM2 as for the structure determination of SRSF1 RRM2 bound to RNA38. Those restraints did not induce any distance violations indicating that they were in perfect agreement with all other experimentally derived restraints.
In total, to calculate the structure of RRM1/2 bound to the 5´-AUCCAGUGGAA-3´ RNA, we used 3788 distance restraints including 189 intermolecular ones and 62 Residual Dipolar Coupling (RDC) derived restraints (Supplementary Table 2). We could reach a high precision with a heavy atom RMSD of 1.23 Å (Fig. 5A). The two RRMs are precisely positioned relative to each other due to an interaction of Npl3 RRM2 with G6 (Fig. 5B) that was not present in the complex formed with the pseudo-RRM in isolation or in SRSF1 RRM2 complex. This nucleotide identity of G6 is strictly conserved or largely dominant in the split-iCRAC consensus sequences (Fig. 1C). G6 adopts a syn conformation and stacks on the Phe229 aromatic ring located on RRM2 β2-strand (Fig. 5B). G6 is also contacted by the C-terminal region of RRM2, with the side chain of Ile 279 contacting its base and sugar (8 intermolecular NOEs between these two residues position the C-terminal end near the RNA and RRM1). These contacts also explain the chemical shift changes seen for Ile 279 and Arg280 upon RNA binding (Supplementary Fig. 3). In fact, in some of the structural conformers, Arg280 and Arg281 are positioned sufficiently close of RRM1 to interact via a salt bridge with the side chains of Glu 153 and Glu 138. These additional protein-protein contacts help rationalizing why the two RRMs adopt a fixed orientation upon RNA binding (Fig. 5C).
A Overlay of the 10 lowest-energy structures superimposed on the backbone of the structured parts of the protein and heavy atoms of RNA. The protein backbone is shown in gray and heavy atoms are shown in orange (P atoms), yellow (C atoms of RNA), red (O atoms) and blue (N atoms). The RRMs (residues 12–198) and the ordered region of RNA (C3 to G6 and G8 to A10) are shown. B The solution structure of the complex is shown in ribbon (protein backbone) and stick (RNA) representation. Protein side-chains or backbone involved in RNA interactions are shown as sticks. C atoms of the protein are in green. H-bonds are in magenta. C The solution structure of the complex is shown from the back with the residues of the C-terminal part of the protein (Ile279, Arg280 and Arg281) involved in interactions with G6 and the Glu138 and Glu153 residues of RRM1.
Although no inter-NOE could be observed between the Arg199 and the base, the structure suggests that this side chain may interact with G6 explaining the specific recognition of a guanine at this position (Fig. 5C). In good agreement, the Arg199 NHƐ disappears upon complex formation. Furthermore, the chemical shift of G6 H8 was shifted in the RRM1/2 complex compared to the complex with the isolated RRM2 indicating that this proton is in a different environment when the two RRMs are bound. Overall, the GNGGA motif is tightly bound by RRM2 via a series of six consecutive stacking interactions (G6/ Phe229/G8/Trp213/Gln214/A10) adopting a “mille-feuille” topology, which certainly contributes to the higher RNA affinity of RRM2 compared to RRM1.
To investigate the importance of the protein-RNA contacts observed in these structures, we measured the binding affinity of several Npl3 RRM1/2 alanine mutants of key residues involved in RRM1 or RRM2 interaction with RNA. Mutations of residues that are important for the specific recognition of the CC dinucleotide binding by RRM1 (R126A and F128A) decreased the binding affinity strongly from a Kd of 0.5 µM to 10.7 and 12.8 µM, respectively (Supplementary Fig. 7). The mutation of R130 that stacks underneath the U2 base had a milder effect on binding affinity (Kd of 0.9 µM). Mutations that affect the recognition of the GG dinucleotide by RRM2 (Q214A and K217A) also showed a moderate decrease in binding affinity with Kd values increasing to 1.7 and 7.5 µM, respectively (Supplementary Fig. 7). As expected, the protein variant carrying both mutations showed weaker binding (Kd of 15.3 µM). Interestingly, the mutation of Phe229 that contacts G6 had also a clear effect on the RNA binding affinity (Kd of 2.3 µM). The importance of this interaction was further corroborated by the drop in binding affinity (Kd of 2.2 µM) observed between WT RRM1/2 and a G6A RNA variant (Supplementary Fig. 8). Additional mutations of the RNA were tested, including the conversion of C3 or C4 to uracil, which decreased the binding affinity to a Kd value of 1.8 and 1.6 µM, respectively. G8 or G9 mutation to adenine resulted in a slightly higher affinity drop (Kd of 1.9 and 2.5 µM, respectively). Surprisingly, shortening the RNA by removing A5 did not affect RRM1/2 binding affinity. We can explain this effect by the presence of a very short inter-RRM linker (only 7 residues), which forces the two spacing nucleotides to adopt a loop to compact the RNA backbone (Fig. 5B). As a consequence, one spacing nucleotide is still sufficient to maintain the interaction of each RRM with their respective sites. In agreement with these in vitro data, these two possible binding registers are seen in the Npl3 first split-iCRAC consensus motifs selected with the full-length protein where A and C nucleotides are equally present before the conserved GNGG motif (Fig. 1C). Nevertheless, the structure reveals that both RRMs are very close from each other due, in addition to the short inter-domain linker, to its involvement in RNA binding. Indeed, RRM1 requires two amino acids from the linker to bind the first cytosine (Fig. 5B). This interaction stabilizes the linker and seems to have some importance for the interaction of RRM2 with RNA, which also requires at least one residue of the linker (Arg199) to bind G6 (Fig. 5B): mutations affecting RRM1 interaction with RNA lead to a Kd value which is higher than for RRM2 in isolation (Kd > 10 instead of 2.2 µM, Supplementary Fig. 7). We therefore investigated the importance of this protein linker for Npl3 interaction with RNA. We mutated the three residues LPA of the linker that were not directly involved in the RNA binding either to GGG to introduce some flexibility or to three repeats of the GGGGS sequence to increase the length of the linker. In both cases we observed a strong decrease in affinity (Kd of 3.8 µM and 6.8 µM, respectively instead of 0.5 µM) (Supplementary Fig. 7 and Fig. 1E) indicating that a short and rather rigid inter-RRM linker is important for Npl3 RRM1/2 interaction with RNA.
Contribution of each RRM toward Npl3 function in vivo
Our in vitro investigation of Npl3 RRM1/2 interaction with RNA permitted to rationally design protein mutants that strongly decrease the binding of RNA to either RRM1 (F128A, F128A + F160A) or RRM2 (Q214A, K217A, Q214A + K217A) (Supplementary Fig. 7) without affecting the folding of the protein (Supplementary Fig. 9A, B). These substitutions were used to investigate the impact of the RNA interaction of each RRM on Npl3 functionality in vivo. In addition, we also tested the importance of the inter-domain linker using the protein variants mentioned above, in which the LPA sequence was mutated to GGG or three repeats of GGGGS (Supplementary Fig. 10A). Yeast strains lacking the npl3 gene (npl3∆) were transformed with plasmids expressing these mutants under the control of the natural Npl3 promoter. We investigated whether they could rescue the slow growth of the npl3∆ strain9. Interestingly, the complementation with Npl3 having single or double substitutions in RRM1 failed to completely rescue the slow growth defects (Fig. 6A) indicating the importance of RRM1 binding to RNA for Npl3 functionality. Conversely, single and double substitutions within RRM2 did rescue the Npl3 deletion phenotype (Fig. 6A) indicating a more critical contribution of the RRM1 RNA binding interface than RRM2 in Npl3 function. Note that the effect is not due to a difference in RNA binding affinity since both double mutants have a similar Kd (Supplementary Fig. 7). Surprisingly, no effect was observed with the two protein variants in which the inter-RRM linker was mutated (Supplementary Fig. 10A) despite a decrease in affinity observed for these protein variants for RNA by a factor of about 10 in vitro (Supplementary Fig. 7). This result suggests that the inter-RRM linker could potentially be less important for the interaction of Npl3 with RNA in the context of the full-length protein or this in vivo assay is not sensitive enough to detect an effect in yeast. In agreement with the second hypothesis, the substitution of the PA motif to DD was tested previously31. Due to the introduction of these two negative charges in the linker of Npl3, a much stronger negative effect was observed on Npl3 binding to RNA and a significant growth defect could then be observed in yeast.
A Mutant growth analysis of npl3Δ strain complemented with vectors expressing different protein variants of Npl3 (marked on the left). Yeast cells were plated on SD-leu plates and incubated at 30 or 37 °C. Two steps of a yeast serial dilution are shown for each condition. Synthetic growth analysis of npl3Δ + lea1Δ, npl3Δ + nam8Δ, npl3Δ + rad6Δ and npl3Δ + bre1Δ double deletion strains complemented with Npl3 protein variants reducing the interaction of RRM1 (F128A, F162A, F128A + F160A) or RRM2 (Q214A, K217A, Q214A + K217A) with RNA is shown. Lea1 and Nam8 are involved in splicing, whereas Rad6 and Bre1 are chromatin-remodeling factors. B Sequence of yeast U2 snRNA stem I. The Isr1 mutation is shown in red. Overlay of 1D NMR spectra recorded at 303 K with U2 SL I free form and at U2 SL I:Npl3 RRM1/2 ratios of 1:0.5, 1:1, 1:1.5 and 1:2. Overlay of 1H-15N HSQC NMR spectra recorded with Npl3 RRM1/2 free form (blue), Npl3 RRM1/2:U2 SL I at a 1:2 ratio (orange) and Npl3 RRM1/2:AUCCAGUGGAA at a 1:1 ratio (red). C Synthetic growth analysis of Isr1Δ, Isr1Δ + lea1Δ and Isr1Δ + nam8Δ deletion strains complemented with the WT or Mut versions of Isr1. D Synthetic growth analysis of the ΔIsr1 + npl3Δ double deletion strain complemented with either the WT or Mut version of Isr1 and the same Npl3 protein double variants as tested in A. Three biological replicates were performed for the yeast experiments.
The slow growth phenotype of npl3Δ yeast mutant was previously reported to be exacerbated when combined with deletion of genes involved in spliceosome assembly (lea1 and nam8)9 or chromatin remodeling (ex. rad6 and bre1)10. We investigated the respective involvement of both Npl3 RRMs in these two functions by testing whether the RRM1 and RRM2 mutants of Npl3 would lead to the same genetic interaction. In agreement with the predominant importance of the RRM1 RNA binding interface, we observed genetic interactions of RRM1 mutated Npl3 with the four tested genes (Fig. 6A). This indicates that the binding of RRM1 to RNA was important for both the splicing and the interaction of Npl3 with chromatin remodeling factors. On the other hand, RRM2 mutants only showed a genetic interaction with lea1 with a predominant effect seen at 37 °C (Fig. 6A). Surprisingly, no effect was observed with nam8 despite the involvement of both lea1 and nam8 in splicing regulation. Lea1 is part of the U2 snRNP, while Nam8 is a component of the U1 snRNP, indicating that RRM2 binding to RNA might be more linked to U2 snRNP function during splicing.
In good agreement with this hypothesis, Npl3 crosslinks were strongly enriched at the 5’ end of U2 snRNA and observed to a lower extent in U1 snRNA (Supplementary Figs. 10B, C and Table 3). In addition, a sequence containing a CC followed by a GG dinucleotide (reminiscent of the motif recognized by both RRMs of Npl3), is found in this cross-linked region (Supplementary Fig. 10B). Interestingly, those nucleotides are part of the U2 snRNA stem-loop I (SL I) in which the CC and GG are base-paired (Fig. 6B). However, in S. cerevisiae, this stem must be melted during the splicing reaction to allow the formation of a duplex between U2 and U6 snRNAs40. Our structure clearly indicates that Npl3 RRMs interact sequence-specifically with single-stranded RNA at the CC (RRM1) and GG (RRM2) sequences (Figs. 4C and 5B) suggesting that the binding of Npl3 to U2 snRNA SL I would induce the melting of this stem-loop. We then in vitro transcribed the 5’- GAGCGAAUCUCUUUGCCUUUUGGCUUAGAUC-3’ RNA containing the initiator codon GAG followed by the sequence forming the U2 stem-loop I (in bold) including the two parts involved in the duplex formation with the U6 snRNA (underlined sequences). Using NMR spectroscopy, we confirmed that this RNA adopted the expected secondary structure based on previous NMR assignments obtained with this stem-loop41. In addition, the titration of this stem-loop with Npl3 RRM1/2 showed that the protein could interact with the U2 SL I sequence and destabilized the stem (Fig. 6B and Supplementary Fig. 10D). Indeed, the intensity of the imino signals observed when the upper part of the stem is formed (G15, G22 and G23) decreased upon protein binding without any chemical shift perturbations indicating that the protein did not interact with the stem but rather unfolds it (Fig. 6B and Supplementary Fig. 10D). The overlay of the 2D 1H-15N HSQC spectra recorded with Npl3 RRM1/2 in the free form and bound to the U2 SL I (Fig. 6B) validated this interaction. As expected, a significant decrease of the Tm value was observed for this RNA in the presence of Npl3 RRM1/2 (Supplementary Fig. 10E). We therefore mutated the CC and GG binding sites to CG to keep the ability to form the stem I but prevent the potential binding of Npl3 to this RNA (Isr1 mut, Fig. 6B) and tested the effect in yeast. The mutation of U2 snRNA did not show any phenotype at any of the tested temperatures (Fig. 6C). However, when combining the mutation with a deletion of the U2 factor Lea1, we could observe a slow growth phenotype at 30 °C and complete lethality of the cells at 37 °C (Fig. 6C). This effect was not seen when combining the same mutation with a deletion of the U1 snRNP component Nam8 (Fig. 6C). This indicated that the putative binding sequence of Npl3 identified in the stem-loop was important for the function of U2 snRNP. To confirm the link with Npl3, we tested a combination of mutations in Npl3 RRM1 or RRM2 with the Isr1 mutant. Interestingly, we observed a slow growth phenotype at lower temperature (20 °C) confirming the link between Npl3 RRM2 and the U2 snRNA (Fig. 6D). Overall, these data indicate that Npl3 favors the U2-U6 duplex formation required for the formation of the spliceosome active site by interacting with and destabilizing the stem-loop I of U2 snRNA.
Discussion
Split-iCRAC reveals the RNA motif(s) recognized by Npl3 in vivo
Despite the pivotal role of Npl3 in RNA metabolism, no RNA binding consensus sequence was identified for this protein yet. Only a preference for this protein in binding to UG-rich RNA sequences was previously reported16,29. Using a modified version of the CRAC method, we identified a clear consensus RNA sequence bound to yeast Npl3. Adding the individual nucleotide resolution of the iCLIP protocol and looking at long enriched sequences allowed us to identify a consensus motif, which were not assessed in previous CRAC data16. In general, the binding profile that we observed correlates well with the available CRAC data (Supplementary Fig. 11A). Interestingly, the consensus RNA sequence obtained with Npl3 (UCCAGUGGA) is different from the motifs identified by PAR-CLIP with the two other SR-like proteins Hrb1 (CuGCU) and Gbp2 (GGUG)42 indicating that these proteins have distinct RNA targets in vivo. Moreover, the “split” version of the iCRAC also permitted the identification of RNA sequences that were directly bound by the RGG/RS domain. This domain generally binds RNA sequences near the binding sites of the RRMs with no apparent sequence preference either upstream or downstream (Supplementary Fig. 11B). Although a weak preference for the GCGUAUAUC motif was found with the RGG/RS domain in isolation (Supplementary Fig. 1B), the selection of a similar consensus sequence with the full-length protein and the RRM1/2 in isolation (Fig. 1C and Supplementary Fig. 1) strongly suggests that the C-terminal disordered region does not participate to the specific interaction of Npl3 with RNA. This result is in good agreement with recent reports showing that the RS domains bind non-specifically to RNA43,44. Another study reported that RS domains of SR proteins could bind directly to RNA sequences containing the splicing branch point45,46. However, the split-iCRAC data did not reveal any specific cross-links of the Npl3 RGG/RS domain around branch point sequences.
Molecular basis of RNA recognition by Npl3 RRMs
Here, we show that Npl3 RRM1 participates to the specific interaction of the protein with RNA by binding to CC dinucleotides (Fig. 4C). Interestingly, the recognition of two consecutive cytosines was also observed in the structure of the human SR protein SRSF2 bound to RNA47. Despite the fact that the two RRMs are only 43% homologous (and 28% identical), the position of the two cytosines on the β-sheet surface and their recognition by the RRM are quite similar (Supplementary Fig. 12A). However, unlike the SRSF2 RRM that recognizes CC, CG, GC and GG, Npl3 RRM favors strictly CC (Fig. 3A).
In addition, we found that RRM2 recognizes a 5´-GNGGA-3´ motif in the context of RRM1/2. The recognition of the GG dinucleotide is identical to what was reported for SRSF1 RRM238. Although pseudo-RRMs share the recognition of a GG dinucleotide, the nucleotides bound on each side of this motif seem to be more specific to each protein. For instance, the adenine bound by SRSF1 downstream of the GG is positioned similarly in Npl3 RRM2. Nevertheless, the stacking interaction of A10 with the His193 observed in the structure of the SRSF1 complex is not possible with Npl3 as the β3-β4 hairpin is shorter and the corresponding aromatic residue is missing (Figs. 2A and 5B). Another difference between the two complexes is the binding of Npl3 RRM2 to G6 upstream of the GG dinucleotide motif (Fig. 5B). Side-chains from RRM2 (Phe 229), the interdomain linker (Arg 199) and the region C-terminal to RRM2 (Ile 279) contribute to the specific recognition of this nucleotide.
RRM2 of Npl3 was previously reported to bind GU rich RNA sequences29. In good agreement, our split-iCRAC motif showed that the sequence bound by RRM2 can contain uracils, as the consensus motif was GU/AGG (Fig. 1C). In the structure of RRM1/2 bound to RNA, the uracil is bulged out (Fig. 5B) and does not give any intermolecular NOEs either in this complex or in the isolated domain bound to the 5´-AGUGGAC-3´ RNA. However, we observed that in the absence of G2 in the 5´-AUGGAC-3´ RNA, additional chemical shift perturbations were observed in the β2-β3 loop of RRM2. Those additional chemical shift perturbations were not observed with the AAGGUC RNA, which hints towards a possible specific recognition of the U2 5´ to the GG dinucleotide. In this context, we could observe intermolecular NOEs between U2 and Val232 and Asn233 from the β2-β3 loop indicating that the uracil is indeed in contact with the protein. However, because of limited spectral quality, we could not precisely position the uracil to infer its specific recognition. This result suggests that RRM2 could either bind to GNGGA or UGGA motifs.
Tandem RRMs bind an extended single-strand RNA via an unusual orientation
In addition to defining the exact RNA motifs recognized by RRM1 and RRM2, our structural studies revealed their relative orientation. Although no contact could be observed between the two RRMs of Npl3 in their free form29,30, their binding to a single RNA molecule was expected to rigidify the orientation of one relative to the other as reported previously with other tandem RRMs48. The preferential binding of RRM1 upstream of the RRM2 binding site is not common among tandem RRMs. In most structures, RRM2 binds RNA upstream of the RRM1 binding site48. The only case reported so far of two RRMs adopting an opposite orientation on RNA was with the tandem RRMs of TDP-43 (Supplementary Fig. 12B)49. To keep this unusual orientation, it was hypothesized that a long inter-domain linker (15 aa) is required to allow the two RRMs to lie side-by-side (β2-β4 type) and form an extended β-sheet RNA binding surface48. Recently, two structures (Dnd150 and Npl3 in this work) revealed new ways for tandem RRMs to bind RNA cooperatively with RRM1 binding to the 5’-end despite having a short interdomain linker (5 and 7 aa, respectively). In such cases, the canonical β-sheet surface of the RRM is not used but rather both sides of the domain. In Dnd1, the α2−β4 edge of the RRM is used while in Npl3, this is the α1−β2 edge (Supplementary Fig. 12B). Having a short interdomain linker presents the advantage to reduce the entropy cost upon RNA binding. This illustrates once more the unusual diversity and rather unpredictable binding mode of RRM-RNA interactions28,48,51.
Another similarity between the structure of TDP43 and Npl3 is the presence of a guanine located in the center of the bound RNA sequence, which is sequence-specifically recognized by both proteins and contributes to establish a fixed orientation of the two RRMs. However, the base adopts an anti-conformation in the TDP-43 complex, whereas the base is syn when bound to Npl3. Surprisingly, despite the fixed orientation of RRMs on RNA, the RGG/RS domain of Npl3 seems to bind non-specifically upstream or downstream of their binding sites (Supplementary Fig. 11B), suggesting a high flexibility of the domain. In addition, it raises an intriguing possibility that Npl3 recruits additional proteins on both sides of its binding site via this disordered region.
Functional insights into the role of Npl3 during RNA splicing
Our genetic interactions implicate that the RRM1-RNA interactions are broadly important for the role of Npl3 during splicing and chromatin remodeling, whereas the RRM2 involvement seems to be rather limited to the splicing process (Fig. 6A). The observed genetic interactions were more obvious when yeast cells were grown at 37 °C (Fig. 6A). In addition, the genetic interaction between RRM2 mutated npl3 and lea1 was only observed at 37 °C (Fig. 6A). One explanation is that at a higher temperature, the lower affinity of the RRM mutants for RNA is thermodynamically disfavored while it can still occur to some extent at lower temperatures.
Previous mutational analyses to study the function of Npl3 RRM2 in vivo used three mutations (L225S, G241N, and E244K)13 mostly in combination23,24. However, it was previously reported that the L225S unfolds the RRM229. The effect of the G241N and E244K on the folding of the RRM was never tested, but these two residues are far away from the RNA binding interface. Therefore, it is difficult to correlate a loss of function from those mutants with the RNA binding properties of RRM2. Similarly, in vivo mutational studies were previously done using the F160L mutation to investigate the function of RRM123. However, the effect of this single mutation on RNA binding was never directly tested. Our structure shows that indeed Phe160 has hydrophobic contacts with the ribose rings of the two recognized cytosines (Fig. 4C). However, a mutation of the phenylalanine to a leucine, another hydrophobic residue, might not be sufficient to prevent the binding of RRM1 to RNA as it may still allow these hydrophobic contacts. This could explain the absence of effect of this protein mutant on Npl3 functions23,24. Our structural-guided analysis uncovered the functional contribution of RRM1 binding to RNA in vivo.
In humans, SR proteins were previously shown to recruit U1 and U2 snRNPs on the 5´SS and 3´SS, respectively52. In yeast, the use of a npl3 Δ strain revealed a general decrease of pre-mRNA splicing suggesting a role in constitutive splicing. Npl3 was reported to facilitate co-transcriptional splicing by recruiting the U1 snRNP on RNA pol II9. In addition, the protein was recently shown to interact with U1 snRNP through protein-protein interaction using its RGG/RS domain53. However, we could not detect in our iCRAC data any specific enrichment of Npl3 binding around spliced introns nor specific enrichment in ribosomal protein genes. In good agreement with this observation, it was previously proposed that the recruitment of Npl3 at these sites might be driven through interactions with other proteins and chromatin modifications9,10. Our split-iCRAC data show a specific binding of Npl3 to the Stem-loop I of the U2 snRNA. Moreover, RNA mutations that prevent the binding of Npl3 without affecting the stability of the Stem-loop I resulted in a slow growth phenotype in combination with the deletion of lea1, as observed with Npl3 mutants preventing its binding to RNA (Fig. 6B, C). We found that SRSF154 and FUS55 could interact directly with the SL3 of the human U1 snRNA. The specific interaction between Npl3 and the U2 snRNA reported here implicates a broader role for the U snRNAs. For example, this interaction could serve as an early and transient binding platform to load splicing factors having a specific function during the splicing reaction. In addition, this interaction of Npl3 with U2 snRNA stem-loop I suggests an unexpected mode of action of this protein in splicing. As Npl3 was shown to genetically interact with Snu66, a component of the tri-snRNP9, its binding to U2 snRNA could play a role at a later stage of the spliceosome assembly. Our structure shows that Npl3 can unfold the stem-loop I by binding to its RNA target sequence (Fig. 6B and Supplementary Fig. 10E). This unfolding is required for the recruitment of the U4/U6.U5 tri-snRNP as this unfolded sequence can then form a duplex with the U6 snRNA upon the spliceosome complex A to B transition, which leads to the active Bact spliceosome complex40,41. Therefore, Npl3 may play an early chaperoning role for U2-U6 hybridization, which would facilitate the formation of the Bact complex. This would also explain why the effect of the stem-loop I mutation on yeast growth is more visible at low temperature, as the dynamics of the RNA rearrangement may be slower and the role of Npl3 more important to unfold U2 stem-loop I. In agreement with its involvement at this stage of the splicing reaction, Npl3 was proposed to stimulate Prp28’s ATPase activity to remove U1 snRNP from the pre-B complex11. Indeed, synthetic sick genetic interactions between Npl3 and components involved in catalytic steps during splicing have been observed9.
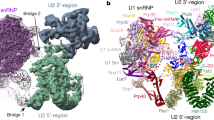
Interestingly, 16 nts of the U2 snRNA encompassing the sequence targeted by Npl3 were not visible in the cryo-EM structure of the yeast B complex56 indicating some flexibility near the U2-U6 helix II duplex formation. In addition, a large empty cavity is present at this location which is large enough to perfectly accommodate the two Npl3 RRMs bound to RNA (Supplementary Fig. 13). Moreover, the α-helix from an unidentified protein was observed at the proximity of this Npl3 binding site in the cryo-EM structure of the yeast spliceosome complex C57. All these biochemical and structural elements point towards an RNA chaperoning activity of Npl3 in the formation of active spliceosomes in yeast and pave the way for mechanistic investigation on the mode of action of other SR- and SR-like proteins in higher eukaryotes.
Methods
Expression and purification of recombinant proteins
Escherichia coli BL21 (DE3) codon plus cells transformed with pET28a::Npl3 RRM1 (residues 114–201), pET28a::Npl3 RRM2 (residues 193–282) or pET28a::Npl3 RRM1/2 (residues 114–282) were grown at 37 °C in M9 minimal medium supplemented with 50 µg/ml kanamycin, 34 µg/ml chloramphenicol, 1 g/l 15NH4 Cl and 4 g/l unlabeled or 2 g/l 13C labeled glucose for 15N or 15N and 13C labeled proteins, respectively. Protein expression was induced at OD600 of 0.9 with 1 mM IPTG at 20 °C. After 18 hours, the cells were harvested, and proteins were purified by two successive nickel affinity chromatography (Qiagen®) steps. The proteins were dialyzed in RRM1 NMR buffer (25 mM Na2HPO4, 25 mM NaH2PO4, pH 6.9), RRM2 NMR buffer (100 mM NaCl, 20 mM NaH2PO4 pH 5.5), or RRM1/2 NMR buffer (25 mM Na2HPO4, 25 mM NaH2PO4, pH 6.9). The concentration of recombinant proteins was carried out using 10-kDa molecular mass cut-off Centricons (Vivascience®). The absence of RNases was confirmed using the RNase Alert Lab Kit (Ambion®).
Preparation of RNA–protein complexes
All RNA oligonucleotides were purchased from Dharmacon®, de-protected according to manufacturer’s instructions, lyophilized and resuspended in the corresponding NMR buffer. NMR titrations were carried at a protein concentration of 0.2 mM. The Npl3 RRM–RNA complexes used for structure calculations were prepared in their corresponding NMR buffer at a protein:RNA stoichiometric ratio of 1:1 and a final concentration of 0.9 mM. The U2 RNA stem-loop (5’-GAGCGAAUCUCUUUGCCUUUUGGCUUAGAUC-3’) was transcribed in vitro and purified by HPLC on an anion exchange column at 85°C and in denaturing conditions (6 M urea). The fraction containing the RNA was precipitated using butanol and dissolved in water. The RNA was renaturated 30 sec at 95 °C followed by a slow cooling step to room temperature.
Isothermal titration calorimetry
ITC experiments were performed on a VP-ITC instrument (Microcal), calibrated according to the manufacturer’s instructions. Protein and RNA samples were dialyzed against the NMR buffer. Concentrations of proteins and RNAs were determined using optical-density absorbance at 280 and 260 nm, respectively. 10 µM of each RNAs were titrated with 200–600 µM of protein by 40 injections of 6 µl every 5 min at 40 °C. Raw data were integrated, normalized for the molar concentration and analyzed using the Origin 7.0 software according to a single site model. A correction of the heats of dilution was only done for the complex formed with Npl3 RRM2 as the effect observed with control experiments was negligeable in the context of the RRM1 and RRM12. All measurements were performed in duplicate.
NMR experiments
All the NMR spectra were recorded at 313 K using Bruker AVIII-500 MHz, 600 MHz, 700 MHz, AVIIIHD-600 MHz, 900 MHz equipped with a cryoprobe, and AVIII-750 MHz spectrometers. Topspin 3.6.2 (Bruker®) was used for data processing and Sparky 3.133 (http://www.cgl.ucsf.edu/home/sparky/) for data analysis.
Protein backbone assignment was achieved using 2D 1H–15N HSQC and 3D HNCACB, while side chain assignments were achieved using 2D 1H–13C HSQC, 3D HcccoNH TOCSY, 3D hCccoNH TOCSY, 3D NOESY 1H–15N HSQC and 3D NOESY 1H–13C HSQC aliphatic. Aromatic protons were assigned using 2D 1H–1H TOCSY and 3D NOESY 1H–13C HSQC aromatic58.
RNA resonance assignments in complex with Npl3 RRMs were performed using 2D 1H–1H TOCSY, natural abundance 2D 1H–13C HSQC and 2D 13C 1F-filtered 2F-filtered NOESY in 100% D2O. Intermolecular NOEs were obtained using 2D 1H–1H NOESY and 2D 13C 2F-filtered NOESY59 in the presence of unlabeled RNA and 15N- and 15N-13C-labeled proteins, respectively.
All NOESY spectra were recorded with a mixing time of 100 ms, the 3D TOCSY spectrum with a mixing time of 17.75 ms and the 2D TOCSY with a mixing time of 60 ms.
The 1D NMR experiments shown in Fig. 6B were recorded at 298 K on a Bruker 700 MHz spectrometer in the RRM1/2 NMR buffer at RNA concentrations of 50 µM.
15N T1 and T2 measurements were recorded at 313 K at a 1H frequency of 600 MHz with established methods60.
Residual dipolar couplings (RDCs) measurement
In order to measure the amide residual dipolar couplings of Npl3 RRM1/2 in its RNA bound state, we used Pf1 phages as alignment medium. The Pf1 phages were previously washed using the NMR buffer according to the manufacturer instructions (Alsa Biotech). The Pf1 phages were then added to the sample at a concentration of 10 mg/ml and the formation of a crystalline medium was monitored by measuring the splitting of the deuterium atoms from D2O. In order to extract amide RDCs, we compared the apparent scalar couplings H-N observed on a 2D 1H-15N IPAP HSQC before and after addition of the phages. The RDC restraints were then added during the cartesian refinement procedure using AMBER.
Structure calculations
AtnosCandid 2.1 software61 was used for peak picking 3D NOESY (15 N- and 13 C-edited) spectra. Preliminary structures and a list of automatically assigned NOE distance constraints were generated through 7 cycles using CYANA noeassign61. Additionally, intra-protein hydrogen bond constraints were added based on hydrogen–deuterium exchange experiments on the amide protons. For these hydrogen bonds, the oxygen acceptors were identified based on preliminary structures calculated without hydrogen bond constraints. Intra- molecular RNA and RNA–protein intermolecular distance restraints were manually assigned and added to the calculation with 62 RDCs restraints in the case of Npl3 RRM1/2. Calculations with the RNA were done using CYANA 3.98.4 in which seven iterations were performed, and 500 independent preliminary structures were calculated at each iteration step. These 50 structures were refined with the SANDER module of AMBER 14.062 by simulated annealing in implicit water using the rna.ff12SB force field63. The 10 best structures based on energy and NOE violations were analyzed with PROCHECK64,65,66,67. Figures were generated with MOLMOL 2 K68 and Pymol 4.6.0. The Ramachandran plot of the Npl3 RRM1 in complex with RNA indicates that 86.9% of the residues are in the most favored regions, 12.9% in the additional allowed regions, 0.1% in the generously allowed regions and 0% in the disallowed regions. The Ramachandran plot of the Npl3 RRM1/2 in complex with RNA indicates that 71.2% of the residues are in the most favored regions, 25.4% in the additional allowed regions, 3.3% in the generously allowed regions and 0.1% in the disallowed regions.
Modified Scaffold independent analysis
The method was adapted from Beuth et al.39. Briefly, 1H–15N HSQC NMR titrations were done with 0.2 mM Npl3 RRM1 protein in the RRM1/2 buffer at 40 °C with successive addition of ssDNA (DNA:protein ratios 0.3:1, 0.6:1, 1:1, 2:1). The chemical shift perturbations observed were calculated for the 1:1 ratio with the formula (Δδ = [(δHN)2 + (δN/6.51)2]1/2). The values calculated for non-overlapping peaks were summed.
Split-iCRAC
The BY4741 (MATa ura3 his3 leu2 met15 TRP1) was used as a parental yeast strain in which the promoter of Npl3 was replaced with an inducible gal promoter by homologs recombination. The strain was complemented with pRS315::Npl3±500kbp::HRV-3C::CterHTP plasmid. In this plasmid, the expression of Npl3 is driven by its endogenous promoter. A HRV-C3 protease cleavage site was inserted between amino acids S282 and N283 using PCR. A His-Trypsin-Protein A (HTP) tandem tag was placed at the C-terminus of the protein by PCR.
The split-iCRAC was based on the original CRAC protocol described by Granneman et al., 2009 with some modifications69. Briefly, the recombinant yeast strains were grown in SD-leu medium to drive Npl3 expression only from the transformed plasmid. 2 L of yeast culture were harvested at an OD600 of ~2. The cells were resuspended in 1 v/w SD-Trp medium and UV-irradiated (1.6 J/cm2) in Petri dishes in a Stratalinker 1600 (Stratagene). Half the cells were not subjected to UV treatment and were kept as the UV minus control. Cells were pelleted and resuspended in 25 ml of lysis buffer (50 mM Tris-HCl pH 7.8, 150 mM NaCl, 0.15% NP-40) with addition of 1.3 mM PMSF, 1 mM DDT, complete protease inhibitor cocktail (Roche®)). The cells were lysed using 25 ml glass beads in Planetary mill for 20 min at 750 × g. 5 ml of lysis buffer was added, and the lysate was cleared by centrifugation (20 min at 7985 × g and 4 °C followed by 2 × 20 min at 43,200 × g and 4 °C). Cleared lysate was incubated with 300 of IgG bead suspension (1:1) for 2 h at 4 °C with rotation. Beads were collected and washed 3× with 10 ml wash buffer (50 mM Tris-HCl pH 7.8, 1 M NaCl, 0.15% NP-40, 0.5 mM DDT) followed by 3× with 10 ml lysis buffer with added 0.5 mM DDT. Beads were resuspended in 5 ml of lysis buffer and rotated with 40 μg of homemade TEV for 18 h at 4 °C. The eluates were concentrated to a volume of 500 μl in 30 kDa cutoff centricons (Millipore®). No RNase treatment was performed in the final experiments for library preparation. 0.4 g of Guanidine-HCl were dissolved in the eluate to yield a final concentration of 6 M. NaCl and imidazole were added to a final concentration of 300 and 10 mM, respectively. 100 μl of pre-equilibrated Ni-beads were added to the samples and incubated for 2 h at 4 °C. Beads were washed 3× with wash buffer (6 M guanidine-HCl, 50 mM Tris-HCl (pH 7.8), 300 mM NaCl, 0.1% NP-40, 40 mM imidazole, and 1 mM DDT) followed by 3× with PNK buffer (50 mM Tris-HCl (pH 7.8), 40 mM MgCl2, 0.1% NP-40, and 1 mM DDT). The beads were then incubated in 50 μl of PNK buffer (pH 6.5) in the presence of 10 units of PNK (NEB®) and 10 units of CIP (NEB®) and 20 units of SUPERase.In (Ambion®) for 30 min at 37 °C. Beads were washed 3× with wash buffer followed by 2× with PNK buffer (pH 7.8) and incubated with 50 pmoles of L3 adapter (rAppAGATCGGAAGAGCGGRRCAG/ddC/) in 50 μl of ligation mixture (1× PNK buffer (pH 7.8) supplemented with 12.5 units of T4 RNA ligase 1 (NEB®), 250 units of T4 RNA ligase 2 truncated K227Q (NEB®), 40 units of SUPERase.In (Ambion®) and 10% PEG 4000). The reaction is incubated for 18 h with mild shaking at 16 °C. Beads are then washed with 3× wash buffer followed by 3× PNK buffer. For the PreScission cleaved version, the beads are then incubated with 1× PNK buffer (pH 7.8) supplemented with 20 units of SUPERase.In (Ambion®) and 10 units of PreScission protease (GE Healthcare®) for 18 h at 4 °C with mild shaking. The mixture is then supplemented with 10 units of PNK and 0.5 μl of γ32P-ATP and incubated for 1 h at 37 °C. 20 μl of 4× LDS is added to the beads, boiled for 10 min and resolved on a 4–12% Bis-Tris NuPage gel (Invitrogen®). Subsequent steps of RNA isolation and library preparation were done as described in Huppertz et al. 2014 Methods32. The negative control sample was generated following the same procedure but starting with WT yeast strain that does not express any tagged protein.
UV melting experiments
UV melting experiments were recorded on a CARY 100 Bio UV spectrophotometer (Varian) equipped with a temperature-controlled heating unit. The heating rate was 5 °C/min from 15 to 85 °C. Data from control experiments (buffer and protein alone) were subtracted from the data presented in the Supplementary Fig. 10E. The fit of the curves was performed with SigmaPlot13 using a sigmoidal equation with 3 parameters: f = a/(1 + exp(−(x − xo)/b)).
High-throughput sequencing and analysis
Four replicates of the full-length protein and RRM1/2 domain, three replicates of the RGG/RS and one negative control sample were Illumina sequenced on a single lane of the NextSeq500 High Output (single-end 75 bp reads) according to manufacturer’s protocol. The reads corresponding to each sample were demultiplexed using the FLEXBAR tool70. At least 25 million reads were generated (up to 50 million) for each sample. After barcode removal and quality trimming, the reads were mapped against the S. cerevisiae reference genome S288C_R64-1-171 using STAR72. Approximately 30% of the reads of each sample were uniquely mapped to the genome. Reads mapping to the same genomic location and with the same Unique Molecular Identifier (UMI) were assumed to arise from PCR duplication and were therefore merged using UMI-tools73. To increase the reliability of identifying significant crosslinking sites, the deduplicated reads from the replicate samples were merged and subsequently used for peak calling using iCount74. A False Discovery Rate (FDR) cut-off of 0.05 was used to identify significant crosslinking sites. Identified crosslinking sites that were less than three nucleotides apart were merged into one cluster. Clusters that overlapped with ones identified in the negative control sample were not included in subsequent analysis steps. HOMER software34 was used for de novo motif discovery using the identified clusters flanked by five nucleotides on each side because of the expected short motif. Motif predictions were made for sizes ranging from 2 up to 10 nucleotides. The HOMER motifs were validated by plotting the density of the top motifs in the different samples and the negative control in a window of ±50 nucleotides around the crosslink cluster centers.
Mutant growth analysis
Genetically modified yeast strains were prepared by homologs recombination according to standard protocols64,65,67. Equal amounts of streaked yeast cells were resuspended in 90 μl H2O and subsequently diluted 10× in a series of 5 steps. 10 μl of the four lowest dilutions were spotted on SD plates. Plates were incubated up to one week at appropriate temperatures and pictures were taken daily using a Coolpix P310 digital camera (Nikon®).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The coordinates of the structures have been deposited in the Protein Data Bank (PDB), under the accession code 7QDD for Npl3 RRM1–AUCCAA and under the accession code 7QDE for Npl3 RRM1/2-AUCCAGUGGAA. The split-iCRAC data have been deposited at ArrayExpress using this accession number E-MTAB-11736. Source data are provided with this paper.
References
Howard, J. M. & Sanford, J. R. The RNAissance family: SR proteins as multifaceted regulators of gene expression. Wiley Interdiscip. Rev. RNA 6, 93–110 (2015).
Jeong, S. SR proteins: binders, regulators, and connectors of RNA. Mol. Cells 40, 1–9 (2017).
Manley, J. L. & Krainer, A. R. A rational nomenclature for serine/arginine-rich protein splicing factors (SR proteins). Genes Dev. 24, 1073–1074 (2010).
Boucher, L., Ouzounis, C. A., Enright, A. J. & Blencowe, B. J. A genome-wide survey of RS domain proteins. RNA 7, 1693–1701 (2001).
Davis, C. A., Grate, L., Spingola, M. & Ares, M. Jr. Test of intron predictions reveals novel splice sites, alternatively spliced mRNAs and new introns in meiotically regulated genes of yeast. Nucleic Acids Res. 28, 1700–1706 (2000).
Schreiber, K., Csaba, G., Haslbeck, M. & Zimmer, R. Alternative splicing in next generation sequencing data of Saccharomyces cerevisiae. PLoS One 10, e0140487 (2015).
Gilbert, W., Siebel, C. W. & Guthrie, C. Phosphorylation by Sky1p promotes Npl3p shuttling and mRNA dissociation. RNA 7, 302–313 (2001).
Hacker, S. & Krebber, H. Differential export requirements for shuttling serine/arginine-type mRNA-binding proteins. J Biol Chem 279, 5049–5052 (2004).
Kress, T. L., Krogan, N. J. & Guthrie, C. A single SR-like protein, Npl3, promotes pre-mRNA splicing in budding yeast. Mol Cell 32, 727–734 (2008).
Moehle, E. A., Ryan, C. J., Krogan, N. J., Kress, T. L. & Guthrie, C. The yeast SR-like protein Npl3 links chromatin modification to mRNA processing. PLoS Genet 8, e1003101 (2012).
Yeh, F. L. et al. Activation of Prp28 ATPase by phosphorylated Npl3 at a critical step of spliceosome remodeling. Nat Commun 12, 3082 (2021).
Sandhu, R., Sinha, A. & Montpetit, B. The SR-protein Npl3 is an essential component of the meiotic splicing regulatory network in Saccharomyces cerevisiae. Nucleic Acids Res 49, 2552–2568 (2021).
Bucheli, M. E. & Buratowski, S. Npl3 is an antagonist of mRNA 3’ end formation by RNA polymerase II. The EMBO journal 24, 2150–2160 (2005).
Bucheli, M. E., He, X., Kaplan, C. D., Moore, C. L. & Buratowski, S. Polyadenylation site choice in yeast is affected by competition between Npl3 and polyadenylation factor CFI. RNA 13, 1756–1764 (2007).
Dermody, J. L. et al. Unphosphorylated SR-like protein Npl3 stimulates RNA polymerase II elongation. PLoS One 3, e3273 (2008).
Holmes, R. K. et al. Loss of the yeast SR protein Npl3 alters gene expression due to transcription readthrough. PLoS Genet 11, e1005735 (2015).
Wong, C. M. et al. Yeast arginine methyltransferase Hmt1p regulates transcription elongation and termination by methylating Npl3p. Nucleic Acids Res. 38, 2217–2228 (2010).
Shen, E. C., Stage-Zimmermann, T., Chui, P. & Silver, P. A. 7The yeast mRNA-binding protein Npl3p interacts with the cap-binding complex. J. Biol. Chem. 275, 23718–23724 (2000).
Zander, G. et al. mRNA quality control is bypassed for immediate export of stress-responsive transcripts. Nature 540, 593–596 (2016).
Baierlein, C. et al. Monosome formation during translation initiation requires the serine/arginine-rich protein Npl3. Mol. Cell. Biol. 33, 4811–4823 (2013).
Estrella, L. A., Wilkinson, M. F. & Gonzalez, C. I. The shuttling protein Npl3 promotes translation termination accuracy in Saccharomyces cerevisiae. J. Mol. Biol. 394, 410–422 (2009).
Santos-Pereira, J. M. et al. The Npl3 hnRNP prevents R-loop-mediated transcription-replication conflicts and genome instability. Genes Dev. 27, 2445–2458 (2013).
Lee-Soety, J. Y. et al. Yeast hnRNP-related proteins contribute to the maintenance of telomeres. Biochem. Biophys. Res. Commun. 426, 12–17 (2012).
Colombo, C. V., Trovesi, C., Menin, L., Longhese, M. P. & Clerici, M. The RNA binding protein Npl3 promotes resection of DNA double-strand breaks by regulating the levels of Exo1. Nucleic Acids Res., https://doi.org/10.1093/nar/gkx347 (2017).
Bossie, M. A., DeHoratius, C., Barcelo, G. & Silver, P. A mutant nuclear protein with similarity to RNA binding proteins interferes with nuclear import in yeast. Mol. Biol. Cell 3, 875–893 (1992).
Russell, I. D. & Tollervey, D. NOP3 is an essential yeast protein which is required for pre-rRNA processing. J. Cell Biol. 119, 737–747 (1992).
Birney, E., Kumar, S. & Krainer, A. R. Analysis of the RNA-recognition motif and RS and RGG domains: conservation in metazoan pre-mRNA splicing factors. Nucl. Acids Res. 21, 5803–5816 (1993).
Clery, A., Blatter, M. & Allain, F. H. RNA recognition motifs: boring? Not quite. Curr. Opin. Struct. Biol. 18, 290–298 (2008).
Deka, P., Bucheli, M. E., Moore, C., Buratowski, S. & Varani, G. Structure of the yeast SR protein Npl3 and Interaction with mRNA 3’-end processing signals. J. Mol. Biol. 375, 136–150 (2008).
Skrisovska, L. & Allain, F. H. Improved segmental isotope labeling methods for the NMR study of multidomain or large proteins: application to the RRMs of Npl3p and hnRNP L. J. Mol. Biol. 375, 151–164 (2008).
Keil, P. et al. Npl3 functions in mRNP assembly by recruitment of mRNP components to the transcription site and their transfer onto the mRNA. Nucleic Acids Res. 51, 831–851 (2023).
Huppertz, I. et al. iCLIP: protein-RNA interactions at nucleotide resolution. Methods 65, 274–287 (2014).
Schneider, C., Kudla, G., Wlotzka, W., Tuck, A. & Tollervey, D. Transcriptome-wide analysis of exosome targets. Mol. Cell 48, 422–433 (2012).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Shetlar, M. D., Carbone, J., Steady, E. & Hom, K. Photochemical addition of amino acids and peptides to polyuridylic acid. Photochem. Photobiol. 39, 141–144 (1984).
Gossert, A. D. & Jahnke, W. NMR in drug discovery: a practical guide to identification and validation of ligands interacting with biological macromolecules. Prog. Nucl. Magn. Reson. Spectrosc. 97, 82–125 (2016).
Rossi, P. et al. A microscale protein NMR sample screening pipeline. J. Biomol. NMR 46, 11–22 (2010).
Clery, A. et al. Isolated pseudo-RNA-recognition motifs of SR proteins can regulate splicing using a noncanonical mode of RNA recognition. Proc. Natl Acad. Sci. USA 110, E2802–E2811 (2013).
Beuth, B., Garcia-Mayoral, M. F., Taylor, I. A. & Ramos, A. Scaffold-independent analysis of RNA-protein interactions: the Nova-1 KH3-RNA complex. J. Am. Chem. Soc. 129, 10205–10210 (2007).
Yan, C., Wan, R., Bai, R., Huang, G. & Shi, Y. Structure of a yeast activated spliceosome at 3.5 A resolution. Science 353, 904–911 (2016).
Sashital, D. G., Venditti, V., Angers, C. G., Cornilescu, G. & Butcher, S. E. Structure and thermodynamics of a conserved U2 snRNA domain from yeast and human. RNA 13, 328–338 (2007).
Baejen, C. et al. Transcriptome maps of mRNP biogenesis factors define pre-mRNA recognition. Mol. Cell 55, 745–757 (2014).
Castello, A. et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell 149, 1393–1406 (2012).
Jarvelin, A. I., Noerenberg, M., Davis, I. & Castello, A. The new (dis)order in RNA regulation. Cell. Commun. Signal 14, 9 (2016).
Shen, H. & Green, M. R. A pathway of sequential arginine-serine-rich domain-splicing signal interactions during mammalian spliceosome assembly. Mol. Cell 16, 363–373 (2004).
Shen, H., Kan, J. L. & Green, M. R. Arginine-serine-rich domains bound at splicing enhancers contact the branchpoint to promote prespliceosome assembly. Mol. Cell 13, 367–376 (2004).
Daubner, G. M., Clery, A., Jayne, S., Stevenin, J. & Allain, F. H. A syn-anti conformational difference allows SRSF2 to recognize guanines and cytosines equally well. EMBO J. 31, 162–174 (2012).
Afroz, T., Cienikova, Z., Clery, A. & Allain, F. H. One, two, three, four! How multiple RRMs read the genome sequence. Methods Enzymol. 558, 235–278 (2015).
Lukavsky, P. J. et al. Molecular basis of UG-rich RNA recognition by the human splicing factor TDP-43. Nat. Struct. Mol. Biol. 20, 1443–1449 (2013).
Duszczyk, M. M. et al. The solution structure of Dead End bound to AU-rich RNA reveals an unprecedented mode of tandem RRM-RNA recognition required for mRNA regulation. bioRxiv, 572156, https://doi.org/10.1101/572156 (2021).
Maris, C., Dominguez, C. & Allain, F. H. The RNA recognition motif, a plastic RNA-binding platform to regulate post-transcriptional gene expression. FEBS J. 272, 2118–2131 (2005).
Long, J. C. & Caceres, J. F. The SR protein family of splicing factors: master regulators of gene expression. Biochem J. 417, 15–27 (2009).
Muddukrishna, B., Jackson, C. A. & Yu, M. C. Protein arginine methylation of Npl3 promotes splicing of the SUS1 intron harboring non-consensus 5’ splice site and branch site. Biochim. Biophys. Acta Gene Regul. Mech. 1860, 730–739 (2017).
Jobbins, A. M. et al. Exon-independent recruitment of SRSF1 is mediated by U1 snRNP stem-loop 3. EMBO J. 41, e107640 (2022).
Jutzi, D. et al. Aberrant interaction of FUS with the U1 snRNA provides a molecular mechanism of FUS induced amyotrophic lateral sclerosis. Nat. Commun. 11, 6341 (2020).
Bai, R., Wan, R., Yan, C., Lei, J. & Shi, Y. Structures of the fully assembled Saccharomyces cerevisiae spliceosome before activation. Science 360, 1423–1429 (2018).
Galej, W. P. et al. Cryo-EM structure of the spliceosome immediately after branching. Nature 537, 197–201 (2016).
Sattler, M. Heteronuclear multidimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog. Nucl. Mag. Res. Sp. 34, 93–158 (1999).
Peterson, R. D., Theimer, C. A., Wu, H. & Feigon, J. New applications of 2D filtered/edited NOESY for assignment and structure elucidation of RNA and RNA-protein complexes. J. Biomol. NMR 28, 59–67 (2004).
Barraud, P. & Allain, F. H. Solution structure of the two RNA recognition motifs of hnRNP A1 using segmental isotope labeling: how the relative orientation between RRMs influences the nucleic acid binding topology. J. Biomol. NMR 55, 119–138 (2013).
Herrmann, T., Guntert, P. & Wuthrich, K. Protein NMR structure determination with automated NOE assignment using the new software CANDID and the torsion angle dynamics algorithm DYANA. J. Mol. Biol. 319, 209–227 (2002).
Case, D. A. et al. The Amber biomolecular simulation programs. J. Comput. Chem. 26, 1668–1688 (2005).
Lindorff-Larsen, K. et al. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins 78, 1950–1958 (2010).
Altvater, M. et al. Targeted proteomics reveals compositional dynamics of 60S pre-ribosomes after nuclear export. Mol. Syst. Biol. 8, 628 (2012).
Janke, C. et al. A versatile toolbox for PCR-based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast 21, 947–962 (2004).
Laskowski, R. A., Rullmannn, J. A., MacArthur, M. W., Kaptein, R. & Thornton, J. M. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR 8, 477–486 (1996).
Longtine, M. S. et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961 (1998).
Koradi, R., Billeter, M. & Wuthrich, K. MOLMOL: a program for display and analysis of macromolecular structures. J. Mol. Graph. 14, 29–32 (1996).
Granneman, S., Kudla, G., Petfalski, E. & Tollervey, D. Identification of protein binding sites on U3 snoRNA and pre-rRNA by UV cross-linking and high-throughput analysis of cDNAs. Proc. Natl Acad. Sci. USA 106, 9613–9618 (2009).
Dodt, M., Roehr, J. T., Ahmed, R. & Dieterich, C. FLEXBAR-flexible barcode and adapter processing for next-generation sequencing platforms. Biology 1, 895–905 (2012).
Engel, S. R. et al. The reference genome sequence of Saccharomyces cerevisiae: then and now. G3 4, 389–398 (2014).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Smith, T., Heger, A. & Sudbery, I. UMI-tools: modeling sequencing errors in Unique Molecular Identifiers to improve quantification accuracy. Genome Res. 27, 491–499 (2017).
Sugimoto, Y. et al. Analysis of CLIP and iCLIP methods for nucleotide-resolution studies of protein-RNA interactions. Genome Biol. 13, R67 (2012).
Acknowledgements
We thank Dr. Alvar Gossert, Dr. Simon Rudisser and Dr. Fred Damberger for the maintenance of the biomolecular NMR spectroscopy platform and Dr Ina Meuskens for the access to the CARY instrument. We also thank the SNF (grant numbers: 310030B-189379 and 31003A-170130) and SNF-NCCR RNA and Disease (grant number: 51NF40-182880). V.G.P. acknowledges financial support from the Swiss National Science Foundation, NCCR RNA & Disease, Novartis Foundation, Olga Mayenfisch Stiftung and a Starting Grant Award (EURIBIO260676) from the European Research Council.
Author information
Authors and Affiliations
Contributions
F.H.T.A., V.G.P., M.D.R. and A.C. designed research. S.G., S.R., A.M., J.M. and A.C. prepared samples. A.M., S.G., S.R., J.M. and A.C. collected data. A.M., A.C., S.G., K.M.B., S.R., S.C., I.B., M.M.D., J.M., V.G.P., M.D.R. and F.H.T.A. analyzed data. A.M., A.C., V.G.P. and F.H.T.A. wrote the manuscript and all authors edited it.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moursy, A., Cléry, A., Gerhardy, S. et al. RNA recognition by Npl3p reveals U2 snRNA-binding compatible with a chaperone role during splicing. Nat Commun 14, 7166 (2023). https://doi.org/10.1038/s41467-023-42962-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-42962-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.