Abstract
The quest for efficient non-Pt/Pd catalysts has proved to be a formidable challenge for auto-exhaust purification. Herein, we present an approach to construct a robust catalyst by embedding single-atom Ru sites onto the surface of CeO2 through a gas bubbling-assisted membrane deposition method. The formed single-atom Ru sites, which occupy surface lattice sites of CeO2, can improve activation efficiency for NO and O2. Remarkably, the Ru1/CeO2 catalyst exhibits exceptional catalytic performance and stability during auto-exhaust carbon particle oxidation (soot), rivaling commercial Pt-based catalysts. The turnover frequency (0.218 h−1) is a nine-fold increase relative to the Ru nanoparticle catalyst. We further show that the strong interfacial charge transfer within the atomically dispersed Ru active site greatly enhances the rate-determining step of NO oxidation, resulting in a substantial reduction of the apparent activation energy during soot oxidation. The single-atom Ru catalyst represents a step toward reducing dependence on Pt/Pd-based catalysts.
Similar content being viewed by others
Introduction
Auto-exhaust carbon particles (mainly containing soot) constitute a major source of atmospheric pollution, leading to severe environmental and health problems1,2. To address this problem effectively, a catalytic after-treatment technique combining particulate filters and oxidation catalyst has been adopted as the most effective strategy3,4. The success of this approach heavily relies on high-efficiency catalysts that facilitate soot oxidation at lower temperature range5. Researchers have explored numerous high-efficient catalysts for soot oxidation, including precious metal and metal oxides6,7,8. Notably, platinum (Pt) and palladium (Pd) metals remain essentially active components in commercial catalysts for soot purification, with their usage exceeding 42 % of the global demand amount, as reported in the Pgm market report (May 2023) by Johnson Matthey. This high reliance on Pt/Pd significantly contributes to the costly nature of auto-exhaust after-treatment systems. Developing high-efficiency, non-Pt/Pd catalysts with lower costs for soot oxidation presents a challenging task9. While the cost of ruthenium (Ru) metal is merely a third of Pt/Pd, the creation of Ru-based catalysts exhibiting both high activity and stability has been rarely reported in vehicle catalysts10,11. The primary issue lies in the volatility of Ru oxides at higher temperatures12,13. Hence, designing and preparing a robust and cost-effective Ru-based catalyst for auto-exhaust applications, capable of inhibiting volatile Ru at elevated temperatures, holds great significance in replacing Pt/Pd-based catalysts in the field of soot purification. Studies have found that the strong interactions between Ru and CeO2 in the Ru/CeO2 catalyst can enhance the catalytic activity and the thermal stability during soot oxidation reaction14. Therefore, employing a strong metal-support interaction (SMSI) emerges as a reasonable strategy to enhance the stability of Ru-based catalysts. Nonetheless, fabricating high-efficiency Ru-based catalysts with optimal atomic configurations continues to present a formidable challenge.
In recent times, single-atom catalysts (SACs) have garnered significant attention, especially precious metal SACs, due to their exceptional atomic utilization and uniform active site structure, making them highly attractive for deep oxidation reactions15,16. Studies further reveal that the architecture interfacial sites in SACs catalysts significantly influence both catalytic performance and thermal stability17,18,19,20. The complexity of soot oxidation occurring at the three-phase interface among solid catalysts, soot particles, and gaseous reaction gases (O2 and NO), adds to the challenge of designing and preparing efficient SACs catalysts21. This complexity demands careful consideration of both the intrinsic activity and stability of the catalyst for adsorbed/activated reactants, and the contact efficiency between soot particles and catalysts, as these factors play crucial roles in the reaction22,23,24,25,26,27. Compared to other oxides, Ceria (CeO2) proves to be an excellent cocatalyst, enhancing the catalytic performance and stability of precious metal active components in auto-exhaust catalysts because of its excellent oxygen storage/release properties28,29,30. In our previous works, we successfully constructed a series of Ce-based oxides as supports for the preparation of highly efficient supported noble metal soot oxidation catalysts31. Furthermore, our works highlight the importance of the strong interaction between Au NPs and CeO2 with the optimal crystal facet, which is crucial to adjust the intrinsic activity for O2 activation32. Despite these advancements, there remains limited research on SACs of single-atomic Ru anchored at the surface lattice of single-crystal CeO2, which holds the potential for replacing Pt/Pd-based catalysts in the field of soot purification.
In this study, we present a simple one-step synthesis of stabilized single-atom Ru sites confined on the surface lattice site of nanoflower-like CeO2 microspheres (Ru1/CeO2) using the gas bubbling-assisted membrane deposition (GBMD) method. These single-atom Ru active sites demonstrate remarkable thermal stability and enhance the activation efficiency of reactants (NO and O2). The Ru1/CeO2 catalyst, with Ru single sites on the CeO2 surface, exhibits excellent intrinsic catalytic performance and stability during soot oxidation under a loose contact model, outperforming both Ru nanoparticle and commercial Pt-based catalysts. Through comprehensive characterizations and density functional theory calculations, we identify the atomically dispersed Ru1O5 active site in the Ru1/CeO2 catalyst, along with the strong interface charge transfer within the Ru-O-Ce bond. The well-constructed active sites facilitate the formation of crucial surface NO2* intermediate species, which play a key role in the rate-determining step of NO oxidation to NO2, resulting in a significant reduction of the apparent activation energy during catalytic soot oxidation. This work emphasizes the advantages of synthesizing well-defined catalytic single sites on nanocrystals, and the combination of in-situ DRIFTS and DFT calculations provides valuable insights into the reaction mechanism. Furthermore, it establishes a methodological foundation for obtaining high-efficiency catalysts for auto-exhaust purification. The single-atom Ru catalyst offers a highly promising and cost-effective solution for auto-exhaust treatment systems.
Results
Chemical structure characterizations of Ru1/CeO2 and Run/CeO2 catalysts
The detailed synthesized processes of nanoflower-like CeO2 microspheres and Ru1/CeO2 catalyst were described (Supplementary Fig. 1). The atomically dispersed Ru1/CeO2 catalyst was synthesized using the GBMD method33, while the reference catalyst of supported Ru nanoparticles (NPs) on nanoflower-like CeO2 (Run/CeO2) was obtained through the gas bubbling-assisted membrane reduction method34. Inductively coupled plasma optical emission spectroscopy (ICP-OES) analysis revealed that the actual Ru loading amounts in Ru1/CeO2 and Run/CeO2 catalysts are 0.46 wt.% and 3.80 wt.%, respectively (Supplementary Table 1). The powder X-ray diffraction (XRD) patterns of all samples exhibit characteristic peaks of CeO2 nanocrystals with a cubic fluorite structure (JCPDS 34-0394). Notably, no characteristic diffraction peaks associated with Ru or RuOx NPs are detected, implying that the Ru species are highly dispersed on the surface of CeO2 (Fig. 1a). The lattice constant of CeO2 in the Ru1/CeO2 catalyst slightly decreases to 5.4084 Å compared to pure CeO2 (5.4101 Å) (Supplementary Table 1). This decrease is attributed to the substitution of Ce-sites on the surface lattice by smaller cation radius Ru ions (Supplementary Fig. 2). The SEM and TEM images provide a detailed view of the monodispersed nanoflower-like CeO2 microspheres, composed of single-crystal nanosheets (Supplementary Figs. 3a–d and 4). The thickness of these nanosheets is about 18 nm (Supplementary Fig. 5a, b), and the distance between two Ce atoms measures 3.1 Å, with clear step sites observed at the edge of the nanosheets (Supplementary Fig. 6).
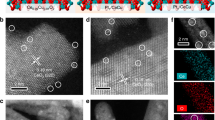
a XRD patterns of all samples and standard card of CeO2. b EXAFS fitting results in R space and the Ru K- edge for Ru1/CeO2 and Run/CeO2 catalysts with RuO2 and Ru foil. The shell radii (R) of Ru-O and Ru-O-Ru are marked. c The Ru K-edge XANES profiles. d and e In-situ DRIFT spectra of CO adsorption at 50 oC for the Ru1/CeO2 and Run/CeO2 catalysts with the extension of purge time (N2 flow, 30 mL min-1). f STEM-ADF image of Ru1/CeO2 catalyst with scale bars of 2 nm. The yellow circles represent atomically dispersed Ru sites. g STEM EDX mapping of Ru1/CeO2 catalyst (Purple, Ru; Orange, O; Mazarine, Ce).
In the N2 adsorption-desorption isotherms, all catalysts exhibit an H4 hysteresis loop in the P/P0 range 0.4-1.0, indicating the presence of a porous structure in the as-prepared samples (Supplementary Fig. 7a). The pore size distribution profiles of nanoflower-like CeO2, Ru1/CeO2, and Run/CeO2 catalysts all exhibit two peaks centered at 4.5 and 13.0 nm, in contrast to the single peak observed for CeO2-C at 8.0 nm (Supplementary Fig. 7b). The formation of these pores is attributed to the interwoven CeO2 nanosheets and stacked CeO2 nanoparticles (Supplementary Fig. 8a, b). To investigate the local electronic structure and coordination environment of Ru in the Ru/CeO2 catalysts, X-ray absorption measurements were conducted. The extended X-ray absorption fine structure (EXAFS) of Ru1/CeO2 shows only a primary peak at 1.49 Å, while the Run/CeO2 catalyst exhibits two peaks at 1.49 and 3.07 Å (Fig. 1b), corresponding to the Ru-O and Ru-O-Ru bond, respectively35,36. Further EXAFS curves fitting analyses provide insights into the coordination environment of Ru species in Ru1/CeO2 and Run/CeO2 catalysts, giving the coordination number of nearest-neighbor O atoms surrounding the isolated Ru atom (Fig. 1b and Supplementary Table 2). The coordination number of O atoms surrounding Ru atom in Ru1/CeO2 catalyst is 5.3, with a mean bond length of 2.01 Å, while the value of Run/CeO2 catalyst is 3.4, with the bond length of 2.04 Å. As a result, the atomically dispersed Ru species in the Ru1/CeO2 catalyst diffuse into the surface lattice of CeO2, forming coordination unsaturated Ru1O5 active sites. The atomic dispersion of Ru species in the Ru1/CeO2 catalyst is further corroborated by subsequent characterizations.
The X-ray photoelectron spectroscopy (XPS) spectra of Ru1/CeO2 and Run/CeO2 catalysts were acquired to determine the status of Ru species. The Ru 3p spin-orbit splits into 3p3/2 and 3p1/2 components, corresponding to the binding energy (BE) of 462.2 and 484.1 eV37,38. The two BE pairs (461.7 and 484.7 eV; 463.3 and 485.3 eV) corresponding to Ru 3p3/2 and 3p1/2 can be assigned to Ru0 and Run+ species, respectively (Supplementary Fig. 9)39,40. In the Ru1/CeO2 catalyst, the Ru 3p XPS only shows the oxidation state, indicating the presence of single positively charged Ru atoms. Conversely, the Ru 3p peak of the Run/CeO2 catalyst exhibits the coexistence of Ru0 and Run+ (4 ≤ n ≤ 6) species, attributed to the formation of a core-shell structured Ru@RuOx layer in Ru sub-nanometric particles (SNPs)41. This suggests that the Ru species in the Ru1/CeO2 catalyst consist of single-atom Ru with a positively charged feature, while the Ru species in Run/CeO2 catalyst contain a certain amount of metallic state. Furthermore, the Ru K-edge adsorption position of the near-edge X-ray absorption fine structure (NEXAFS) spectra over the Ru1/CeO2 catalyst is slightly higher than that of Run/CeO2 and RuO2 observed through local magnification (Fig. 1c). This observation indicates that the valence state of single-atom Ru species in the Ru1/CeO2 catalyst is higher than +4, consistent with the result obtained from the Ru 3p XPS analysis.
Diffuse reflectance infrared Fourier transform spectrum of CO adsorption (CO-DRIFTS) on noble metal catalysts is widely used to investigate the atomic and electronic structures of metal-support binding sites. In the case of Ru/CeO2 catalysts, CO-DRIFTS can differentiate between active Ru sites in single atoms and nanoparticles. The CO-DRIFTS spectrum of Ru1/CeO2 catalyst shows four strong adsorption peaks (Fig. 1d), whereas the spectrum of Run/CeO2 catalyst shows only two strong adsorption peaks (Fig. 1e). The strong adsorption peaks centered at 2170 and 2117 cm-1 are assigned to the R and P branches of the rotation vibrational spectra of gas-phase CO species42. The strongest peak centered at 2057 cm-1 is ascribed to the C-O stretching vibration of dicarbonyl CO species (Run+(CO)2) adsorption on single atomically dispersed Run+ sites43,44,45. The peak centered at ~2018 cm-1 can be assigned to C-O vibration of CO linearly bound Ru sites with high coordination46, while the peak at ~1991 cm-1 could be ascribed to the CO adsorbed on oxygen vacancies or Ru-doped CeO247. These findings indicate that the Ru species in Ru1/CeO2 catalyst exist as a single atomically dispersed ionic state, forming a Ru-O-Ce bond originating from the surface lattice site of CeO2, which has been substituted by a single Ru atom. On the other hand, the CO adsorption peak of the Run/CeO2 catalyst, centered at 2057 cm-1, is difficult to detect, but it demonstrates two weak bands at 1830 and 1860 cm−1, which can be attributed to the bridged adsorption of CO on two and three Ru atoms46,48, indicating the presence of Ru sub-nanometric particles (SNPs). With increasing purging time, the intensity of CO adsorption band on single atomic Ru sites decreases significantly, whereas that on Ru SNPs remains relatively unchanged. This suggests that the binding strength of CO to Ru1 is lower than that of Ru SNPs.
To further visualize the existence and dispersion state of the Ru species, aberration-corrected STEM images of the catalysts were obtained. Upon the introduction of the Ru species, Ru1/CeO2 catalyst maintains the initial nanoflower-like morphology of CeO2 (Supplementary Fig. 10a, b). The atomically dispersed Ru species in Ru1/CeO2 catalyst are faintly observed and coincided with the atomic lattice of CeO2 (Fig. 1f). The interplanar crystal spacing of CeO2 paralleled to the edge is 3.1 Å, as determined from the fast Fourier transform (FFT) pattern in the inset (A) of Fig. 1f, and corresponds to the exposed CeO2 (111) crystal facets. STEM images and corresponding Energy-dispersive X-ray (EDX) element mapping of the Ru1/CeO2 catalyst demonstrate the homogeneous dispersion of single-atom Ru species on the surface of CeO2 support (Fig. 1g). In contrast, the Ru species in Run/CeO2 catalyst exhibit a certain degree of aggregation and form small Ru SNPs (Supplementary Fig. 11a, b). The size distribution and EDX element mapping analysis in STEM-ADF images show the uniformed dispersion of Ru SNPs (d = 0.9 ± 0.2 nm) deposited on the surface of CeO2 nanosheets (Supplementary Fig. 12). Based on the results of EXAFS, in-situ CO-DRIFTS and STEM characterizations, it is evident that the state of Ru species in Ru1/CeO2 catalyst is predominantly isolated single-atom dispersion, occupying the surface lattice sites of CeO2 nanocrystals to form Ru1O5 structure. Conversely, the state of Ru species in the Run/CeO2 catalyst is RuO2 SNPs.
Catalytic performance and kinetics of Ru/CeO2 catalysts in soot oxidation
The performance of all catalysts for soot oxidation was evaluated under the loose contact mode. To highlight the catalytic performance of the CeO2-M nanosheet catalyst, conventional powder-type CeO2 (CeO2-C) was synthesized as the reference sample. The relative reaction rates (R) of catalyzing soot oxidation were calculated through an isothermal oxidation reaction at 280 oC (Fig. 2a). The CeO2-M catalyst exhibits a higher R value (0.94 μmol g−1 min−1) compared to the CeO2-C sample (0.79 μmol g−1 min−1), indicating that the nanoflower-like morphology effectively enhances catalytic performance for soot oxidation. Additionally, the tight soot-catalyst contact mode over the same catalyst showed higher performance compared with the loose contact mode, which approximates real conditions (Supplementary Fig. 13a, b). Upon the introduction of Ru species, the R values of Ru1/CeO2 and Run/CeO2 catalysts remarkably increased to 2.59 and 2.38 μmol g-1 min-1, respectively. The actual Ru content obtained by ICP-OES allowed the representation of the reaction rate per each Ru atom, represented as the turnover frequency (TOFRu). The TOFRu value of Ru1/CeO2 catalyst (0.218 h-1) is approximately nine-fold higher than that of Run/CeO2 catalyst (0.023 h-1) (Fig. 2b). This suggests that a single atomic Ru site possesses both high activity and atom utilization. Moreover, the selectivity of CO2 product (SCO2) over the Ru1/CeO2 catalyst is close to 100 %, allowing for the rapid removal of CO product emitted from vehicle exhaust. Furthermore, the Ru1/CeO2 catalyst exhibits excellent intrinsic catalytic performance during auto-exhaust soot oxidation compared to the commercial Pt-based catalysts (Supplementary Table 3).
a Soot conversion amount as a function of times by isothermal oxidation reaction at 280 oC. b TOFRu over Ru1/CeO2 and Run/CeO2 catalysts. c Active oxygen amount by isothermal anaerobic titration at 280 oC. d Ozawa plots of ln[-ln(1-α)/T2] vs T-1 for different soot conversion. e Time-TOFRu and the selectivity of CO2 over Ru1/CeO2 and Run/CeO2 catalysts (Reaction condition: Soot, 20 mg; Catalyst, 200 mg; Reactant gas flow, 50 mL min-1; At 280 oC). f NO2 concentration curves of NO temperature-programmed oxidation.
The amount of active surface oxygen species plays a crucial role in catalytic performance for deep oxidation reactions, and it was determined by isothermal anaerobic titration at 280 oC (Supplementary Fig. 14a-d). The presence of single atomic Ru species in the Ru1/CeO2 catalyst significantly increased the active oxygen amounts from 31.3 (CeO2-M) to 48.6 μmol g-1 (Ru1/CeO2), owing to the contribution of interface oxygen species in Ru-O-Ce bond (Fig. 2c and Supplementary Fig. 14a-d). To explore the reaction energy barrier on single atomic Ru sites during soot oxidation, the Arrhenius plots of the catalysts were analyzed (Fig. 2d). The Ru1/CeO2 catalyst exhibited the lowest apparent activation energy (Ea, 75.2 kJ mol−1) in comparison with CeO2 and Run/CeO2 catalysts (Supplementary Table 4), indicating that single atomic Ru sites play a pivotal role in boosting the catalyzing soot oxidation.
Addressing the stability concerns associated with single-atom catalysts, we further investigated the stability of Ru species in the catalysts via the TOFRu and SCO2 values versus time (Fig. 2e). The TOFRu and SCO2 values of the Ru1/CeO2 catalyst remain relatively stable during 400 min, whereas those of the Run/CeO2 catalyst gradually decrease. In line with the cyclic test results of soot oxidation (Supplementary Figs. 15 and 16a, b), this confirms that the Ru1/CeO2 catalyst demonstrates higher catalytic stability compared with the Run/CeO2 catalyst. Additionally, the morphology and crystal phase structure remain unchanged during six-cycle TPO tests (Supplementary Fig. 17). On the other hand, ICP-OES analysis revealed a significant loss of Ru content on the used Run/CeO2 catalyst, which could be attributed to the volatilization of Ru species (Supplementary Fig. 18). The Ru1/CeO2 catalyst, with the surface lattice confinement single atom Ru, effectively inhibited the volatilization of Ru species. The CO-DRIFTS of the used Ru1/CeO2 catalyst demonstrated Ru species maintain a single atomically dispersed ionic state (Supplementary Fig. 19a, b), and STEM EDX mapping of Ru1/CeO2 catalyst also showed high dispersion of Ru species (Supplementary Fig. 20a, b). Conversely, the Ru species in the Run/CeO2 catalyst had aggregated into larger particles (Supplementary Fig. 21). The Ru 3p XPS spectra of the used Ru1/CeO2 catalyst still maintained the oxidation state with peaks located at 463.3 and 485.3 eV, corresponding to Run+ species (Supplementary Fig. 22). Raman spectra of the used Ru1/CeO2 revealed that the lattice-confined single-atom Ru1/CeO2 structure remains stable (Supplementary Fig. 23a, b). However, for the used Run/CeO2 catalyst, the peak intensity of RuO2 species (~323 cm−1) increased, indicating the aggregation of RuO2 into larger particles after six cycles of soot-TPO tests. After six cycles of tests, the ID/IF2g value (area ratio of D peak to F2g peak for Raman spectra) of the Run/CeO2 catalyst significantly decreased. This suggests that the interaction between the Ru species and CeO2 has changed, resulting in a decrease in the number of oxygen vacancies on the CeO2 surface. Additionally, the surface plasmon resonance (SPR) peak of UV-Vis red-shifted for the used Run/CeO2 catalyst (Supplementary Fig. 24), further indicating an increase in the average size of RuO2 nanoparticle during the reaction49. Consequently, the weakening of the interaction facilitates the volatility and migration of Ru species, leading to the formation of large particles, which in turn reduces the number of active sites and deactivates the catalyst during soot oxidation. These results demonstrate that the single-atom Ru sites anchored on the surface lattice of CeO2 nanocrystals exhibit high thermal and structural stability during soot oxidation.
Nitric oxide (NO) emissions from automobile exhaust are inevitable and can promote the removal efficiency of soot particles through NO oxidation into NO2, enhancing soot oxidation via a NO2-assisted mechanism50. We also investigated the role of NO over single-atom Ru active sites during catalytic soot oxidation (Supplementary Fig. 25a, b). Interestingly, the catalytic activity of Run/CeO2 catalyst is higher than that of the Ru1/CeO2 catalyst under the sole presence of O2. However, with the addition of NO, the T50 values significantly shift to the lower temperature for both Ru1/CeO2 and Run/CeO2 catalysts. The temperature difference (ΔT50) of Ru1/CeO2 and Run/CeO2 catalysts is 87 and 54 oC under the sole presence of 5 vol% O2, and 5 vol% O2 + 0.2 vol% NO, respectively. This observation indicates that the presence of NO has a more pronounced effect on soot oxidation, emphasizing the critical role of NO activation and oxidation in enhancing catalytic activity for soot oxidation. It suggests that the Ru1/CeO2 catalyst can significantly promote the activation and oxidation of NO, thereby enhancing catalytic activity for soot oxidation via the NO2-assistant soot purification mechanism. The catalytic performance for NO oxidation was further evaluated using NO-temperature programmed oxidation (NO-TPO, Fig. 2f). It is noted that the Ru1/CeO2 catalyst has a higher NO2 concentration than the Run/CeO2 catalyst. Moreover, the Ru1/CeO2 (305 oC) catalyst exhibits a lower temperature of NO2 concentration peak comparison with the Run/CeO2 catalyst (315 oC). Therefore, the single-atom Ru sites anchored on the surface lattice of CeO2{111} facets exhibit improved catalytic performance for low-temperature NO oxidation compared with the Run/CeO2 catalyst. The relatively low temperature of NO oxidation over the single-atom Ru catalyst indicates that the Ru1/CeO2 catalyst demonstrates excellent activation and oxidation of NO molecules, contributing to its super-catalytic performance for NO2-assistant soot oxidation.
Identifying active species and reaction pathways during soot oxidation
The performance of the catalysts during deep oxidation strongly depends on the presence of surface-active oxygen species, which are generated from activated O2 molecules by the low-coordinatively unsaturated cation (CUC) sites. Identifying the active species involved in catalyzing soot oxidation is crucial to understanding the reaction mechanism. We investigated the surface density of CUC sites induced by single-atom Ru species using Raman scattering spectra (Fig. 3a). The strong vibration peak at ~464 cm−1 corresponds to the first-order F2g symmetry of CeO2 nanocrystals. Interestingly, the presence of Ru in the Ru1/CeO2 catalyst causes this peak to shifts down by ~7 cm−1 (Supplementary Fig. 26), suggesting that the atomically dispersed Ru species either lowers the symmetry of Ce-O bond on the Ru1/CeO2 catalysts or facilitates the transfer of electrons from Ru species to CeO2, affecting the electronic structure of the catalyst51,52. Additionally, the Raman peak centered at ~598 cm−1 corresponds to the defect-induced (D) mode of CeO2, and its intensity noticeably increases with the introduction of Ru species53. For Ru1/CeO2 and Run/CeO2 catalysts, two exclusive peaks at ~682 and ~975 cm−1 indicate the formation of the Ru-O-Ce bond, further confirming the presence state of Run+ species in the catalysts54. Moreover, a weak peak at ~323 cm−1 can be attributed to RuO2 formation in Run/CeO2 catalyst55. Furthermore, the peak at ~831 cm−1 is assigned to isolated two-electron surface defect sites on oxidized surfaces of Ce-based oxide56. To evaluate the surface density of oxygen vacancies, we examined the area ratio of D peak to F2g peak (denoted as ID/IF2g)57. The Ru1/CeO2 catalyst exhibits the highest ID/IF2g value (Supplementary Fig. 27), indicating that the formation of the Ru-O-Ce bond induces electronic transfer from Ru to Ce4+, leading to two Ce4+ ions replaced by two Ce3+ ions for creating each oxygen vacancy. As a result, the surface density of CUC sites is increased.
a Raman spectrum with an excitation wavelength of 532 nm. b H2-TPR profiles. c XPS spectra of O 1 s. d The ratio of oxygen species determined by XPS. e Temperature-dependent in-situ DRIFT spectra and (f) corresponding contour projection results of NO oxidation on Ru1/CeO2 catalyst (Catalyst, 10 mg; 5 vol% O2 and 0.2 vol% NO).
The amount of active oxygen species generated by CUC sites can be evaluated using temperature-programmed reduction with H2 (H2-TPR). CeO2-M catalyst exhibits a lower reduction temperature (457 and 781 oC) and higher H2 consumption (1.389 mmol g-1) compared to CeO2-C catalyst (Fig. 3b and Supplementary Fig. 28), indicating that the exposed CeO2{111} facets enhance the oxidation property of surface oxygen species. In the H2-TPR profile of the Run/CeO2 catalyst, the first peak located at 127 oC is assigned to the reduction of RuOx. However, the reduction of Ru species in the Ru1/CeO2 catalyst occurs at a higher temperature, 173 oC, indicating a stronger interaction between Ru species and the support compared to that in the Run/CeO2 catalyst56. The reduction temperature of Ru species in the Ru1/CeO2 catalyst is higher than in the Run/CeO2, indicating two different existing states of Ru species. The initial H2 consumption rate reflects the activity of low-temperature surface oxygen species, and for supported Ru catalysts, their values increase more than thirteen-fold compared to bare CeO2-M (Supplementary Fig. 29), which results in the relatively low ignition temperature (T10) during catalytic soot oxidation (Supplementary Fig. 13). XPS was used to investigate the surface element state in the catalysts. The O 1 s XPS can be deconvoluted into three peaks (Fig. 3c). Surface peroxy-(O22-) and super-oxygen (O2-) species, activated by CUC sites, are considered active oxygen species rather than lattice oxygen (O2-) during deep oxidation reactions. The percentage of active oxygen species in the single-atom Ru catalyst is the highest (37%) (Fig. 3d), suggesting that the CUC sites induced by Ru species greatly enhance the adsorption-activation of O2 molecules, leading to the formation of active oxygen species. This finding is consistent with the analysis result of Ce 3d XPS which shows that the Ru1/CeO2 catalyst has the highest percent (27.6%) of coordinatively unsaturated Ce3+ species, resulting from the strong Ru-O-Ce electronic interaction (Supplementary Fig. 30 and Supplementary Table 5). This electronic interaction is crucial for boosting the catalytic activity of soot oxidation56.
The transformation pathways of surface-active species over the catalysts were studied using in-situ DRIFTS. At 50 oC, a series of NOx-containing species is observed on surface of the Ru1/CeO2 catalyst after introduction of NO and O2 into the reactor (Fig. 3e). These species include N2O4 dimer (1766 cm-1), bridging nitrates (1627 cm−1), monodentate nitrites (1422 and 1360 cm-1), ionic nitrites (1390 cm-1) and nitrates (836 cm-1) (Supplementary Table 6). As the reaction temperature increases, the three characteristic peaks of ionic nitrites and monodentate nitrites gradually merge into one new peak (1372 cm-1), and its intensity increases significantly. At the same time, the peak of bridged nitrates gradually weakens and eventually disappears, while new peaks of anionic (1134 cm−1) and bidentate (1043 and 965 cm-1) nitrates appear (Fig. 3f). These changes suggest that the adsorbed NOx species gradually transform into labile NO3- intermediates, which subsequently decompose into NO2. The Run/CeO2 catalyst exhibits similar evolution processes of in-situ DRIFTS spectra for NO oxidation (Supplementary Fig. 31), and the Ru1/CeO2 catalyst exhibits the largest relative intensity of surface NO3- at 200 oC (Supplementary Fig. 32), indicating that single atomic Ru species significantly promote the transfer from surface active oxygen to NO3- intermediate. Finally, the gaseous NO2 produced by surface nitrate decomposition boosts catalyzing soot oxidation in the NO2-assisted mechanism. The electron and energy evolution processes of surface-active intermediate over the catalysts were further investigated through DFT calculations in the following discussion.
Insight into the mechanism of single atomic Ru catalyst for soot oxidation
To gain insight into NO2-assisted catalytic mechanism for soot oxidation over Ru catalysts, the reaction pathways were investigated by DFT calculations. Model active sites of single atom Ru1 and Ru10 SNPs were constructed on the surface of CeO2 (110) facets (Supplementary Fig. 33a-c). The charge density difference maps of Ru1/CeO2 and Ru10/CeO2 were used to investigate the strong interaction between Ru and CeO2, and the extra number of transferred electrons was calculated using Bader charge analyses. The results showed that the Ru1 model can donate 1.46 e- to CeO2 support (Supplementary Fig. 34a-c), while Ru10 model transfers a total of 1.26 e-, indicating a stronger electronic interaction between Ru1 and CeO2, which boosts the formation of CUC sites compared to Ru10 SNPs (Supplementary Fig. 35a-c).
The reaction pathways and relative energy (E) during catalyzing NO oxidation can be divided into six stages as follows (Fig. 4): first, a gaseous O2 molecule adsorbs on the unsaturated coordination Ce site (Ce-O-Ru) over the catalyst surface through O-O-Ce bond to form surface adsorption O2* (IM1). The adsorption energy of O2 (Eads(O2)) is -0.9 and -0.53 eV for Ru1/CeO2 and Ru10/CeO2, respectively, indicating that the single Ru site easily adsorbs O2 molecule. The calculated charge density difference shows that the O2 molecule can be adsorbed on coordination unsaturated Ce atom of Ru1/CeO2 and Ru10/CeO2, with a total net charge transfer from Ru1/CeO2 surface to O2 being 0.56 e- (Supplementary Fig. 36a, b) and 0.44 e- for Run/CeO2 catalyst (Supplementary Fig. 37a, b). The adsorbed O2 molecule over Ru1/CeO2 catalyst gains the more electrons, facilitating the activation of O = O bond. Second, one NO molecule adsorbs on the Ru site of the catalyst surface through the O-N-Ru bond to form surface NO species (IM2). The NO adsorption energy (Eads(NO)) of Ru10/CeO2 (-2.82 eV) is lower than that of Ru1/CeO2 (-1.63 eV), and this strong adsorption capacity is not conducive to the subsequent reaction over Ru10/CeO2. Third, the O-O bond of the adsorbed O2 is activated to dissociate into atomic O* species, and adsorbed NO combines with O* to form the surface intermediate NO2* species (IM3). The reaction barriers for these steps are 0.9 and 1.23 eV with the corresponding transition states (TS1) of Ru1/CeO2 and Ru10/CeO2, respectively. Fourth, the NO2 species desorb from the catalyst surface (IM4). The NO2 desorption energy (Edes(NO2)) for Ru1/CeO2 (1.01 eV) is lower than that of Ru10/CeO2 (2.60 eV), indicating that the desorption of NO2 molecules over the single atomic Ru site is easier than that of Ru10 site. This result in the rapid formation of NO2 on the surface of Ru1/CeO2 catalyst, a crucial step during soot oxidation. Fifthly, an additional NO molecule adsorbs on the O* to form ONO* (IM5). Finally, the formation of NO2* species desorbs from the catalyst surface, completing the reaction cycle (FS). The NO2 desorption energy (Edes(NO2)) for Ru1/CeO2 (1.70 eV) is lower than that of Ru10/CeO2 (2.15 eV), indicating that the NO2 molecule was easily desorbed from the catalyst surface. Based on the above results, we found that NO molecules prefer to adsorb on Ru active sites on the surface of the catalysts, while O2 molecules are strongly adsorbed on the CUC sites. The rate-determining step of NO oxidation at the Ru-CeO2 interface is the formation of NO2* intermediate species. The stronger oxidizing NO2 can migrate to the soot surface along with the reaction gas flow and oxidize them to form CO2 via the indirect pathway (NO + 1/2O2 → NO2 and NO2 + soot → CO2). These DFT calculation results for NO oxidation are consistent with our experimental findings. The single active Ru site easily boosts NO oxidation to NO2 intermediate, which is beneficial to further promote soot oxidation.
Discussion
We successfully fabricated stabilizing single-atom Ru sites on the surface lattice of nanoflower-like CeO2. The Ru1O5 coordination structure in the Ru1/CeO2 catalyst demonstrated remarkable thermal stability and activity in boosting the adsorption and activation of NO and O2 molecules, resulting in the formation of the crucial NO2 intermediate, which plays a key role in the NO2-assisted catalytic mechanism for soot oxidation. Therefore, the Ru1/CeO2 catalyst exhibits excellent intrinsic catalytic performance with a high turnover frequency (TOFRu=0.218 h−1) and low apparent activation energy (Ea = 75.2 kJ mol-1) during auto-exhaust soot oxidation, surpassing both Ru nanoparticle (TOFRu=0.023 h-1) and commercial Pt-based catalysts. Moreover, the single-atom Ru1/CeO2 catalyst demonstrates exceptional selectivity of the CO2 product (>99%) and remarkable durability during catalytic soot oxidation. This high-efficiency of the single-atom Ru catalyst offers a promising avenue for designing a considerably low-cost auto-exhaust treatment system, moving away from the reliance on costly Pt and Pd-based catalysts. The findings are also of significant importance for the further development of single-atom catalysts in the areas of deep oxidation and activated O2 reactions. The single-atom Ru catalyst paves the way for environmentally friendly and efficient exhaust treatment technologies, contributing to cleaner air and sustainable development.
Methods
Chemicals
Cerium (III) nitrate hexahydrate, acrylamide, and glucose were purchased from Aladdin Technology Co., Ltd. Standard ammonia solution (25 wt%) was purchased from Macklin Technology Co., Ltd. Ruthenium (III) chloride hydrate was purchased from J&K Scientific Ltd. Ethanol was purchased from Sigma Aldrich. All reagents and solvents were of analytical grade and used as received without additional purification.
Catalysts preparation
Syntheses of nanoflower-like CeO2 microspheres and prepared conventional CeO2 nanoparticles
the nanoflower-like CeO2 microsphere was synthesized by the hydrothermal method58. The glucose (1.98 g, 0.010 mol) was dissolved into 80 mL of deionized water, and the addition of acrylamide (1.05 g, 0.015 mol) and hydrated cerium (III) nitrate (2.17 g, 0.005 mol) was stirred to form a transparent solution. Then, the standard ammonia solution (3.2 mL, 25 wt%) was dropwise to the above solution, and the solution became the sol with vigorous stirring at room temperature for 5 h. The color of the gelatinous mixture turned dark brown at the pH value of 10. Subsequently, the mixture was transferred into a 100 mL Teflon-lined autoclave and kept at 180 oC for 72 h. After the natural cooling of the autoclave to room temperature, the sediment was separated by centrifugation, and the sample was washed with deionized water and ethanol three times. The nanoflower-like CeOHCO3 microsphere was obtained by drying at 80 oC for 12 h, and was further two-steps calcined at 600 oC for 4 h in N2 and at 500 oC for 4 h in air. Finally, nanoflower-like CeO2 microsphere (denoted as CeO2-M) was obtained. Conventional CeO2 nanoparticles (CeO2-C) as reference sample was synthesized by the deposition-precipitation method. Cerium (III) nitrate hexahydrate (0.50 g) was dissolved into 20 mL of deionized water. Then, a standard ammonia solution (5 mL) was added dropwise under vigorous stirring. After aging for 30 min, the obtained product was washed with deionized water and ethanol. The product was dried at 80 oC for 12 h and calcined at 500 oC for 4 h in an air atmosphere to obtain general CeO2-C.
Syntheses of nanoflower-like Ru1/CeO2 and Run/CeO2 catalysts
The nanoflower-like CeO2-supported single-atom Ru (Ru1/CeO2) catalyst was synthesized by a gas bubbling-assisted membrane deposition (GBMD) method33. CeO2-M support (50 mg) was dispersed into deionized water (400 mL) with vigorous stirring, and 0.646 mL RuCl3·3H2O solution (0.01 g mL−1) was dropwise added into the above light-yellow slurry (denoted as Beaker I). A stabilizer (polyvinyl pyrrolidone, the molar ratio of Ru/PVPunit is 1/100) was then transferred to Beaker I. A peristaltic pump with a rotation speed of 200 rpm was developed to form tubal cycling of the above solution mixture between the membrane reactor and Beaker I at a flow rate of 400 mL min−1. In the membrane reactor, the solution mixture flowed in the glass tube and outside the ceramic tubes. Prepared ammonia solution (1 mL standard ammonia solution was added to 40 mL deionized water) as a precipitant was immitted into the membrane reactor by a constant flow pump at a flow rate of 0.2 mL min−1. The ammonia solution infiltrated through the abundant holes on the walls of the two ceramic tubes into the glass tube, and the deposition of Ru ions occurred immediately until all the ammonia solution was completely consumed. The color of the slurry changed from light brown to dark gray, indicating that the Ru species were deposited. The mixture was filtered and washed with deionized water and ethanol three times, and the product was dried at 60 oC for 12 h. Finally, the sample was calcined to obtain a single-atom Ru catalyst at 500 oC for 4 h in air, denoted as Ru1/CeO2. The CeO2-M supported Ru nanoparticle (Run/CeO2) catalyst was prepared by using the gas bubbling-assisted membrane reduction method. This process is similar to GBMD, except that the high stoichiometric regents of RuCl3·3H2O (0.01 g mL−1, 5.167 mL) and PVP (Ru/PVPunit is 1/100). Moreover, NaBH4 solution as a reductant (the molar ratio of NaBH4/Ru is 5/1) was immitted into the membrane reactor by a constant flow pump at a flow rate of 0.2 mL min−1. The NaBH4 solution (40 mL) infiltrated through the abundant holes on the walls of the two ceramic tubes into the glass tube, and the reduction of Ru ions occurred immediately until all the NaBH4 solution was completely consumed. The mixture was filtered and washed with deionized water and ethanol three times, and the product was dried at 60 oC for 12 h. Finally, the sample was calcined at 500 oC for 4 h in air, denoted as Run/CeO2.
Characterizations
Powder X-ray (XRD) patterns were obtained by a diffractometer (Bruker D8 advance) using Cu-Kα radiation to obtain the phase structure of all as-prepared catalysts. The Ru K-edge X-ray absorption near edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) experiments were carried out on the experiment assist system of SSRF and beamline BL13SSW and BL06B of the Shanghai Synchrotron Radiation Facility (SSRF). Raman spectra of all catalysts were measured on an inVia Reflex-Renishaw spectrometer with an excitation wavelength of 532 nm. Scanning electron microscopy (SEM), transmission electron microscope (TEM) and high-resolution transmission electron microscope (HRTEM) images were obtained by ZEISS Gemini SEM 300 and JEOL JEM LaB6 2100, respectively. STEM-ADF images and EDX mapping were obtained by Hitachi HF5000, working at an accelerating voltage of 200 kV. The actual loading amounts of Ru in catalysts were determined by ICP-OES (Perkin Elmer, OPTIMA 7300 V). The pore size and specific surface area of catalysts were characterized by nitrogen adsorption−desorption experiments (Micromeritics TriStar-II 3020). Temperature-programmed reduction with H2 (H2-TPR) was carried out on a fixed-bed device. The surface element valence state was detected by X-ray photoelectron spectra (XPS, XPSPHI−1600 ESCA spectrometer). NO-TPO experiments were carried out on a fixed-bed tubular quartz reactor with flowed the gaseous contained O2 (5 vol%) and NO (0.2 vol%) balanced with N2 (50 mL min−1), and the products can be detected by online FT-IR. In situ diffuse infrared Fourier transforms spectra (in-situ DRIFTS) were carried on a Bruker FT-IR spectrometer (TENSOR II) equipped with a liquid nitrogen-cooling mercury-cadmium-telluride (MCT) detector. Before the in-situ CO-DRIFTS adsorption and desorption experiment, the sample (10 mg) was loaded into high-temperature IR cell with a ZnSe window (Pike Technologies), and pretreated in a 10 vol% H2 and balanced with N2 flow rate of 30 mL min−1 at 300 oC for 30 min. After cooling down to 50 oC under N2 flow (30 mL min−1) for 10 min, the background was collected. For the CO adsorption step, the CO (10 vol% CO in N2 balance) gas was fed into the cell at a flow rate of 30 mL min−1 for 30 min up to adsorption saturation. For the CO desorption, N2 flow (30 mL min−1) was purged into the cell to remove adsorbed CO, and the spectra were recorded at an apart of 2 min. In-situ DRIFTS of NO oxidation test, the catalyst (10 mg) was added into high-temperature IR cell with ZnSe window, and heated in N2 flow at 200 oC for 30 min to remove adsorbed H2O and other materials. After cooling down to 50 oC with N2 atmosphere, the background spectrum was recorded. Finally, the O2 (5 vol%), NO (0.2 vol%) and balanced with N2 were fed into cell at a flow rate of 50 mL min−1. IR spectra of the catalysts were recorded in a flow of 0.2 vol% NO/5 vol% O2/N2 balance (50 mL min−1) under heating from 50 to 400 oC.
Catalytic activity and kinetic tests
The catalytic activity for soot oxidation was evaluated by soot-TPO in a tubular quartz reactor using Printex-U as model soot particles. The loose contact was obtained by mixing the catalyst (100 mg) and soot (10 mg) with a spoon for 10 min, and the tight contact was obtained by grinding the above mixture for 10 min. The reaction temperature in soot-TPO rises from 150 to 550 oC with a rising rate of 2 oC min−1. The reaction gases were composed of 5 vol% O2 and 0.2 vol% NO balanced with Ar, and the total flow rate was 50 mL min−1 passed through the mixture. The gas product of CO2 and CO in the outlet gas was monitored by an online gas chromatograph (GC 9890B, Shanghai). The catalytic performance was evaluated by the TOFRu, which can be defined by the ratio of the isothermal reaction rate (R) to the actual amount of Ru-supported in the catalysts. The temperature at 10%, 50%, and 90% of soot conversion from soot-TPO test denotes T10, T50, and T90, respectively. The selectivity of CO2 (SCO2) can be calculated by the ratio of CO2 concentration to the sum of CO and CO2 concentration, and the SCO2 was calculated by the following formula:
Here, the [CO2]out and [CO]out represent the outlet CO2 and CO concentration (ppm), respectively. SCO2m was defined as SCO2 with the maximum value of CO2 concentration.
The R values can be obtained by isothermal reaction at 280 oC for soot oxidation, the conversion of soot remains basically unchanged and the conversion rate is less than 10%. The R values for all catalysts were calculated by the slopes of the soot conversion amount with time, which are reflected in the concentration of CO2 per unit of time. The soot conversion rate (R) was calculated as following59:
Where Q represents the gas flow rate (mL min−1), C represents the concentration of CO2 measured by isothermal reactions (ppm), and m represents the weight of the catalyst (g).
The active oxygen (Oa) amount can be obtained by isothermal anaerobic titrations31. The TOFRu value of the Ru/CeO2 catalyst can be calculated by the following equation:
Here, the R2 and R1 represent the isothermal reaction rate for Ru/CeO2 and CeO2 catalysts (μmol g−1 min−1), respectively. M is the atomic weight of Ru (101.07 g mol−1), m is the mass of the catalyst (g) and the w (wt.%) is the actual loading amount of Ru species on the surface of CeO2.
The apparent activation energy (Ea) can be calculated by the Coats-Redfern interregnal method60. The Ea values are obtained by the following equation:
Here, the α is the conversion value of soot, %. T is the reaction temperature, K. A is the pre-exponential factor, s−1. Ea is the apparent activation energy, kJ mol−1. R represents the ideal gas constant, 8.314 J mol−1 K−1. β represents the heating rate, K min−1.
The stability of catalysts was evaluated by Time-TOFRu and recycling soot-TPO tests. For the Time-TOFRu test, a spatula was used to mix soot (20 mg) and catalyst (200 mg) thoroughly to form loose contact. The mixture was heated on a fixed-bed tubular quartz microreactor with an inner diameter of 6 mm, and heated from 50 to 280 oC at a heating rate of 2 oC min−1 under a reactant gas flow (50 mL min−1) of O2 (5 vol%) and NO (0.2 vol%) balanced with Ar. The reaction temperature was kept at 280 oC (lasting for 400 min) in an approximate kinetic regime because the conversion of soot oxidation was low (10%) and nearly constant over time. The outlet gas concentration of CO and CO2 is detected by an online gas chromatograph. When the temperature reaches 280 oC, the outlet gas concentration is detected for the first time as the starting point. Based on the above process, the production CO2 concentration of Ru/CeO2 and CeO2 catalysts can be obtained at specific times, respectively. The CO2 produced by the bare Ru species can be defined as the concentration produced by Ru/CeO2 subtraction of the concentration of CO2 produced by the CeO2. The TOFRu value was calculated as follows:
Where the\({{{{{{\rm{C}}}}}}}_{CO2}^{p}\) represents the produced CO2 concentration (ppm) by the bare Ru species, the value can be defined as the concentration produced by Ru/CeO2 subtraction of the concentration of CO2 produced by the support CeO2. Q represents the gas flow rate (mL min−1), M is the atomic weight of Ru (101.07 g mol−1), m is the mass of the catalyst (g) and the w (wt.%) is the actual loading amount of Ru species on the surface of CeO2. Finally, the profile of Time-TOFRu and the selectivity of CO2 over Ru1/CeO2 and Run/CeO2 catalysts were gained within 400 min.
For the cyclic stability test, the catalyst was recycled after the first test of soot-TPO. The detailed process is as follows: firstly, the catalyst was taken out from the reaction quartz tube and then the quartz cotton was cleaned off from the surface of the catalyst (this process loses about 2% of the catalyst mass). Each time, the used catalyst was mixed with 10 mg soot particles, and the next activity test (soot-TPO) was performed, the values of T10, T50, T90 and SCO2m for Ru/CeO2 catalysts can be obtained. The above experimental process was repeated six times, and six groups of activity data were finally acquired, while no additional catalyst was added for each test during six cycles of soot-TPO.
Density functional theory (DFT) calculations
DFT calculations were carried out by using the VASP package code61. The exchange-correlation energy function was described in the Pardew-Burke-Ernzerhof (PBE) generalized gradient approximation (GGA)62. The kinetic cutoff energy is 400 eV for the plane-wave basis set, and the k point was set to the γ point in the Brillouin zone. All calculations for CeO2 models were performed by the DFT + U method for the present study. The value of Ueff was set to 5 eV for Ce 4 f electrons. CeO2 supercells (2 x 4) were built, with (110) surfaces exposed. The bottom two atomic layers were fixed during all calculations. The thinness of the vacuum layer for all the models was set as 15 Å. Combined with the results of STEM-ADF and EXAFS characterizations, one of the Ce atoms in the surface layer was replaced by a Ru atom to simulate the single-atom Ru structure, and the Ru nanoparticles contain 10 atoms in the Run/CeO2 model63. The adsorption and desorption energy were calculated by the equation:
Where Eads is the adsorption energy, Edes is the desorption energy, EMS is the total energy of a surface slab with the adsorbate, and ES is the energy of pure substrate. EM represents the energy of an adsorbate molecule.
The climbing image-nudged elastic band (CI-NEB) code was used to identify the reaction coordinates from IS to FS64,65, which located the transition state (TS). The activation energy (Ea) and reaction energy (Er) were determined with the following equation:
Where EIS, ETS, and EFS represent the energy of IS, TS, and FS, respectively.
Data availability
The data that support the findings of this study are included in the published article (and its Supplementary Information). Source data are provided with this manuscript and is available from the corresponding author upon request. Source data are provided with this paper.
References
Niessner, R. The Many Faces of Soot: Characterization of soot nanoparticles produced by engines. Angew. Chem. Int. Ed. 53, 12366–12379 (2014).
Wang, M., Zhang, Y., Yu, Y., Shan, W. & He, H. Synergistic effects of multicomponents produce outstanding soot oxidation activity in a Cs/Co/MnOx catalyst. Environ. Sci. Technol. 55, 240–248 (2021).
Yang, Y. et al. Interface interaction induced oxygen activation of cactus-like Co3O4/OMS-2 nanorod catalysts in situ grown on monolithic cordierite for diesel soot combustion. Appl. Catal. B 286, 119932 (2021).
Gryboś, J. et al. Mechanistic insights into oxygen dynamics in soot combustion over cryptomelane catalysts in tight and loose contact modes via 18O2/16O2 isotopic variable composition measurements – A hot ring model of the catalyst operation. ACS Catal. 11, 9530–9546 (2021).
Cui, B. et al. Holey Co-Ce oxide nanosheets as a highly efficient catalyst for diesel soot combustion. Appl. Catal. B 267, 118670 (2020).
Wu, Q. et al. High-efficient catalysts of core-shell structured Pt@transition metal oxides (TMOs) supported on 3DOM-Al2O3 for soot oxidation: The effect of strong Pt-TMO interaction. Appl. Catal. B 244, 628–640 (2019).
Xiong, J. et al. Efficiently multifunctional catalysts of 3D ordered meso-macroporous Ce0.3Zr0.7O2-supported PdAu@CeO2 core-shell nanoparticles for soot oxidation: Synergetic effect of Pd-Au-CeO2 ternary components. Appl. Catal. B 251, 247–260 (2019).
Jeong, H. et al. Highly durable metal ensemble catalysts with full dispersion for automotive applications beyond single-atom catalysts. Nat. Catal. 3, 368–375 (2020).
Muravev, V. et al. Interface dynamics of Pd–CeO2 single-atom catalysts during CO oxidation. Nat. Catal. 4, 469–478 (2021).
Wu, X. et al. Solvent-free microwave synthesis of ultra-small Ru-Mo2C@CNT with strong metal-support interaction for industrial hydrogen evolution. Nat. Commun. 12, 4018 (2021).
Aouad, S., Abi-Aad, E. & Aboukaïs, A. Simultaneous oxidation of carbon black and volatile organic compounds over Ru/CeO2 catalysts. Appl. Catal. B 88, 249–256 (2009).
Kobylinski, T. P., Taylor, B. W. & Young, J. E. Stabilized ruthenium catalysts for NOx reduction. SAE Trans. 83, 1089–1095 (1974).
Hess, F. Is there a stable deacon catalyst? Computational screening approach for the stability of oxide catalysts under harsh conditions. ACS Catal. 12, 497–511 (2022).
Zheng, C., Mao, D., Xu, Z. & Zheng, S. Strong Ru-CeO2 interaction boosts catalytic activity and stability of Ru supported on CeO2 nanocube for soot oxidation. J. Catal. 411, 122–134 (2022).
Lu, Y. et al. Identification of the active complex for CO oxidation over single-atom Ir-on-MgAl2O4 catalysts. Nat. Catal. 2, 149–156 (2018).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Kadam, R. G. et al. Single Co-atoms as electrocatalysts for efficient hydrazine oxidation reaction. Small 17, 2006477 (2021).
Ro, I., Resasco, J. & Christopher, P. Approaches for understanding and controlling interfacial effects in oxide-supported metal catalysts. ACS Catal. 8, 7368–7387 (2018).
Jones, J. et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 353, 150–154 (2016).
Liu, S., Yu, J. & Jaroniec, M. Anatase TiO2 with dominant high-energy {001} facets: Synthesis, properties, and applications. Chem. Mater. 23, 4085–4093 (2011).
Yu, X. et al. Enhanced activity and sulfur resistance for soot combustion on three-dimensionally ordered macroporous-mesoporous MnxCe1-xOδ/SiO2 catalysts. Appl. Catal. B 254, 246–259 (2019).
Mei, X. et al. Decreasing the catalytic ignition temperature of diesel soot using electrified conductive oxide catalysts. Nat. Catal. 4, 1002–1011 (2021).
Li, Y. et al. Facilitating catalytic purification of auto-exhaust carbon particles via the Fe2O3{113} facet-dependent effect in Pt/Fe2O3 catalysts. Environ. Sci. Technol. 55, 16153–16162 (2021).
Zhang, T. et al. A blinking mesoporous TiO2−x composed of nanosized anatase with unusually long-lived trapped charge carriers. Angew. Chem. Int. Ed. 59, 15000–15007 (2020).
Liu, Z. et al. Tuning the selectivity of catalytic nitriles hydrogenation by structure regulation in atomically dispersed Pd catalysts. Nat. Commun. 12, 6194 (2021).
Serve, A., Boreave, A., Cartoixa, B., Pajot, K. & Vernoux, P. Synergy between Ag nanoparticles and yttria-stabilized zirconia for soot oxidation. Appl. Catal. B 242, 140–149 (2019).
Liu, S. et al. Soot oxidation over CeO2 and Ag/CeO2: Factors determining the catalyst activity and stability during reaction. J. Catal. 337, 188–198 (2016).
Aneggi, E., Wiater, D., de Leitenburg, C., Llorca, J. & Trovarelli, A. Shape-dependent activity of ceria in soot combustion. ACS Catal. 4, 172–181 (2014).
Fu, Q., Saltsburg, H. & Flytzani-Stephanopoulos, M. Active nonmetallic Au and Pt species on ceria-based water-gas shift catalysts. Science 301, 935–938 (2003).
Chen, L. et al. Insights into the mechanism of methanol steam reforming tandem reaction over CeO2 supported single-site catalysts. J. Am. Chem. Soc. 143, 12074–12081 (2021).
Xiong, J. et al. Synergetic effect of K sites and Pt nanoclusters in an ordered hierarchical porous Pt-KMnOx/Ce0.25Zr0.75O2 catalyst for boosting soot oxidation. ACS Catal. 10, 7123–7135 (2020).
Wei, Y. et al. Boosting the removal of diesel soot particles by the optimal exposed crystal facet of CeO2 in Au/CeO2 catalysts. Environ. Sci. Technol. 54, 2002–2011 (2020).
Xiong, J. et al. Fabrication of spinel-type PdxCo3–xO4 binary active sites on 3D ordered meso-macroporous Ce-Zr-O2 with enhanced activity for catalytic soot oxidation. ACS Catal. 8, 7915–7930 (2018).
Wei, Y. et al. Highly active catalysts of gold nanoparticles supported on three-dimensionally ordered macroporous LaFeO3 for soot oxidation. Angew. Chem. Int. Ed. 50, 2326–2329 (2011).
Liu, K. et al. Strong metal-support interaction promoted scalable production of thermally stable single-atom catalysts. Nat. Commun. 11, 1263 (2020).
Ji, K. et al. Electrocatalytic hydrogenation of 5-hydroxymethylfurfural promoted by a Ru1Cu single-atom alloy catalyst. Angew. Chem. Int Ed. 61, e202209849 (2022).
Liang, G., Zhou, Y., Zhao, J., Khodakov, A. Y. & Ordomsky, V. V. Structure-sensitive and insensitive reactions in alcohol amination over nonsupported Ru nanoparticles. ACS Catal. 8, 11226–11234 (2018).
Sun, Y. et al. Modulating electronic structure of metal-organic frameworks by introducing atomically dispersed Ru for efficient hydrogen evolution. Nat. Commun. 12, 1369 (2021).
Hao, J. et al. Unraveling the electronegativity-dominated intermediate adsorption on high-entropy alloy electrocatalysts. Nat. Commun. 13, 2662 (2022).
Ji, H. et al. Boosting polyethylene hydrogenolysis performance of Ru-CeO2 catalysts by finely regulating the Ru sizes. Small 19, 2300903 (2023).
Herd, B., Goritzka, J. C. & Over, H. Room temperature oxidation of ruthenium. J. Phys. Chem. C. 117, 15148–15154 (2013).
Wang, X. Y. et al. Atomically dispersed Ru catalyst for low-temperature nitrogen activation to ammonia via an associative mechanism. ACS Catal. 10, 9504–9514 (2020).
Sato, K. et al. Operando Spectroscopic Study of the Dynamics of Ru Catalyst during Preferential Oxidation of CO and the Prevention of Ammonia Poisoning by Pt. JACS Au 2, 1627–1637 (2022).
Kang, L. et al. Design, identification, and evolution of a surface ruthenium(II/III) single site for CO activation. Angew. Chem. Int Ed. 60, 1212–1219 (2021).
Sarma, B. B. et al. Tracking and understanding dynamics of atoms and clusters of late transition metals with in-situ DRIFT and XAS spectroscopy assisted by DFT. J. Phys. Chem. C. 127, 3032–3046 (2023).
Xu, W. et al. In situ studies of CeO2-supported Pt, Ru, and Pt–Ru alloy catalysts for the water–gas shift reaction: Active phases and reaction intermediates. J. Catal. 291, 117–126 (2012).
Li, J. et al. Distribution and valence state of Ru species on CeO2 supports: Support shape effect and its influence on CO oxidation. ACS Catal. 9, 11088–11103 (2019).
Qiu, J.-Z. et al. Pure siliceous zeolite-supported Ru single-atom active sites for ammonia synthesis. Chem. Mater. 31, 9413–9421 (2019).
Khan, M. A., Han, D. H. & Yang, O. B. Enhanced photoresponse towards visible light in Ru doped titania nanotube. Appl Surf. Sci. 255, 3687–3690 (2009).
Andana, T. et al. Ceria-supported small Pt and Pt3Sn nanoparticles for NOx-assisted soot oxidation. Appl. Catal. B 209, 295–310 (2017).
Lin, J. et al. In situ calorimetric study: Structural effects on adsorption and catalytic performances for CO oxidation over Ir-in-CeO2 and Ir-on-CeO2 Catalysts. J. Phys. Chem. C. 115, 16509–16517 (2011).
Lin, B. et al. Enhanced ammonia synthesis activity of ceria-supported ruthenium catalysts induced by CO activation. ACS Catal. 11, 1331–1339 (2021).
Wang, F. et al. Catalytic behavior of supported Ru nanoparticles on the {100}, {110}, and {111} facet of CeO2. J. Catal. 329, 177–186 (2015).
Huang, H., Dai, Q. & Wang, X. Morphology effect of Ru/CeO2 catalysts for the catalytic combustion of chlorobenzene. Appl. Catal. B 158-159, 96–105 (2014).
Dai, Q., Bai, S., Wang, Z., Wang, X. & Lu, G. Catalytic combustion of chlorobenzene over Ru-doped ceria catalysts. Appl. Catal. B 126, 64–75 (2012).
Pushkarev, V. V., Kovalchuk, V. I. & d’Itri, J. L. Probing defect sites on the CeO2 surface with dioxygen. J. Phys. Chem. B 108, 5341–5348 (2004).
Wang, F. et al. Active site dependent reaction mechanism over Ru/CeO2 catalyst toward CO2 methanation. J. Am. Chem. Soc. 138, 6298–6305 (2016).
Sun, C. et al. Mesoscale organization of nearly monodisperse flowerlike ceria microspheres. J. Phys. Chem. B 110, 13445–13452 (2006).
Xing, L. et al. Highly efficient catalytic soot combustion performance of hierarchically meso-macroporous Co3O4/CeO2 nanosheet monolithic catalysts. Catal. Today 351, 83–93 (2020).
Zhao, M. et al. Roles of surface-active oxygen species on 3DOM cobalt-based spinel catalysts MxCo3–xO4 (M = Zn and Ni) for NOx-assisted soot oxidation. ACS Catal. 9, 7548–7567 (2019).
Kresse, G. & Furthmüller, J. Furthmüller Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 6, 15–50 (1996).
Xu, X. et al. Facile Cr3+-doping strategy dramatically promoting Ru/CeO2 for low-temperature CO2 methanation: Unraveling the roles of surface oxygen vacancies and hydroxyl groups. ACS Catal. 11, 5762–5775 (2021).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Henkelman, G. & Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113, 9978–9985 (2000).
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (2022YFB3504100, 2022YFB3506200, 2021YFA1500300, 2022YFA1500146), National Natural Science Foundation of China (21972166, 22376217, 22072090), Beijing Natural Science Foundation (2202045). This work was supported by “Photon Science Research Center For Carbon Dioxide”. The authors wish to thank the facility support of the experiment Assist System of SSRF and beamline BL13SSW and BL06B.
Author information
Authors and Affiliations
Contributions
Y.L. and Y.W. conceived, synthesized the catalyst and completed most of the experiments and characterizations, and wrote the paper. T.Q., X.L. and L.C. completed the electron microscopy test. J.X. and P.Z. helped with the catalytic performance and carried out theoretical calculations and analyses. K.L., H.C. and L.L. helped analyze the experiment data. X.Y., Y.W., Z.Z. and J.L. modified the manuscript. All the authors discussed the results in the manuscript. All authors have approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Agustin Bueno Lopez and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Y., Qin, T., Wei, Y. et al. A single site ruthenium catalyst for robust soot oxidation without platinum or palladium. Nat Commun 14, 7149 (2023). https://doi.org/10.1038/s41467-023-42935-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-42935-7
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.