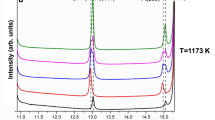
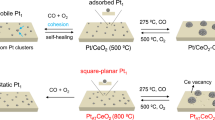
Abstract
Reversibly adjusting the active structures of supported metal catalysts in response to dynamic working conditions has long been pursued. Here we report the reaction-environment-modulated transformations of subnanometre-sized Pd on CeO2 for efficient methane removal, leveraging the reaction environments at different stages of automotive exhaust aftertreatment. During the cold start of vehicles, inactive Pd1 single atoms are readily transformed into PdOx subnanometre clusters by CO even at room temperature with excess O2, resulting in boosted low-temperature CH4 oxidation. At elevated temperatures, dispersion of PdOx cluster into Pd1 against metal sintering renders outstanding hydrothermal stability to the catalyst, to be activated during the next vehicle start. Combined experimental and computational studies elucidate the dynamically evolved Pd speciation on CeO2 at an atomic level. Modulating the reversible nature of supported metals helps overcome the long-existing trade-off between low-temperature activity and high-temperature stability, also providing a new paradigm for designing intelligent catalysts that brings single-atom/cluster catalysts closer to real applications.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files or from the corresponding authors upon reasonable request. All optimized structure and corresponding energetics of DFT calculations have been uploaded to the open data base catalysis-hub.org62 via the link https://www.catalysis-hub.org/publications/JiangDynamic2023. Source data are provided with this paper.
References
Jiang, D., Khivantsev, K. & Wang, Y. Low-temperature methane oxidation for efficient emission control in natural gas vehicles: Pd and beyond. ACS Catal. 10, 14304–14314 (2020).
Pan, D. et al. Methane emissions from natural gas vehicles in China. Nat. Commun. 11, 4588 (2020).
Cargnello, M. et al. Exceptional activity for methane combustion over modular Pd@CeO2 subunits on functionalized Al2O3. Science 337, 713–717 (2012).
Monai, M., Montini, T., Gorte, R. J. & Fornasiero, P. Catalytic oxidation of methane: Pd and beyond. Eur. J. Inorg. Chem. 2018, 2884–2893 (2018).
Chen, H.-Y., Lu, J., Fedeyko, J. M. & Raj, A. Zeolite supported Pd catalysts for the complete oxidation of methane: a critical review. Appl. Catal. A 633, 118534 (2022).
Farrauto, R. J. Low-temperature oxidation of methane. Science 337, 659–660 (2012).
DeRita, L. et al. Structural evolution of atomically dispersed Pt catalysts dictates reactivity. Nat. Mater. 18, 746–751 (2019).
Yuan, W. et al. In situ manipulation of the active Au-TiO2 interface with atomic precision during CO oxidation. Science 371, 517–521 (2021).
Wan, G. et al. Reaction-mediated transformation of working catalysts. ACS Catal. 12, 8007–8018 (2022).
Maurer, F. et al. Tracking the formation, fate and consequence for catalytic activity of Pt single sites on CeO2. Nat. Catal. 3, 824–833 (2020).
Yan, G. et al. Reaction product-driven restructuring and assisted stabilization of a highly dispersed Rh-on-ceria catalyst. Nat. Catal. 5, 119–127 (2022).
Tang, Y. et al. Rh single atoms on TiO2 dynamically respond to reaction conditions by adapting their site. Nat. Commun. 10, 1–10 (2019).
Jiang, D. et al. Tailoring the local environment of platinum in single-atom Pt1/CeO2 catalysts for robust low-temperature CO oxidation. Angew. Chem. Int. Ed. 60, 26054–26062 (2021).
Parkinson, G. S. et al. Carbon monoxide-induced adatom sintering in a Pd–Fe3O4 model catalyst. Nat. Mater. 12, 724–728 (2013).
Gänzler, A. M. et al. Tuning the structure of platinum particles on ceria in situ for enhancing the catalytic performance of exhaust gas catalysts. Angew. Chem. Int. Ed. 56, 13078–13082 (2017).
Wang, H. et al. Surpassing the single-atom catalytic activity limit through paired Pt–O–Pt ensemble built from isolated Pt1 atoms. Nat. Commun. 10, 1–12 (2019).
Li, Y. et al. Dynamic structure of active sites in ceria-supported Pt catalysts for the water gas shift reaction. Nat. Commun. 12, 914 (2021).
Lambert, C. K. Current state of the art and future needs for automotive exhaust catalysis. Nat. Catal. 2, 554–557 (2019).
Lee, B.-H. et al. Reversible and cooperative photoactivation of single-atom Cu/TiO2 photocatalysts. Nat. Mater. 18, 620–626 (2019).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Xiong, Y. et al. Single-atom Rh/N-doped carbon electrocatalyst for formic acid oxidation. Nat. Nanotechnol. 15, 390–397 (2020).
Zhu, C., Fu, S., Shi, Q., Du, D. & Lin, Y. Single-atom electrocatalysts. Angew. Chem. Int. Ed. 56, 13944–13960 (2017).
Wang, A., Li, J. & Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2, 65–81 (2018).
Jeong, H. et al. Highly durable metal ensemble catalysts with full dispersion for automotive applications beyond single-atom catalysts. Nat. Catal. 3, 368–375 (2020).
Goodman, E. D. et al. Catalyst deactivation via decomposition into single atoms and the role of metal loading. Nat. Catal. 2, 748–755 (2019).
Pereira-Hernández, X. I. et al. Tuning Pt–CeO2 interactions by high-temperature vapor-phase synthesis for improved reducibility of lattice oxygen. Nat. Commun. 10, 1358 (2019).
Xiong, H., Datye, A. K. & Wang, Y. Thermally stable single-atom heterogeneous catalysts. Adv. Mater. 33, 2004319 (2021).
Liu, K. et al. Strong metal-support interaction promoted scalable production of thermally stable single-atom catalysts. Nat. Commun. 11, 1263 (2020).
Jones, J. et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 353, 150–154 (2016).
Matam, S. K., Aguirre, M., Weidenkaff, A. & Ferri, D. Revisiting the problem of active sites for methane combustion on Pd/Al2O3 by operando XANES in a lab-scale fixed-bed reactor. J. Phys. Chem. C 114, 9439–9443 (2010).
Jiang, D. et al. Elucidation of the active sites in single-atom Pd1/CeO2 catalysts for low-temperature CO oxidation. ACS Catal. 10, 11356–11364 (2020).
Zammit, M. et al. Future Automotive Aftertreatment Solutions: The 150 °C Challenge Workshop Report (U.S. DRIVE, 2013).
White, J. J. et al. Natural Gas Converter Performance and Durability. Report No. 0148-7191 (SAE Technical Paper, 1993).
Tan, W. et al. Tuning single-atom Pt1-CeO2 catalyst for efficient CO and C3H6 oxidation: size effect of ceria on Pt structural evolution. ChemNanoMat 6, 1797–1805 (2020).
Su, Y., Liu, J.-X., Filot, I. A., Zhang, L. & Hensen, E. J. Highly active and stable CH4 oxidation by substitution of Ce4+ by two Pd2+ ions in CeO2 (111). ACS Catal. 8, 6552–6559 (2018).
Spezzati, G. et al. Atomically dispersed Pd–O species on CeO2 (111) as highly active sites for low-temperature CO oxidation. ACS Catal. 7, 6887–6891 (2017).
Xu, J. et al. Operando and kinetic study of low-temperature, lean-burn methane combustion over a Pd/γ-Al2O3 catalyst. ACS Catal. 2, 261–269 (2012).
Su, Y.-Q., Filot, I. A., Liu, J.-X. & Hensen, E. J. Stable Pd-doped ceria structures for CH4 activation and CO oxidation. ACS Catal. 8, 75–80 (2017).
Jiang, D., Wang, W., Gao, E., Sun, S. & Zhang, L. Highly selective defect-mediated photochemical CO2 conversion over fluorite ceria under ambient conditions. Chem. Commun. 50, 2005–2007 (2014).
Filtschew, A., Hofmann, K. & Hess, C. Ceria and its defect structure: new insights from a combined spectroscopic approach. J. Phys. Chem. C 120, 6694–6703 (2016).
Xiong, H. et al. Design considerations for low-temperature hydrocarbon oxidation reactions on Pd based catalysts. Appl. Catal. B 236, 436–444 (2018).
Lott, P., Dolcet, P., Casapu, M., Grunwaldt, J.-D. & Deutschmann, O. The effect of prereduction on the performance of Pd/Al2O3 and Pd/CeO2 catalysts during methane oxidation. Ind. Eng. Chem. Res. 58, 12561–12570 (2019).
Xiong, H. et al. Engineering catalyst supports to stabilize PdOx two-dimensional rafts for water-tolerant methane oxidation. Nat. Catal. 4, 830–839 (2021).
Martin, N. M. et al. Intrinsic ligand effect governing the catalytic activity of Pd oxide thin films. ACS Catal. 4, 3330–3334 (2014).
Alcala, R. et al. Atomically dispersed dopants for stabilizing ceria surface area. Appl. Catal. B 284, 119722 (2021).
Gremminger, A. et al. PGM based catalysts for exhaust-gas after-treatment under typical diesel, gasoline and gas engine conditions with focus on methane and formaldehyde oxidation. Appl. Catal. B 265, 118571 (2020).
Gélin, P. & Primet, M. Complete oxidation of methane at low temperature over noble metal based catalysts: a review. Appl. Catal. B 39, 1–37 (2002).
Hellman, A. et al. The active phase of palladium during methane oxidation. J. Phys. Chem. Lett. 3, 678–682 (2012).
Huang, W. et al. Steam-created grain boundaries for methane C-H activation in palladium catalysts. Science 373, 1518–1523 (2021).
Kim, R. S. et al. Rapid electrochemical methane functionalization involves Pd–Pd bonded intermediates. J. Am. Chem. Soc. 142, 20631–20639 (2020).
Lu, Y. et al. Unraveling the intermediate reaction complexes and critical role of support-derived oxygen atoms in CO oxidation on single-atom Pt/CeO2. ACS Catal. 11, 8701–8715 (2021).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 12, 537–541 (2005).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Dudarev, S. L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 57, 1505–1509 (1998).
Fabris, S., de Gironcoli, S., Baroni, S., Vicario, G. & Balducci, G. Taming multiple valency with density functionals: a case study of defective ceria. Phys. Rev. B 71, 041102 (2005).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Peterson, A. A., Abild-Pedersen, F., Studt, F., Rossmeisl, J. & Nørskov, J. K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 3, 1311–1315 (2010).
Winther, K. T. et al. Catalysis-Hub.org, an open electronic structure database for surface reactions. Sci. Data 6, 75 (2019).
Acknowledgements
This work was supported by the US Department of Energy (DOE), Office of Basic Energy Sciences (SC), Division of Chemical Sciences (grant DE-FG02-05ER15712). C.E.G. and G.W. acknowledge additional support from the US DOE, Office of Energy Efficiency and Renewable Energy, Vehicle Technology Office. G.W. acknowledges the inspiration and support of A. Majumdar and P. A. Pianetta. This research used resources of the Advanced Photon Source, an Office of Science User Facility operated for the US DOE Office of Science by Argonne National Laboratory and was supported by the US DOE under contract DE-AC02-06CH11357, and the Canadian Light Source and its funding partners. F.A.P. and J.H.S acknowledge support from the US DOE, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division, Catalysis Science Program to the SUNCAT Center for Interface Science and Catalysis as well as a postdoctoral fellowship from the Knut and Alice Wallenberg Foundation (grant 2019.0586). Use of Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the US DOE, Office of Science, Office of Basic Energy Sciences under contract DE-AC02-76SF00515. Computational support is acknowledged from the National Energy Research Scientific Computing Center (computer time allocation m2997), a DOE Office of Science User Facility supported by the Office of Science of the US DOE under contract DE-AC02-05CH11231. The authors also acknowledge W. Huang, A. Hoffman and A. Cho for their valuable discussion on data analysis, and M. H. Engelhard for the help with X-ray photoelectron spectroscopy measurements.
Author information
Authors and Affiliations
Contributions
D.J., G.W., C.J.T., F.A.-P. and Y.W. conceived the research. G.W., C.S. and C.J.T. performed XAS studies. J.H.S. and F.A.-P. performed DFT computation. D.J. and Y.W. planned and supervised the rest of the experimental work. C.E.G.-V. performed AC-STEM and part of catalytic measurements. J.L. performed TEM measurements. J.Z. performed XRD measurements. D.J. synthesized the catalysts and performed catalytic and other analytical measurements. D.J., G.W., J.H.S., C.J.T., F.A.-P. and Y.W. wrote the paper. G.W. and J.H.S. contributed to polishing the paper. All authors discussed the results and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Andrey A. Saraev, Ivo Filot and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, Notes 1–5, Figs. 1–30, Tables 1–8 and References 1–48.
Source data
Source Data Fig. 1
Source data for Figs. 1–4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, D., Wan, G., Halldin Stenlid, J. et al. Dynamic and reversible transformations of subnanometre-sized palladium on ceria for efficient methane removal. Nat Catal 6, 618–627 (2023). https://doi.org/10.1038/s41929-023-00983-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-00983-8
This article is cited by
-
Reverse water gas-shift reaction product driven dynamic activation of molybdenum nitride catalyst surface
Nature Communications (2024)