Abstract
The Mycobacterium tuberculosis complex (MTBC) includes several human- and animal-adapted pathogens. It is thought to have originated in East Africa from a recombinogenic Mycobacterium canettii-like ancestral pool. Here, we describe the discovery of a clinical tuberculosis strain isolated in Ethiopia that shares archetypal phenotypic and genomic features of M. canettii strains, but represents a phylogenetic branch much closer to the MTBC clade than to the M. canettii strains. Analysis of genomic traces of horizontal gene transfer in this isolate and previously identified M. canettii strains indicates a persistent albeit decreased recombinogenic lifestyle near the emergence of the MTBC. Our findings support that the MTBC emergence from its putative free-living M. canettii-like progenitor is evolutionarily very recent, and suggest the existence of a continuum of further extant derivatives from ancestral stages, close to the root of the MTBC, along the Great Rift Valley.
Similar content being viewed by others
Introduction
Tuberculosis (TB), a disease caused by Mycobacterium tuberculosis and other members of the M. tuberculosis complex (MTBC), has been a major killer throughout human history1,2. Based on the current knowledge, the MTBC is comprised of nine human-adapted lineages (L), representing M. tuberculosis sensu stricto (L1-4 and L7-8) and M. africanum (L5-6 and L9), plus at least nine animal-adapted lineages3,4,5. Strains belonging to these lineages share highly conserved genomes, differing by no more than ~2000 single nucleotide polymorphisms (SNPs), and with no significant trace of genetic exchange among them, suggesting that this entire population expanded clonally from a single bacterial progenitor6,7,8. As seen in host adaptation of other bacterial pathogens9, this single-cell bottleneck might reflect a drastic change in ecology and selective pressures that putatively coincided with the jump to an obligate pathogen lifestyle in a mammalian host10.
In accordance with this scenario, the current view is that the MTBC progenitor clone emerged from a diversified and recombinogenic pool of free-living mycobacteria resembling extant strains of M. canettii, which is the phylogenetically closest relative of the MTBC11,12,13. In contrast to the worldwide distribution of the MTBC, clinical isolates of M. canettii are geographically restricted to the Horn of Africa, and fewer than 150 isolates have been described, mainly obtained from Djibouti residents. There is no evidence of inter-human transmission of M. canettii13,14, and M. canettii strains are less persistent and virulent than MTBC strains in infection models12,15, indicating a lower degree of pathoadaptation. Moreover, in contrast with MTBC members, M. canettii strains show marked genome mosaicism indicating multiple intraspecies horizontal gene transfer (HGT) events, explained by a distributive conjugal transfer (DCT) mechanism and consistent with frequent inter-strain contacts in an environmental reservoir11,12,16. Furthermore and consistently with the hypothesized M. canettii-like progenitor of the MTBC, recent phylogenomic analyses suggested that HGT events have also happened with ancestors of extant M. canettii strains in the ancestral evolutionary branch leading to the MTBC genesis. This finding provides additional support for a shared gene pool and ecological niche between both parental populations17.
This hypothesis is further supported by the observation of homologous recombination spontaneously occurring in vitro between two adjacent pks5 genes in M. canettii, resulting in abrogation of lipooligosaccharides (LOS) production and a change in colony morphotype from smooth to rough. This recombination results in a single pks5 configuration similar to that conserved among all MTBC members, which lack LOS and show a rough morphotype. This conservation suggests a unique recombination event in the MTBC progenitor after separation from its M. canettii-like ancestor18. Importantly, such M. canettii morphotype variants show increased virulence in cellular- and animal-infection models, suggesting that pks5 recombination generated a beneficial genetic background in the MTBC ancestor for colonization and spread in mammalian hosts18.
However, when this emergence from the recombinogenic M. canettii pool and these key ecological and adaptive changes happened in the ancestral branch of the MTBC remains unknown. Indeed, previous analysis showed that M. canettii strains can differ by up to 65,000 SNPs from each other, and at least 14,000 SNPs separate the hitherto known closest lineages from the currently acknowledged most recent common ancestor (MRCA) of the MTBC12.
Here, we report the discovery of a TB clinical isolate (hereafter referred to as ET1291) from Ethiopia, which shares the characteristic smooth colony morphology and twin-pks5 configuration of M. canettii strains, but is phylogenetically much closer to the MTBC clade. We used short and long read sequencing to reconstruct the complete circular genome of the ET1291 strain and performed in-depth comparative genomics with all previously available and other newly sequenced M. canettii genomes and representatives of all known MTBC lineages. The obtained findings provide proximal insights into the genomic and biological changes involved in the emergence of the MTBC, and on its original lifestyle.
Results
Patient with the ET1291 infection in Ethiopia
ET1291 was isolated from a newly diagnosed 20-year-old HIV-negative male patient living in the rural part of central Tigray, Ethiopia. The strain was identified within the national anti-TB drug resistance survey 2017–2019, designed as a cross-sectional health facility-based study as per WHO recommendations19, enrolling for 16 months newly registered and previously treated bacteriologically confirmed pulmonary TB cases. The primary culture of ET1291 became positive after 6 days using BD BACTEC MGIT liquid culture and colonies were observed after 5 weeks on the Löwenstein–Jensen medium. Such colonies showed the archetypal smooth morphotype that distinguishes M. canettii strains from MTBC strains. Extended drug susceptibility testing showed monoresistance to pyrazinamide, consistent with the natural resistance of M. canettii to this drug20. Moreover, ET1291 showed a faster growth rate in liquid medium (Middlebrook 7H9 supplemented with OADC and glycerol) than M. tuberculosis H37Rv, similarly to a reference M. canettii strain STB-A (CIPT 140010059) (Supplementary Fig. 1) and as also seen with other M. canettii strains12.
WGS analysis, genotypic resistance, SNP profile, and phylogenetic position of ET1291
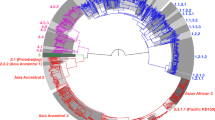
Analysis with the MTBseq pipeline21 of Illumina-based whole-genome sequencing (WGS) data obtained from ET1291 (Fig. 1) was unable to provide a M. canettii or MTBC phylogenetic classification. Phylogenetic SNPs detected across the genome were shared with evolutionarily early branching MTBC lineages (L1, 5, 6, 7, 8 and animal lineages; to note, reference phylogenetic SNPs have not been defined for L9 yet) and/or M. canettii (Supplementary Data 1). Of note, multiple non-synonymous SNPs (17 and 2, respectively; Supplementary Data 2) were found in the fgd1 and Rv0678 genes, associated with resistance to the newest anti-TB drugs delamanid/pretomanid and bedaquiline, respectively; however, the minimum inhibitory concentrations of ET1291 for all three drugs were below the proposed respective critical concentrations. Targeted next-generation sequencing (tNGS) using Deeplex Myc-TB testing22 resulted in M. canettii identification with a predicted pyrazinamide mono-resistant profile, based on hsp65 sequencing data and detection of specific phylogenetic SNPs including pncA A46A (Fig. 1). Like for previous M. canettii strains12,23, no spoligotype spacers were detected, indicating absence of spacers in common with those in the CRISPR-Cas locus of reference MTBC genomes, which was confirmed by analysis of the WGS data using CRISPRCasFinder (https://crisprcas.i2bc.paris-saclay.fr/). This M. canettii classification was supported by multiple other archetypal characteristics shared with previously described M. canettii strains, as described below.
The hybrid strategy combining Illumina and Oxford Nanopore sequencing used to obtain the complete assembly and functional annotation of the genome of M. canettii ET1291, and to perform comparative genomics analyses with previously known M. canettii and MTBC strains, are represented in the center. Illumina whole-genome sequencing data were also used to perform SNP-based phylogenetic reconstructions and to determine the ratio of recombination versus mutation in the strain dataset. Results of targeted next-generation sequencing using the Deeplex Myc-TB assay, identifying ET1291 as M. canettii, are represented on the circular map on the right. Information on hsp65 best match-based and phylogenetic SNP-based identification of M. canettii is shown inside the circle, along with information on spoligotype (without any spacer detected). Target gene regions in the map are grouped within sectors according to the anti-tuberculous drug resistance with which they are associated. Sectors in red (for pyrazinamide (PZA) here, as typically expected for M. canettii) and green indicate targets in which resistance-associated mutations or either no mutation or only mutations not associated with resistance (shown in gray) are detected, respectively. Blue sectors refer to regions where as yet uncharacterized mutations are detected (see ref. 22 for further details on the Deeplex map). gDNA, genomic DNA; MTBC, M. tuberculosis complex, MCAN, M. canettii; MAP, M. tuberculosis (MTB)-associated phylotype. Abbreviations of anti-tuberculous drugs on the Deeplex map: RIF: rifampicin, INH: isoniazid, PZA: pyrazinamide, EMB: ethambutol, SM: streptomycin, FQ: Fluoroquinolones, KAN: kanamycin, AMI: amikacin, CAP: capreomycin, ETH: ethionamide, LIN: linezolid, BDQ: bedaquiline, CFZ: clofazimine. Figure created using BioRender.com by Arash Ghodousi with license to publish.
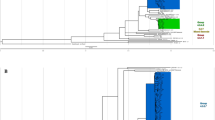
To determine the phylogenetic position of ET1291, a maximum likelihood phylogeny was inferred from a whole-genome alignment of 80 MTBC genomes, including representatives of all known human- and animal-adapted lineages, as well as 39 known publicly available or newly sequenced M. canettii strains. This reference dataset of M. canettii strains represents the closest outgroup to ET1291 strain and the MTBC, as shown by in-depth analysis of the ET1291 genome (see below) and recent genomic analysis of phylogenetically closest non-tuberculous mycobacterial species, comprising M. decipiens, M. shinjukuense, M. lacus, and M. riyadhense (forming a clade defined as the “MTB-associated phylotype”)24 (Supplementary Data 3). A unique phylogenetic position was thereby discovered for ET1291, revealing an intermediate branch between the reconstructed common ancestral node of the MTBC lineages and the closest node of the other, currently known M. canettii strains (Fig. 2a). This median position was confirmed by a reconstructed phylogeny based on 1000 single copy core genes, additionally including strains of the MTB-associated phylotype as more external outgroups (Supplementary Fig. 2). Genome-wide SNP data based on 82,637 positions indicated that ET1291 was separated by 5948 SNPs from the reconstructed ancestral genome of the MTBC, and at least 8984 SNPs from the next closest, previously described M. canettii strain, indicating a substantially closer genetic distance of ET1291 relatively to the MTBC compared to M. canettii strains documented thus far.
a Maximum likelihood phylogenetic tree including 121 genomes before accounting for recombination. Bootstrap support values are shown only for values below 100%; the value is not shown for branches with 100% bootstrap support, which represent the majority of the long branches as expected. b Maximum likelihood phylogenetic tree including the same genomes after correction for recombination. Branches are colored according to the estimated ratios of recombination versus mutation (r/m). r/m values are only shown for branches longer than 6e-5 substitutions/site, because of inherent stochasticity in signal to noise ratios in the shortest branches. The intermediate phylogenetic position of ET1291 between the MTBC and the other, currently known M. canettii strains is indicated by a red arrow. c ClonalFrameML analysis of the 121 genomes, showing the extent and segments of recombination mapped on the branches of ET1291 and other M. canettii strains and the branch leading to the common ancestor of the MTBC. Note that the red dotted lines indicate two branches that are ancestral to the progenitor of the MTBC and to the progenitor of the MTBC and ET1291, respectively, and are thus not part of the MTBC clade. MTBC, M. tuberculosis complex; MCAN, M. canettii. ET1291 indicated by a red arrow.
Estimated ratios of recombination versus mutation for ET1291, M. canettii, and MTBC
We then investigated whether ET1291 was part of the recombinogenic population of M. canettii, and sought to evaluate the impact of recombination on the genetic relationships identified above. To do so, we used ClonalFrameML, which also estimates a phylogeny accounting for recombination, thereby correcting for skewing effects due to potential imports of genomic segments25. This analysis detected multiple recombination segments in the core genome of ET1291 strain, as in previously identified M. canettii strains, corresponding to HGT events that occurred after the separation from these M. canettii lineages (Fig. 2b, c). Moreover, subsequent HGT events were mapped on the branch leading to the MTBC ancestor, indicating continuation of gene exchanges even after its divergence from the ET1291 lineage. Of note, substantial variations were observed in the estimated ratios of recombination vs mutation (r/m) between different branches of the phylogeny (Fig. 2b, c). Strikingly, the r/m ratio fell to 1 or below (0.7–1.0) in the branches leading to ET1291 and the MTBC ancestor, in contrast to the main, earlier branching M. canettii lineages (2.0–8.7), thus indicating reduced lateral gene flow closer to the emergence of the MTBC progenitor (Fig. 2b). Consistently with current evidence supporting strict clonality8,16,26, virtually no trace of recombination was detected in the MTBC. After correction for multiple recombination, lengths of the branches leading to M. canettii strains and the MTBC ancestor were considerably shorter in the ClonalFrameML tree (Fig. 2b). Based on the inferred clonal genealogy, the distance between ET1291 and the last common ancestor of the MTBC was reduced to 3389 SNPs, which was only two-fold larger than the maximal distance separating any two MTBC strains (1735 SNPs commonly covered in all studied genomes, Fig. 2b).
Defining features of the complete ET1291 genome
To comprehensively identify the genomic features associated with the proximal phylogenetic position of ET1291 relatively to the MTBC, the ET1291 strain was subjected to Oxford Nanopore Technologies (ONT) MinIon long read sequencing, followed by polishing and hybrid assembly using Illumina short reads. This hybrid approach resulted in a single complete circular genome of 4,515,631 bp containing 4086 predicted genes (Supplementary Fig. 3, Supplementary Data 4). This genome size is ~85–175 kb larger than the 4.34–4.43 Mb size range of the MTBC genomes (4.41 Mb for M. tuberculosis H37Rv), similarly to M. canettii genomes (currently culminating at 4.53 Mb for STB-K) previously identified12. As reductive evolution is a hallmark of mycobacterial pathogens26,27, this larger genome size relative to the MTBC further supports an ancestral status shared with M. canettii strains.
Additional ancestral characters were found in common with known M. canettii genomes. Consistent with its typical smooth colony morphotype, ET1291, displayed the dual polyketidesynthase-encoding pks5 gene configuration with an intervening pap gene shared by all M. canettii strains, instead of the single pks5 gene found in all MTBC members (Fig. 3)18. Moreover, the orthologues of four interrupted coding sequences (ICDS) in the MTBC (Supplementary Data 5), reflecting frameshifts or stop codons acquired during pseudogenization of the MTBC genomes28, were intact in ET1291, as in the formerly characterized M. canettii genomes and the non-tuberculous mycobacterial species outgroup12. Therefore, these frameshifts, and the recombination in the pks5 locus that is thought to have resulted in surface remodeling and increased virulence in the MTBC MRCA18, likely occurred only after the separation of the ancestral branch leading to the MTBC from the ET1291 lineage. Further like all M. canettii strains, ET1291 contains the cobF region, involved in the cobalamin (vitamin B12) synthesis, only shared by strains of the MTBC L8, representing a sister clade compared to the rest of the MTBC4.
a Similar smooth colony morphotypes shared by ET1291 and M. canettii STB-A, contrasting with the rough colony morphotype of M. tuberculosis H37Rv. b Aligned genome segments showing the dual polyketide synthase-encoding pks5 gene configuration with an intervening pap gene shared by ET1291 and M. canettii STB-A (and all other M. canettii strains), instead of the single pks5 gene found in M. tuberculosis H37Rv (and all other MTBC members). Colors show the correspondence between gene orthologues among ET1291, M. canettii STB-A, and M. tuberculosis H37Rv.
Nevertheless, other genome features are consistent with an evolutionarily closer branching point relatively to the MTBC. The average nucleotide identity versus M. tuberculosis H37Rv of the ET1291 genome is 99.55%, incrementally above the 97.97–99.37% range observed with all the previously described genomes of M. canettii strains12 (Supplementary Data 6). Moreover, the ET1291 genome contains the orthologues of eight of the 50 MTBC genes that were not found in the previously identified M. canettii genomes12 (Supplementary Data 7) and that partially overlap with genomic islands likely acquired by HGT in the MTBC29. Excluding the MTBC, the sequences of this gene subset show best hits with ~70–90% identity at the nucleotide level with counterparts in different environmental mycobacteria, including M. shinjukuense and M. riyadhense part of the MTB-associated phylotype, or mycobacteria part of the M. avium complex (Supplementary Data 8). Proteins encoded by the 8-gene set are a probable transcriptional regulatory protein (Rv1990c; the corresponding gene is essential for in vitro growth of M. tuberculosis H37Rv30), a member of the PE_PGRS protein family (Rv0746c, PE_PGRS9), a probable fatty-acid-CoA synthetase (Rv3513c, fadD18), as well as proteins of unknown functions. The corresponding genes were thus probably acquired already by lateral transfer in a common ancestor of ET1291 and the MTBC, before the emergence of the MTBC MRCA.
Further in contrast to formerly known M. canettii strains, the ET1291 genome also contains prophage regions of ~9–10 kb found in the same genomic locations as the PhiRv1 and PhiRv2 prophage regions of MTBC strains (Fig. 4). However, only a fraction of these phage genes show substantial identity (83–96%, lower than the 99–100% seen for the core genes) with the MTBC PhiRv1 and PhiRv2 genes, suggesting that these prophages were acquired independently from those in the MTBC. Interestingly, a third, 7.1 kb prophage region was additionally found, located between the orthologues of Rv1719-Rv1720c of H37Rv (Supplementary Fig. 4). In addition to genes with 83–91% identity with those in the PhiRv1 and PhiRv2 prophages in H37Rv, other genes encoded by this region are 70–75% identical to those found in non-tuberculous mycobacteria such as the MTB-associated phylotype member M. shinjukuense, and M. koreense, both with an unknown reservoir (Supplementary Data 9).
a PhiRv1 prophage region. b PhiRv2 prophage region. These two prophage-like regions of ~9–10 kb found are located in the same genomic locations as the PhiRv1 and PhiRv2 prophage-like regions of MTBC strains. Percent identities are shown for genes with at least 70% identity with an orthologue in M. tuberculosis H37Rv. Only a fraction of these phage gene sequences show substantial identity (76–96%, lower than the 99–100% seen for the core genes) with the MTBC PhiRv1 and PhiRv2 genes, suggesting that these prophages were acquired independently from those in the MTBC.
As another prominent imprint of HGT, ET1291 contains a CRISPR-associated protein (CRISPR-Cas) locus encoding a system of type I-E, also present with 98.7-100% identical cas genes in M. canettii STB-G, STB-I12 and Mc157 (ERR266126) (Fig. 5). However, the 115 spacers found in the CRISPR-Cas locus of ET1291 appear strain-specific. ET1291 is phylogenetically distant from M. canettii STB-G, STB-I, and Mc157 (Supplementary Fig. 5), indicating that the locus was horizontally exchanged among these strains or from a common donor, instead of having been inherited from a common ancestor shared by these four strains. The distribution of the other types of CRISPR-Cas systems mapped on a phylogenetic tree (Supplementary Fig. 5) was similarly homoplasic, indicating multiple independent HGT-mediated acquisition events, including possibly for the system of type III-A acquired in the same genomic region in the ancestral branch of the MTBC.
Percentages of identities are indicated with arrows between cas2, cas1e, cas6/cse3/casE, cas5/casD and cas7/cse4/casC genes of ET1291, and STB-G/I. CRISPR repeats sequences (shown above the respective CRISPR arrays) are identical between ET1291, STB-G, and STB-I (as shown as same black vertical bars in CRISPR arrays), while CRISPR spacers are strain-specific (as shown by different colors interspaced between vertical bars among respective CRISPR arrays). Colors show the correspondence between gene orthologues among ET1291, STB strains, and M. tuberculosis H37Rv.
HGT-mediated exchanges or acquisitions from a common ancestral gene pool between M. canettii and the MTB-associated phylotype were further denoted by the finding that genomic regions of the MTBC members such as Rv0402c-Rv0406, Rv1041c-Rv1050, Rv1499–1510 and Rv2307c-Rv2309c were replaced in the genomes of ET1291 and/or M. canettii STB-J and -K by only distantly related or unrelated gene segments, with coding sequences sharing ~90–99% identity at nucleotide level with their equivalents in M. shinjukuense (or >80% identity with homologs in M. riyadhense) (Supplementary Figs. 6–8).
Discussion
Tracing the origins and conditions of emergence of the MTBC provides fundamental clues to understand the causes of its outrageous evolutionary success8,12,27,31,32,33. Whether lineages of MTBC strains, now extinct, could have existed and caused TB in humans and animals much earlier (maybe by two orders of time magnitude) than the MRCA of known extant lineages, is an open and largely debated question10,34,35. The discovery in East Africa of a TB clinical isolate that shares typical features of M. canettii strains but is phylogenetically much closer to the MTBC reveals that this was likely not the case, indicating that the emergence of the ancestral clone of the MTBC from its putative free-living M. canettii-like progenitor pool is evolutionarily very recent.
After correction for recombination, the clonally inherited SNP distance separating ET1291 from the currently known MTBC MRCA is only about two-fold the maximal distance separating any two known extant MTBC strains. Consistent with this proximal phylogenetic position, while ET1291 shares archetypal phenotypic and genomic characteristics of M. canettii, including its smooth colony morphotype associated with the twin-pks5 gene configuration, genome-wide imprints of HGT and a larger genome size compared to the MTBC, it distinctively harbors some specific genes likely acquired in a recent common ancestor shared with the MTBC MRCA. By hypothesizing similar molecular clocks across the respective evolutionary branches, this inferred relative phylogenetic distance would thus imply that the original MTBC progenitor that emerged from a M. canettii-like ancestor in East Africa is perhaps only twice as old evolutionarily than the currently reconstructed MRCA of the MTBC. More data would be required for estimating a dating for this emergence, also in the view of the debated dating of the MTBC MRCA32,35.
Regardless of temporal estimation, the multiple traces of HGT in the ET1291 genome and those inferred, after the separation of the ET1291 lineage, in the evolutionary branch leading to the MTBC represent evidence for continued exposure to lateral gene flow until the emergence of the MTBC progenitor clone. Consistent with previous presumption17, these results imply at least partially shared habitats between ancestors of M. canettii strains, including those part of the ancestral branch of the MTBC, but also other environmental (myco)bacterial (including members of the MTB-associated phylotype) and/or phage populations. However, our data suggest an increased barrier to gene flow during evolution in the branches phylogenetically closest or leading to the MTBC, before becoming extreme within the MTBC16,17, post-emergence. This raises intriguing questions of whether such increase already reflected partial adaptation to a new, potentially host-associated ecological niche separate from the rest of the ancestral free-living M. canettii strain pool, and/or from relative reduction of natural abilities of some ancestral strains to receive extrinsic DNA. Both hypotheses are consistent with certain experimental findings on previously known M. canettii strains. Indeed, some correlation was observed between phylogenomic distance of these strains from the MTBC and degrees of virulence/persistence in animal infection models12. Moreover, some, but not all, extant M. canettii strains can act in vitro as recipients of chromosomal segments transferred from MTBC or other M. canettii donor strains, while MTBC strains appear incapable to do so36. Further experiments would be needed to determine the degree of virulence/persistence of ET1291 in infection models and to which extent this strain has retained a recombinogenic property.
ET1291 was the sole M. canettii-like strain identified in the national drug resistance survey conducted in Ethiopia. Likewise, only two M. canettii strains were identified in another national survey recently conducted in the same geographic region37, including one representing an additional intermediate lineage between the MTBC and the formerly identified M. canettii strains, albeit with a branching point more distant from the MTBC (and thus closer to the previously known M. canettii strain pool) compared to ET1291 (in preparation). M. canettii strains represented a more notable, but still small, proportion (i.e., 6%) of the TB isolates only in a national survey previously done in Djibouti38. These population-based proportions represent evidence of the infrequency of human TB caused by M. canettii even in the Horn of Africa, further arguing for spillover infection from a local environmental reservoir. It is thus all the more fascinating that extant representatives of M. canettii lineages reflecting distinct ancestral stages towards the obligate MTBC pathogens can still be sporadically isolated from tuberculosis patients living in different places of this region. When also combined with the recent identification of a new sister clade of the MTBC in the African Great Lakes region4, these findings suggest that a continuum of extant derivatives from further ancestral stages near the root of the MTBC may well exist along the Great Rift Valley. With wider use of genome sequencing in the region, more strains might thus be uncovered, which will help further dissect and resolve the different molecular and evolutionary events leading to the emergence of M. tuberculosis. Last but not least in this respect, we showed that M. canettii strains even genetically very distant from the MTBC have the potential to rapidly evolve towards enhanced, M. tuberculosis-like virulence and persistence phenotypes, via spontaneous pks5 recombination in vitro18 or by different mutational pathways during in vivo experimental evolution33, respectively. Taken altogether, these data thus suggest a possibility of (re-)emergence of a (novel) prototype of professional TB pathogen.
Methods
The ET1291 strain was isolated from a patient with TB in the framework of a national drug resistance survey in Ethiopia according to WHO recommendations.
Strain characterization
The ET1291 strain was isolated from a patient with TB in the framework of a national drug resistance survey in Ethiopia (manuscript in preparation) according to WHO recommendations19. Phenotypic drug susceptibility testing (DST) for first-line drugs was performed at the Ethiopian Public Health Institute (EPHI) and a subculture of all survey strains including ET1291 strain were shipped to the TB Supranational Reference laboratory (SRL) at the San Raffaele Scientific Institute in Milan for further phenotypic tests and whole-genome sequencing. Morphological and growth properties were assessed on Löwenstein–Jensen (BD BBL™, cat. no. 220909), Middlebrook 7H10 (BD Difco™, cat. no. 262710), and MGIT media (BD BBL™, cat. no. 245113). Growth rate in the Middlebrook 7H9 medium (BD Difco™, cat. no. 271310) supplemented with OADC (BD Difco™ BBL™, cat. no. 211886) and 0,5% glycerol (Sigma-Aldrich, Product No. G5516) was monitored using optical density measurements39. Extended DST to first- and second-line anti-TB drugs was performed using the BACTEC MGIT 960 System according to the manufacturer’s instructions and WHO’s recommendations40. Minimum inhibitory concentrations (MICs) for bedaquiline, delamanid, and pretomanid were performed with the BACTEC MGIT 960 system41.
Targeted next-generation sequencing
Targeted next-generation sequencing analysis was performed on DNA from cultured isolates using the Deeplex Myc-TB kit and the associated web application as per the manufacturer’s instructions22.
Whole-genome sequencing
Illumina short-read sequencing, SNP calling, and phylogenetic analysis
Genomic DNA was extracted and purified using the Maxwell® 16 Cell DNA Purification kit (Promega, USA). Paired-end DNA libraries were prepared using the Nextera XT DNA Library Prep Kit (Illumina, CA, USA) and sequenced on an Illumina NextSeq 500 platform with 150-bp read lengths. The corresponding sequence reads, as well as the Nanopore reads, the associated sequence assembly and annotation (see below), were submitted to the NCBI Sequence Read Archive with Project number PRJNA823537. In order to detect drug resistance-associated mutations and identify strain (sub)lineages, the sequenced raw reads underwent adapter trimming with Trimmomatic42 and reads shorter than 20 bp were discarded. The resulting reads were aligned to M. tuberculosis H37Rv ATCC 27294 (NC_000962.3) as reference genomes using the MTBseq pipeline21 by application of the mem algorithm of the Burrows-Wheeler alignment tool v0.7.1743. Duplicated reads were marked using the Picard tool v2.23.4-0 (https://github.com/broadinstitute/picard) and local realignment of reads around InDels was performed using the Genome Analysis Toolkit v3.8. SNPs were called with Samtools mpileup v1.644 using the following thresholds: minimum mapping quality of 20, minimum base quality at a position of 20, minimum read depth at a position of 8X, maximum strand bias for a position of 90%.
Genome-wide SNP calling of ET1291 and 120 other genomes including 80 representatives of all known human- and animal-adapted lineages of the MTBC (including the recently identified lineage 95), as well as 39 available and one newly sequenced genomes of previously known M. canettii strains (Supplementary Data 3), was performed using the CLC Genomics Workbench (version 21.0.5; Qiagen). Reads were mapped on the reconstructed ancestral genome sequence of the MTBC obtained by Comas et al.45, after masking repetitive regions as defined by self-self BLAST46, resulting in a final reference sequence of 4,087,113 bp. Reads with more than one match were ignored. Variants were called using the basic variant calling tool with a minimum frequency filter set to 90% (min. coverage 10, min. count 3), and with other parameters set to default. The false positive variant calling rate with this analysis pipeline was verified by mapping Illumina sequence reads from M. tuberculosis H37Rv genomic DNA against a previously assembled reference genome obtained after PacBio sequencing of the same genomic DNA preparation, resulting in absence of any detectable SNP. Positions of single nucleotide variants (SNV) identified in the whole dataset were defined using the “Merge Variant Tracks and Identify Known Mutations” tool. A custom script was used to extract 82,637 positions commonly covered in all genomes, to avoid biases due to variable read coverages across the genome dataset, and to analyze recombination and compare inter-strain SNP distances based on a same, normalized number of sequence positions. The utilization of a reconstructed ancestral genome sequence of the MTBC as a common reference for SNP analysis was further justified by previous findings that M. canettii and MTBC strains share >89% of their genomes, excluding repetitive regions such as PE_PGRS- and PPE_MPTR-encoding regions (accounting for ~8% of the coding capacity of M. tuberculosis), and that individual accessory genomes of M. canettii strains show very few genes in common12.
These SNPs, along with their positions on the reference genome, were used to generate a whole-genome alignment of the same length as the reference genome (4,087,113 bp). This alignment was used as input to build the phylogeny using PhyML v3.347 (Fig. 2a). No Lewis correction was necessary since a whole-genome alignment was used as input, rather than an alignment of SNPs. Bootstrap support values were computed using the PhyML default Shimodaira-Hasegawa-like (SH-like) procedure. This phylogeny was then used as the starting point to build a recombination-corrected phylogeny using ClonalFrameML v1.1225 (Fig. 2b). For each branch, the relative effect of recombination versus mutation (r/m) was computed using the formula r/m = (d1−d2)/d2 where d1 and d2 are the lengths of the branches before and after correcting for recombination, respectively. These values of r/m are illustrated by the redness of each branch in Fig. 2b. The genomic locations of the recombination events detected by ClonalFrameML on each branch are shown in Fig. 2c. When SNP differences were computed based on positions covered between genome pairs instead of using positions commonly covered in the entire dataset, the maximum distance between any two MTBC genomes was 2417 SNPs (between M. orygis and L7 genomes), and 2096 to 2263 SNPs separated M. bovis from M. tuberculosis (L1-L4, L7-L8) genomes, in line with previous results8.
A phylogeny additionally including more distantly related genomes of the MTB-associated phylotype was inferred by using the Codon Tree pipeline of the Bacterial and Viral Bioinformatics Resource Center (BV-BRC, including tools from the previous PATRIC resources), available at https://www.bv-brc.org/app/PhylogeneticTree. The pipeline uses concatenated nucleotide and encoded amino acid sequences from up to 1000 single-copy core genes, identified via detection of cross-genus BV-BRC global Protein Families (PGFams) homology groups48, for constructing a RAxML tree. Out of 1160 single-copy core genes identified in all 59 genomes analyzed, 1000 were picked randomly. Corresponding protein and nucleotide coding sequences were aligned using MUSCLE49 and the Codon_align function of BioPython, respectively. The concatenated alignment of all protein and nucleotide sequences written in a PHYLIP file was partitioned for describing the alignment in terms of protein sequences and first, second, and third codon positions of nucleotide sequences. The resulting file was used for constructing a maximum-likelihood tree using RAxML-NG v1.0.2 with ‘–model GTR + G + ASC_LEWIS50. We used the general time reversible model of nucleotide substitution under the gamma model of rate heterogeneity and performed 1000 alternative runs on distinct starting trees. Support values were generated using 100 rounds of the “Rapid” bootstrapping and the best-scoring maximum-likelihood topology was “midpoint rooted” using FigTree v1.4.4 (https://github.com/cdeanj/figtree). The topology was annotated and colored using the Evolview v3 online tool51.
Oxford Nanopore Technologies long-read sequencing and complete genome assembly
Genomic DNA of ET1291 was purified from culture with the Genomic DNA Clean & Concentrator kit (Zymo Research, Irvine, CA, USA) to recover large-size DNA, according to https://files.zymoresearch.com/protocols/. Input genomic DNA prepared with the Rapid Barcoding Sequencing (SQK-RBK004) kit (ONT, Oxford, UK) was sequenced on a MinION device using a flow cell R9.4.1.
In order to reconstruct a high-quality assembled genome based on the Illumina short reads and ONT long reads, a hybrid genome assembly was constructed as follows. Nanopore long reads were corrected using the Ratatosk de novo error correction tool v0.7.6.352 using Illumina paired-end short reads. Corrected long reads were assembled using Flye de novo assembler v2.9.1 (https://github.com/fenderglass/Flye). Initial genome annotation was done using the BV-BRC RASTtk-enabled genome annotation service53. A circular map of the chromosome was visualized with BRIG54. Comparative alignments and final genome annotation was performed similarly to the strategy described in4, against an ensemble of genome sequences including representatives of MTBC L1-4 and L7-8 (M. tuberculosis sensu stricto; comprising H37Rv), L5-6 &9 (M. africanum), M. bovis, M. canettii strains STB-A, -D, -E, -G, -H, -I, -J and -K, M. kansasii, M. marinum, M. lacus, M. shinjukuense M. decipiens, and M. riyadhense. These steps were performed based on BLAST searches and analysis of gene synteny, using Artemis and Artemis comparison tool55 as well as a custom Multiple Annotation of Genomes and Differential Analysis (MAGDA) software previously used for annotation of MTBC L8, M. canettii and Helicobacter pylori genomes4,12,56. Where applicable, annotations were transferred from those of M. tuberculosis or M. canettii orthologues in the TubercuList/Mycobrowser database or from data from Supply et al.12, using BLAST matches of >90% protein sequence identity, an alignable region of >80% of the shortest protein length in pairwise comparisons and visual inspection of the gene synteny. Finally, in order to estimate the potential false indel rate in the complete genome assembly, we determined the number of frameshift indels among coding sequences of the orthologues of the 461 genes identified as being essential for in vitro growth of M. tuberculosis H37Rv, by comprehensive essentiality analysis30. Only three (0.7%) occurrences were detected, indicating a low false indel rate.
Analysis of average nucleotide identity (ANI) of the ET1291 genome versus the genome of M. tuberculosis H37Rv ATCC 27294 was done using the ANI calculator online tool with default parameters (http://enve-omics.ce.gatech.edu/).
In silico spoligotyping, detection, and annotation of CRISPR-Cas loci
In silico spoligotyping was performed with Illumina sequence reads using the kvarQ software v 0.12.257. To detect clustered regularly interspaced short palindromic repeats (CRISPR) -Cas loci in assembled genomes, the CRISPRCasFinder58 pipeline (https://github.com/dcouvin/CRISPRCasFinder) was used with default parameters. CRISPR-Cas type and subtype were assigned by using CRISPRmap59.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The genome sequencing data of ET1291 generated in this study have been deposited in the NCBI database under Bioproject accession code PRJNA823537, with SRR23035463 and SRR18636082 accession codes for Oxford Nanopore- and Illumina-derived genome sequences, respectively. Source data for Supplementary Fig. 1 are provided with this paper. Source data are provided with this paper.
References
Houben, R. M. G. J. & Dodd, P. J. The global burden of latent tuberculosis infection: a re-estimation using mathematical modeling. PLoS Med. 13, e1002152 (2016).
Cohen, A., Mathiasen, V. D., Schön, T. & Wejse, C. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur. Respir. J. 54, 1900655 (2019).
Brites, D. et al. A new phylogenetic framework for the animal-adapted Mycobacterium tuberculosis complex. Front. Microbiol. 9, 2820 (2018).
Ngabonziza, J. C. S. et al. A sister lineage of the Mycobacterium tuberculosis complex discovered in the African Great Lakes region. Nat. Commun. 11, 2917 (2020).
Coscolla, M. et al. Phylogenomics of Mycobacterium africanum reveals a new lineage and a complex evolutionary history. Micro. Genom. 7, 000477 (2021).
Sreevatsan, S. et al. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc. Natl Acad. Sci. USA 94, 9869–9874 (1997).
Coscolla, M. & Gagneux, S. Consequences of genomic diversity in Mycobacterium tuberculosis. Semin. Immunol. 26, 431–444 (2014).
Gagneux, S. Ecology and evolution of Mycobacterium tuberculosis. Nat. Rev. Microbiol. 16, 202–213 (2018).
Bacigalupe, R., Tormo-Mas, M. Á., Penadés, J. R. & Fitzgerald, J. R. A multihost bacterial pathogen overcomes continuous population bottlenecks to adapt to new host species. Sci. Adv. 5, eaax0063 (2019).
Smith, N. H., Hewinson, R. G., Kremer, K., Brosch, R. & Gordon, S. V. Myths and misconceptions: the origin and evolution of Mycobacterium tuberculosis. Nat. Rev. Microbiol. 7, 537–544 (2009).
Gutierrez, M. C. et al. Ancient origin and gene mosaicism of the progenitor of Mycobacterium tuberculosis. PLoS Pathog. 1, e5 (2005).
Supply, P. et al. Genomic analysis of smooth tubercle bacilli provides insights into ancestry and pathoadaptation of Mycobacterium tuberculosis. Nat. Genet. 45, 172–179 (2013).
Blouin, Y. et al. Progenitor “Mycobacterium canettii” clone responsible for lymph node tuberculosis epidemic, Djibouti. Emerg. Infect. Dis. 20, 21–28 (2014).
Koeck, J.-L. et al. Clinical characteristics of the smooth tubercle bacilli ‘Mycobacterium canettii’ infection suggest the existence of an environmental reservoir. Clin. Microbiol. Infect. 17, 1013–1019 (2011).
López, B. et al. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin. Exp. Immunol. 133, 30–37 (2003).
Boritsch, E. C. et al. Key experimental evidence of chromosomal DNA transfer among selected tuberculosis-causing mycobacteria. Proc. Natl Acad. Sci. USA 113, 9876–9881 (2016).
Chiner-Oms, A. ́ et al. Genomic determinants of speciation and spread of the Mycobacterium tuberculosis complex. Sci. Adv. 5, eaaw3307 (2019).
Boritsch, E. C. et al. pks5-recombination-mediated surface remodelling in Mycobacterium tuberculosis emergence. Nat. Microbiol 1, 15019 (2016).
World Health Organization. Guidance for the Surveillance of Drug Resistance in Tuberculosis, sixth edition (2020).
Feuerriegel, S., Köser, C. U., Richter, E. & Niemann, S. Mycobacterium canettii is intrinsically resistant to both pyrazinamide and pyrazinoic acid. J. Antimicrob. Chemother. 68, 1439–1440 (2013).
Kohl, T. A. et al. MTBseq: a comprehensive pipeline for whole genome sequence analysis of Mycobacterium tuberculosis complex isolates. PeerJ 6, e5895 (2018).
Jouet, A. et al. Deep amplicon sequencing for culture-free prediction of susceptibility or resistance to 13 anti-tuberculous drugs. Eur. Respir. J. 57, 2002338 (2021).
Fabre, M. et al. Molecular characteristics of ‘Mycobacterium canettii’ the smooth Mycobacterium tuberculosis bacilli. Infect. Genet Evol. 10, 1165–1173 (2010).
Sapriel, G. & Brosch, R. Shared pathogenomic patterns characterize a new phylotype, revealing transition toward host-adaptation long before speciation of Mycobacterium tuberculosis. Genome Biol. Evol. 11, 2420–2438 (2019).
Didelot, X. & Wilson, D. J. ClonalFrameML: efficient inference of recombination in whole bacterial genomes. PLOS Comput. Biol. 11, 1–18 (2015).
Brosch, R. et al. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc. Natl Acad. Sci. USA 99, 3684–3689 (2002).
Veyrier, F. J., Dufort, A. & Behr, M. A. The rise and fall of the Mycobacterium tuberculosis genome. Trends Microbiol. 19, 156–161 (2011).
Deshayes, C. et al. Detecting the molecular scars of evolution in the Mycobacterium tuberculosis complex by analyzing interrupted coding sequences. BMC Evol. Biol. 8, 78 (2008).
Becq, J. et al. Contribution of horizontally acquired genomic islands to the evolution of the tubercle bacilli. Mol. Biol. Evol. 24, 1861–1871 (2007).
DeJesus, M. A. et al. Comprehensive essentiality analysis of the Mycobacterium tuberculosis genome via saturating transposon mutagenesis. mBio 8, e02133–16 (2017).
Comas, I. et al. Out-of-Africa migration and Neolithic co-expansion of Mycobacterium tuberculosis with modern humans. Nat. Genet 45, 1176–1182 (2013).
Bos, K. I. et al. Pre-Columbian mycobacterial genomes reveal seals as a source of New World human tuberculosis. Nature 514, 494–497 (2014).
Allen, A. C. et al. Parallel in vivo experimental evolution reveals that increased stress resistance was key for the emergence of persistent tuberculosis bacilli. Nat. Microbiol. 6, 1082–1093 (2021).
Chisholm, R. H., Trauer, J. M., Curnoe, D. & Tanaka, M. M. Controlled fire use in early humans might have triggered the evolutionary emergence of tuberculosis. Proc. Natl Acad. Sci. USA 113, 9051–9056 (2016).
Menardo, F., Duchêne, S., Brites, D. & Gagneux, S. The molecular clock of Mycobacterium tuberculosis. PLoS Pathog. 15, e1008067 (2019).
Madacki, J. et al. ESX-1-independent horizontal gene transfer by Mycobacterium tuberculosis complex strains. mBio 12, e00965–21 (2021).
Mesfin, A. B. et al. First molecular-based anti-TB drug resistance survey in Eritrea. Int J. Tuberc. Lung Dis. 25, 43–51 (2021).
Tagliani, E. et al. Culture and Next-generation sequencing-based drug susceptibility testing unveil high levels of drug-resistant-TB in Djibouti: results from the first national survey. Sci. Rep. 7, 17672 (2017).
Peñuelas-Urquides, K. et al. Measuring of Mycobacterium tuberculosis growth. A correlation of the optical measurements with colony forming units. Braz. J. Microbiol. 44, 287–289 (2013).
World Health Organization. Technical Report on Critical Concentrations for Drug Susceptibility Testing of Medicines Used in the Treatment of Drug-resistant Tuberculosis (2018).
Ghodousi, A. et al. Acquisition of cross-resistance to bedaquiline and clofazimine following treatment for tuberculosis in Pakistan. Antimicrob. Agents Chemother. 63, e00915–e00919 (2019).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 27, 2987–2993 (2011).
Comas, I. et al. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat. Genet. 42, 498–503 (2010).
Walker, T. M. et al. Whole-genome sequencing for prediction of Mycobacterium tuberculosis drug susceptibility and resistance: a retrospective cohort study. Lancet Infect. Dis. 15, 1193–1202 (2015).
Guindon, S. et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321 (2010).
Davis, J. J. et al. PATtyFams: protein families for the microbial genomes in the PATRIC database. Front. Microbiol. 7, 118 (2016).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Stamatakis, A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014).
Subramanian, B., Gao, S., Lercher, M. J., Hu, S. & Chen, W.-H. Evolview v3: a webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res. 47, W270–W275 (2019).
Holley, G. et al. Ratatosk: hybrid error correction of long reads enables accurate variant calling and assembly. Genome Biol. 22, 28 (2021).
Brettin, T. et al. RASTtk: a modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci. Rep. 5, 8365 (2015).
Alikhan, N.-F., Petty, N. K., Ben Zakour, N. L. & Beatson, S. A. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genom. 12, 402 (2011).
Carver, T. et al. Artemis and ACT: viewing, annotating and comparing sequences stored in a relational database. Bioinformatics 24, 2672–2676 (2008).
Li, H. et al. East-Asian Helicobacter pylori strains synthesize heptan-deficient lipopolysaccharide. PLoS Genet. 15, e1008497 (2019).
Steiner, A., Stucki, D., Coscolla, M., Borrell, S. & Gagneux, S. KvarQ: targeted and direct variant calling from fastq reads of bacterial genomes. BMC Genom. 15, 881 (2014).
Couvin, D. et al. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res. 46, W246–W251 (2018).
Lange, S. J., Alkhnbashi, O. S., Rose, D., Will, S. & Backofen, R. CRISPRmap: an automated classification of repeat conservation in prokaryotic adaptive immune systems. Nucleic Acids Res. 41, 8034–8044 (2013).
Acknowledgements
Timothy Walker is acknowledged for sharing the set of repetitive regions in the genome of M. tuberculosis H37Rv defined by self-self BLAST. Mayam Omrani, Shahab Saghaie, and Shahram Saghaie are gratefully thanked for their expertise and assistance in Bioinformatics analysis. We thank Alberto Trovato for his assistance in whole-genome sequencing.
Author information
Authors and Affiliations
Contributions
D.C., P.S., A.G., B.Y. and A.M.C. designed the study. A.G. and P.S. analyzed data and wrote the manuscript with comments from all authors. A.G., M.C. and M.GE. conducted phenotypic and molecular characterization of ET1291. A.G. and C.G. performed the assembly of sequences. A.G. and M.M. annotated the ET1291 genome. A.G., C.G. and X.D. performed SNP analyses and phylogenetic reconstruction. X.D. performed recombination analyses. A.G., M.M. and P.S. conducted comparative analyses of complete mycobacterial genomes. B.Y., G.D., E.T., A.M.C., M.A., S.M., A.A., B.D., W.S., H.M., A.M., M.T., D.F.G., Y.A., G.S., B.Z., M.G., G.T., S.A., A.K., M.GE., Z.M. and D.C. ran or contributed to the tuberculosis drug resistance national survey.
Corresponding authors
Ethics declarations
Competing interests
P.S. is a consultant for GenoScreen, which developed the Deeplex Myc-TB kit. C.G. was a GenoScreen employee. The other authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks David Couvin and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yenew, B., Ghodousi, A., Diriba, G. et al. A smooth tubercle bacillus from Ethiopia phylogenetically close to the Mycobacterium tuberculosis complex. Nat Commun 14, 7519 (2023). https://doi.org/10.1038/s41467-023-42755-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-42755-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.