Abstract
Ion transport in nanochannels is crucial for applications in life science, filtration, and energy storage. However, multivalent ion transport is more difficult than the monovalent analogues due to the steric effect and stronger interactions with channel walls, and the ion mobility decreases significantly as temperature decreases. Although many kinds of solid ionic conductors (SICs) have been developed, they can attain practically useful conductivities (0.01 S cm−1) only for monovalent ions above 0 °C. Here, we report a class of versatile superionic conductors, monolayer CdPS3 nanosheets-based membranes intercalated with diverse cations with a high density up to ∼2 nm−2. They exhibit unexpectedly similar superhigh ion conductivities for monovalent (K+, Na+, Li+) and multivalent ions (Ca2+, Mg2+, Al3+), ∼0.01 to 0.8 S cm−1 in the temperature range of −30 ‒ 90 °C, which are one to two orders of magnitude higher than those of the corresponding best SICs. We reveal that the high conductivity originates from the concerted movement of high-density cations in the well-ordered nanochannels with high mobility and low energy barrier. Our work opens an avenue for designing superionic conductors that can conduct various cations and provides possibilities for discovering unusual nanofluidic phenomena in nanocapillaries.
Similar content being viewed by others
Introduction
Developing artificial pore structures with angstrom-scale channels to mimic the ion transport in biological systems is crucial for applications in life science, filtration, and energy storage1,2,3. The rapidly growing diversity and safety concern of electrochemical energy storage technologies calls for solid ionic conductors (SICs) that can conduct various ions in the nanochannels, such as Li+, Na+, K+, Ca2+, Mg2+, and Al3+, which provide ionic current to support the cell reactions4,5. In particular, batteries based on multivalent ion transport and storage offer a promising path to reach higher energy density and/or capacity density than current Li-ion batteries, along with the advantages of long-term cost and sustainable resource availability4,5,6. Furthermore, with the continuous upsurge in demand for energy storage, batteries are increasingly required to operate under temperature extremes7. Various materials, including inorganic solids, polymers, metal-organic frameworks (MOFs), and two-dimensional (2D) materials have been developed for SICs2,3,4,5,6,7,8,9,10,11,12,13,14. However, multivalent ion transport in the nanochannels is much more difficult than the monovalent analogs due to the steric effect and stronger interactions with channel walls2,5,6,8, and ion mobility decreases significantly as temperature decreases5,7. Thus, the conductivities of multivalent SICs are at least one order of magnitude smaller than those of monovalent analogs, typically lower than 10−3 S cm−1 at room temperature (RT). So far, nearly none of the existing SICs are able to attain practically useful conductivities (0.01 S cm−1) below 0 °C, even for the most common monovalent ions such as Li+ and Na+.
Recently, we reported a class of membranes assembled from monolayer MPX3 nanosheets (M = Cd, Mn, Fe, Co, Ni, Zn or Cr; X = S or Se), showing exceptionally high proton and Li+ conductivities in the temperature range of 30 to 90 °C15. Unlike MOFs and other lamellar membranes, such as graphene oxide (GO) and MXene membranes, the nanochannel walls of MPX3 membranes contain a huge number of negatively charged transition-metal vacancies15. In this paper, we show that the vacancy-containing CdPS3 nanosheet-based membranes can be intercalated with diverse cations with a high density of up to 2 nm−2 for versatile superionic conductors. They exhibit similar superhigh conductivities for monovalent (Li+, Na+, K+) and multivalent ions (Ca2+, Mg2+, Al3+), ∼0.01 to 0.8 S cm−1 in the temperature range of −30 to 90 °C, which are one to two orders of magnitude higher than those of the corresponding best SICs.
Results
Fabrication and characterizations of CdPS3-Z membranes
We first fabricated Cd-vacancy-containing monolayer Cd0.85PS3Li0.3 nanosheet-based membranes following the approach described previously in ref. 15 (Supplementary Figs. 1, 2). The Cd0.85PS3Li0.3 nanosheet dispersion shows a zeta potential of −51.8 mV (Supplementary Fig. 3), indicating the nanosheets are highly negatively charged. Membranes with other cations were obtained through ion exchange by immersing the Cd0.85PS3Li0.3 membranes into different salt solutions to replace Li+ with other desired cations, including K+, Na+, Ca2+, Mg2+, and Al3+ (Fig. 1a, Methods). The resulting membranes were denoted as CdPS3-Z, where Z represents the cations in the membranes. It should be noted that the ion exchange process of the CdPS3-Li membrane is different from that of the GO-based membrane in which cations can control the interlayer distance by strong cation-π interactions and exclude other cations that have larger hydrated volumes16. There seems to be no clear order for the ion exchange process of the CdPS3-Z membrane (Supplementary Figs. 4–6), which we attribute to the weak electrostatic interactions between cations and the surface of the nanochannels15, similar to the ion exchangeable materials such as vermiculite17,18.
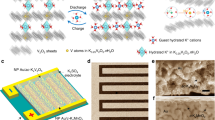
a Schematic illustration of the synthesis process of CdPS3-Z membranes. b Scanning electron microscopy (SEM) images and the corresponding EDS elemental mappings of CdPS3-Z membranes. Scale bars, 10 μm. c Wettability of CdPS3-Z membranes toward the water. Scale bars, 500 μm. d XRD patterns of CdPS3-Z membranes measured at 98% RH. e Comparison of h of the 2D nanochannels in CdPS3-Z membranes with DH. Dashed lines are guides for the eye.
On the basis of the crystal structure and chemical composition of CdPS3-Li, the surface charge density of the 2D nanochannels was estimated to be on the order of 2.0 e nm−2, which is one to two orders of magnitude higher than those reported for carbon and h-BN nanotubes, silica channels, GO laminates, and pores in monolayer crystals, typically ranging from ~0.06 to 0.6 e nm−2, and three to four orders of magnitude larger than angstrom-scale 2D slits with graphite walls2. Such an exceptionally high density of negative charges attracted a huge number of cations from the salt solution to intercalate into the nanochannels while repelled ions of the same charge. Importantly, these membranes have good stability in water, except for the CdPS3-Li membrane (Supplementary Figs. 7,8). Moreover, the stability of CdPS3-Li membrane in aqueous solutions can be significantly improved by the incorporation of Li-containing salts (Supplementary Fig. 9). Figure 1b and Supplementary Fig. 10 show that all the CdPS3-Z membranes have well-ordered lamellar structures with uniform distribution of corresponding cations, while no Cl was observed within the detection limit of energy dispersive X-ray spectroscopy (EDS). X-ray photoelectron spectroscopy (XPS) measurements suggest the complete exchange of Li+ by other cations with the valence states of Cd, P, and S being maintained in the CdPS3-Z membranes (Supplementary Figs. 4, 11,12). Inductively coupled plasma atomic emission spectroscopy (ICP-AES) measurements confirm that Li+ has been completely exchanged with the desired cations on the basis of the charge balance principle (Supplementary Fig. 13 and Supplementary Table 1).
The presence of abundant negatively charged vacancies makes CdPS3-Z membranes highly hydrophilic with a contact angle of 31°, 20°, 23°, 32°, 36°, and 65° under ambient conditions for CdPS3-K, CdPS3-Na, CdPS3-Li, CdPS3-Ca, CdPS3-Mg, and CdPS3-Al, respectively (Fig. 1c and Supplementary Fig. 14), enabling easy adsorption of water molecules in the nanochannels (Supplementary Fig. 15). X-ray diffraction (XRD) measurements reveal the nanochannels in CdPS3-Z membranes. Generally, CdPS3 crystals show a sharp XRD peak at 13.5°, corresponding an interlayer distance (d) of 0.65 nm (Supplementary Fig. 16). Such a peak was also observed in the membranes under ambient conditions (Supplementary Fig. 16), which arises from the sheet restacking of some regions in the case of low RH (∼30–50%). It is worth noting that at 98% RH, this XRD peak disappears and all the membranes show a sharp XRD peak around 7° and the corresponding second-order diffraction peak around 14° (Fig. 1d). According to these XRD data, d corresponds to 1.30, 1.26, 1.32, 1.27, 1.30, and 1.32 nm for CdPS3-K, CdPS3-Na, CdPS3-Li, CdPS3-Ca, CdPS3-Mg, and CdPS3-Al membranes, respectively (Fig. 1e). Taking into account the d in CdPS3 crystal of 6.5 Å, these values yield 2D nanochannels with a height (h) of ∼6.1 to 6.7 Å (Fig. 1e). Note that h is comparable to or much smaller than the hydrated diameter of intercalated cations (DH) (Fig. 1e), thus these ions shredded or flattened their hydration shells when entering and staying in the confined 2D nanochannels2,19,20.
Note that in comparison with the other membranes, the adsorbed water in the CdPS3-Al membrane is easier to be removed at low RH, possibly due to the much stronger electrostatic attraction between Al3+ and vacancies (Supplementary Fig. 17a). The reduced d further increases the electrostatic attraction between Al3+ and vacancies, which leads to the re-occupation of vacancies by Al3+ along with the removal of hydration shells and consequently the decrease in the number of surface charges. Thus, the CdPS3-Al membrane shows a larger contact angle than other membranes under ambient conditions, as shown above, but it shows a similar contact angle to other membranes at high RH (Supplementary Fig. 17b).
Ion transport in CdPS3-Z membranes
To investigate the ion transport within the 2D nanochannels of CdPS3-Z membranes, two-probe alternating current impedance was measured at different temperatures in the range of −40 to 90 °C and 98% RH. Notably, the impedance plots display a semicircle and a spike in the high-frequency and low-frequency regions, respectively (Supplementary Figs. 18–23), which are characteristics of pure ionic conductors9. The ionic transference numbers (tion) of CdPS3-Z membranes were determined to be above 0.99 at 30 °C (Supplementary Fig. 24). The ion conductivities σ were calculated by the following equation
where L, S, and R are the length, cross-sectional area, and resistance of the membranes, respectively. It is important to note all the membranes show similarly superhigh conductivities for monovalent (Li+, Na+, K+) and multivalent ions (Ca2+, Mg2+, Al3+) at the same temperature (Fig. 2). The ion conductivities are in the range of 0.43‒0.78 and 0.17‒0.32 S cm−1 at 90 and 30 °C, respectively, which are dozens of times higher than the practically useful values (0.01 S cm−1) (Fig. 2b). The monovalent K+ with the smallest DH has the highest ion conductivity and the ion conductivities only show a slight decrease as DH and charges of cations increase (Fig. 2b). Even for the multivalent ions with DH much larger than h, including Ca2+, Mg2+ and Al3+, the conductivities still can reach very high values over 0.43 and 0.17 S cm−1 at 90 and 30 °C, respectively (Fig. 2b). This is in sharp contrast to the reported ionic conductors2,5,6, where the conductivities of multivalent ions are suppressed by over ten times than those of monovalent ions. Another remarkable characteristic is that our membranes still maintain practically useful conductivities even at temperatures as low as −30 °C for K+, Li+, and Ca2+ and −20 °C for Na+, Mg2+, and Al3+ (Fig. 2b).
a Arrhenius conductivity plots of CdPS3-Z membranes measured in the temperature range of −40 to 90 °C and 98% RH. b Conductivities of CdPS3-Z membranes measured at 90, 30, −20, and −30 °C and 98% RH. Dashed lines are guides for the eye, and the practically useful conductivity (0.01 S cm−1) was given for comparison.
Figure 3 and Supplementary Tables 2–7 further illustrate the advantages of CdPS3-Z membranes over the existing ionic conductors, including liquid, quasi-solid and solid electrolytes. Generally, liquid and hydrogel ionic conductors can reach high ion conductivity over 0.1 S cm−1 at RT. However, the conductivity decreases sharply when the temperature is lower than the freezing point of water. For example, aqueous Na2SO4 (1 M) shows ion conductivity around 0.12 S cm−1 at RT but less than ~10−4 S cm−1 at a temperature below 0 °C. Although SICs do not show a serious conductivity decay as the temperature decreases, their conductivities are lower than the practically useful values below 0 °C as well because of the low baseline conductivities. For multivalent cations, including Ca2+, Mg2+, and Al3+, no SICs reaching the practically useful conductivity are available at RT. Even for the well-known divalent βʺ-aluminas SICs, the conductivities are on the order of 10−6 S cm−1 at 40 °C (Ca2+, Zn2+, Sr2+, Ba2+, and Pb2+)6. To the best of our knowledge, the ion conductivities of our CdPS3-Z membranes are the highest among all kinds of ionic conductors, which are several times higher than those of aqueous salt-based ionic conductors including aqueous solutions, water-in-salt electrolytes and hydrogels21,22, and at least one order of magnitude higher than those reported for practical organic liquid electrolytes, quasi-/solid-state polymers, and inorganic solid electrolytes over a broad range of temperatures from −40 to 90 °C5,23. We also investigated the ion conductivities of CdPS3-Z membranes under low RHs (from 30% to 75% RH) at 60 °C. As shown in Supplementary Fig. 25, at comparable ambient humidity (~55% RH), the conductivity of the membranes ranges from about 0.24 to 0.02 S cm−1, which is still higher than the practically useful conductivity.
Ion transport mechanism of CdPS3-Z membranes
To explain the remarkable ion transport behavior in CdPS3-Z nanochannels, we use the concerted ion migration model previously suggested to account for the fast Li+ diffusion in superionic conductors24, in which concerted migration is activated at high mobile-ion concentration and in unique mobile-ion configuration with high-energy site occupancy enabled by Coulomb repulsion between adjacent ions (Fig. 4a). Fourier transform infrared (FTIR) spectra show that the asymmetric stretching band of PS3 is split into two absorption bands for all the CdPS3-Z membranes (Fig. 4b), which verifies the presence of Cd vacancies in CdPS3 layers15. Thus, individual layers in CdPS3-Z membranes have two types of regions: charge-free pristine CdPS3 and negatively charged Cd vacancies, which construct high-energy and low-energy sites for cation occupancy, respectively, in the 2D nanochannels (Fig. 4a). At a low charge density, the cations tend to gather around the low-energy sites by electrostatic attraction, thus a high energy barrier need to be overcome for cations’ transport, and this barrier is significantly increased for multivalent cations with more charges. We estimated the ion density on the basis of the chemical composition of CdPS3-Z membranes, which yielded ∼2, 1, and 0.7 nm−2 for monovalent (K+, Na+, Li+), divalent (Ca2+, Mg2+), and trivalent (Al3+) ions, respectively. Notably, such ion densities are quite high and compare well with the measurements in the electrochemical charging of bilayer graphene25, reduced GO flake19, and ultrathin graphite26. The high packing density of cations within the nanochannels could impart strong repulsive Coulomb interaction among adjacent cations to overcome the electrostatic attractions from vacancies and enable stable high-energy site occupancy, which promotes concerted movements of multiple ions. The ions located at the high-energy sites migrate downhill, which cancels out a part of the energy barrier felt by other uphill-climbing ions (Fig. 4a), leading to a low migration energy barrier and high mobility. The presence of hydration shells also plays an important role in this process since it prevents the cations from being trapped in the vacancies, the low-energy sites.
a Schematic illustration (top) and the corresponding energy landscape (bottom) for the concerted migration of multiple hydrated ions along the direction of applied voltage in CdPS3-Z membranes. Here, monovalent cations were used as an example, and the in-plane atomic structure (middle) of the CdPS3 layer shows the positions of Cd vacancies. b FTIR spectra, showing peak splitting in CdPS3-Z membranes. c Ion mobilities in CdPS3-Z nanochannels as a function of DH of cations at 30 °C. Values for the bulk solutions and the angstrom-scale 2D slits (∼6.6 Å in h) at RT were presented for comparison2. Dashed lines and curves are guides for the eye. d Ea at normal- and low-temperature stages for CdPS3-Z membranes. Error bars represent s.d. e Raman O-H stretching bands at different temperatures for CdPS3-Li membranes. f The corresponding proportion of strongly H-bonded water and non-H-bonded water.
To support our interpretation, the mobilities of cations in the 2D nanochannels of CdPS3-Z membranes at different temperatures were estimated based on the equation
where F is Faraday constant, and c+/c- and μ+/μ− are the concentration and mobility of cations/anions, respectively. As mentioned above, no anions’ entry was allowed, and thus c- equals 0. This is different from bulk solutions and chemically inert carbon nanotubes/2D nanoslits, where cations and anions would simultaneously move along opposite directions to generate ionic current2,27. Notably, all the cations confined in 2D nanochannels show higher mobilities than bulk solutions with a weak dependence on DH and charges of cations (Fig. 4c and Supplementary Fig. 26). At 30 °C, the mobility of K+ is ∼1.64 times higher than the bulk value at RT. This value is lower than the prediction by mean-field theory (15-fold improvement), but it is reasonable given that the mean-field model assumes cations inside a smooth, defect-free nanochannel28, and does not take into account the abundant negatively charged vacancies on the channel walls of CdPS3-Z membranes, which are a hindrance to ultrafast ion transport. In particular, the confined Al3+ shows mobility ∼1.03 times higher than that of the bulk solution, although it has three times more charges with DH 1.4 times larger than d. In sharp contrast, the mobility of Al3+ in angstrom-scale charge-free slits of similar h is substantially decreased by ∼10 times compared to its bulk mobility due to the steric effect2. Furthermore, the mobilities of cations in our membranes are over two orders of magnitude higher than those of the well-known Li+ superionic conductor Li10GeP2S12 (refs. 9,24). Even at −30 °C, they still maintain values about one order of magnitude higher than the latter at RT (Supplementary Fig. 26). Thus, our membrane shows one to two orders of magnitude higher conductivity in the temperature range of −40 to 90 °C despite of much lower Li-ion concentration. The screened electrostatic attraction by the hydration shells of cations inside the well-ordered nanochannels of CdPS3-Z membranes may play an important role in their extraordinarily higher mobility compared to Li10GeP2S12 (ref. 6).
Molecular dynamics simulations previously suggested that as DH is larger than h, the ions under quantum confinement have to overcome the hydration energy to reconfigure their hydration shells that become effectively squashed2. The hydration energy is on the order of 3‒50 eV for the ions with DH between K+ and Al3+ (ref. 29). It has been experimentally observed that μ+ values evolve approximately exponentially with the hydration energy2,20, which is in conceptual agreement with the dependence of ions’ conductivities on the activation energy barrier Ea. We calculated Ea for ion transport in CdPS3-Z membranes by using the equation
where σ0 is a preexponential factor, R is the gas constant, and T is temperature. The evolution of ion conductivity with temperature is divided into two stages: normal-temperature stage (90‒0 °C) and low-temperature stage (−10 to −40 °C) (Supplementary Fig. 27). As shown in Fig. 4d, the fitted Ea for K+, Na+, Li+, Ca2+, Mg2+, and Al3+ transport at the normal-temperature stage is 0.18 ± 0.01, 0.28 ± 0.04, 0.18 ± 0.02, 0.18 ± 0.02, 0.20 ± 0.02, and 0.20 ± 0.04 eV, respectively. These values are very similar and compare well with the migration barrier for concerted migration of K ions in biological channels (<0.2 eV)30 and Li ions in superionic conductors (∼0.2–0.4 eV)24, further demonstrating the significant role of Coulomb repulsion between high-density cations in accelerating ion transport inside the nanochannels. At a low-temperature stage, a relatively larger Ea reveals the increase in the ions’ migration barrier. Fortunately, it is still small enough (0.36–0.61 eV), about one order of magnitude lower than that of frozen liquid electrolyte31.
Taking CdPS3-Li membranes as an example, Raman spectra were recorded to reveal the structure evolution of water molecules in the 2D nanochannels in the temperature range of −40 to 30 °C at 98% RH. Generally, the O-H stretching vibration of water molecules shows a broad peak at 3000‒3700 cm−1, which is composed of three components related to different intermolecular bonding degrees of water31,32. For pure water, the proportion of strongly H-bonded water (at ~3230 cm−1) greatly increases while non-H-bonded water (at ~3600 cm−1) disappears as the temperature decreases below 0 °C (Supplementary Figs. 28, 29a, b), suggesting the formation of ordered ice. However, the proportion of strongly H-bonded and non-H-bonded water molecules in CdPS3-Li membranes only slightly increases and decreases, respectively, even down to −40 °C (Fig. 4e, f and Supplementary Fig. 29c, d). The H-bond is formed between the partially positively charged H atoms and partially negatively charged O atoms of neighboring H2O mainly by electrostatic interaction31. Taking into account the high-density cations confined within the 2D nanochannels, which contain at most bilayer water, we expect that the coordination of water molecules with cations would destroy the H-bond interactions between water molecules. The destruction of H-bonds enables the survival of non-H-bonded water molecules, which ensures practically useful conductivities even down to −30 °C. This is in stark contrast to the increased freezing point for confined pure water molecules in 2D nanochannels, where the van der Waals pressure leads to water-to-ice transformation at RT33.
Discussion
Our work demonstrates that the high-density cations inside the well-ordered 2D nanochannels of CdPS3-Z membranes enable concerted ion migrations, which overcome the steric effect, strong interaction with channel walls, and freezing of aqueous solutions that limit the migration of cations in nanochannels. Thus, the membranes exhibit superhigh conductivities for both monovalent and multivalent ions over a broad range of temperatures (−30 to 90 °C), which are over one order of magnitude higher than those of the corresponding best SICs. These findings not only provide insights into the nanofluidic phenomena in the confined nanocapillaries but also open an avenue for designing superionic conductors that can conduct various cations under temperature extremes, which are essential for the development of diverse electrochemical devices with expanded applications.
Methods
Synthesis of CdPS3-Z membranes
Cd0.85PS3Li0.3 nanosheets with Cd vacancies were synthesized by a two-step ion intercalation and exchange method using CdPS3 crystals as raw material15. In brief, 0.1 g of CdPS3 crystals were stirred in a 10 mL solution of 1 M KCl, 2 M K2CO3, and 1 M EDTA at 55 °C for 3 h and then filtered and washed with deionized water several times. The as-obtained product was mixed with 1 M LiCl at 35 °C for 2 h, followed by repeated washing to remove residual salts. The resulting product was redispersed in deionized water and sonicated for several minutes to facilitate exfoliation. After centrifuging at 10,000 rpm for 5 min to remove unexfoliated crystals, the stable Cd0.85PS3Li0.3 dispersion obtained was filtered through polycarbonate membranes in a vacuum filtration cell to fabricate Cd0.85PS3Li0.3 (CdPS3-Li) membranes.
To obtain other CdPS3-Z membranes, CdPS3-Li membranes were immersed in 0.5 M aqueous chloride solution of desired cations (K+, Na+, Ca2+, Mg2+, Al3+) for more than 48 h, and then thoroughly rinsed with deionized water before measurements and characterizations. During this process, Li ions were completely exchanged with desired cations (Supplementary Table 1).
Structure characterizations
The thickness and lateral size of the exfoliated nanosheets were measured using an atomic force microscope (AFM, Bruker Multimode 8). The Zeta potential of Cd0.85PS3Li0.3 nanosheet suspensions were measured on Malvern Zetasizer Nano-ZS90. The morphology and thickness of CdPS3-Z membranes were characterized by SEM (Verios G4 UC, 10 kV). The cross-sectional element distribution of the membranes was analyzed by EDS (Oxford Instrument Ultim Max 100). XPS measurements were conducted on ESCALAB 250 with an excitation source of Al Kα X-ray (1486.6 eV). The elemental compositions of the membranes were characterized by ICP-AES (PerkinElmer, OPTIMA 8300DV). More than three samples were measured to determine the chemical composition of each material. The hydrophilicity of the membranes was evaluated by a contact angle analyzer at 298 K (Dataphysics, OCA20). FTIR spectra were measured using a UV-vis absorption spectrometer (JACSO V-550).
The nanochannels of CdPS3-Z membranes at 98% RH were characterized by XRD (Rigaku, D/MAX 2400 and PANalytical, X’Pert PRO using Cu Kα radiation). CdPS3-Z membranes were first taken out from the salt solution and washed with deionized water. After the surface water was removed, the CdPS3-Z membranes were stored in a sealed homemade container with 98% RH for overnight to make sure that the membranes were under equilibrium conditions. The measurements were carried out at RT as quickly as possible to avoid the dehydration of the membranes.
To reveal the structural evolution of water molecules in the nanochannels of CdPS3-Li membranes with temperature, Raman measurements were performed in the temperature range of −40 to 30 °C using a Raman spectrometer (Witec alpha 300 R, excited by 532 nm laser). Before measurements, the membranes were kept at 98% RH for over 48 h to ensure complete hydration. During the measurements, the CdPS3-Li membrane was sandwiched between two quartz plates to minimize the change in the humidity of the membrane. The ratio of strongly H-bonded and non-H-bonded water was calculated based on the areas of fitted peaks.
Measurements of ion transport
All the samples used in our study had a dimension of 15 mm × 5 mm (length × width) and slightly varied thickness, which was determined by using cross-sectional SEM imaging. Before measurements, the membranes were fixed on a homemade device with two sides attached to platinum electrodes. To ensure that the samples reached a near-equilibrium state, they were kept at room temperature in a 98% RH environment overnight for 98% RH and variable temperature experiments. For 60 °C and variable humidity experiments, we found no significant changes in conductance after exposing samples to a certain RH for 2 and 20 h at 60 °C. Therefore, samples were exposed to various humidity environments for over 2 h before testing. The impedance measurements were carried out on Autolab electrochemical workstation (PGSTAT204) with frequencies ranging from 20 Hz to 1 MHz and an alternating potential of 150 mV. The conductivities at temperatures from 30 to 90 °C and 98% RH were tested in a constant temperature and humidity chamber (Shenzhen Hongjian Instruments Co. Ltd), and those from −40 to 30 °C and 98% RH, as well as low RH experiments were tested on a constant temperature and humidity chamber (Votschtechnik, LabEvent). At least three samples were measured for evaluating the ion conductivities for each kind of membrane.
Estimation of ionic transference number
Chronoamperometry was used to differentiate the electron and ion conductance in the membranes at 98% RH and 30 °C. This electrochemical technique measures the current as a function of time by applying a constant potential to the electrodes. An inert Pt electrode was used to block ion transport in our experiments. As shown in Supplementary Fig. 24a, after the device was subjected to a direct voltage, ions accumulated at the interface between the tested membrane and Pt electrode, creating a significant internal inverse electric field that inhibited ion transport. As ions were blocked, the current was primarily carried by electrons, allowing us to distinguish between the ion and electron contributions. At the initial stage of measurement, the current (iT) was derived from both ionic and electronic currents. However, in the static state, the ionic conductance was blocked and only the electronic current was measured (ie) (Supplementary Fig. 24b). Thus, the tion was calculated as follows:
Note that the tion were nearly identical regardless of the polarity of the applied direct voltage.
Data availability
The authors declare that the experimental data supporting the results of this study can be found in the paper and its Supplementary Information file. The detailed data for the study is available from the corresponding author upon request.
References
Bocquet, L. & Charlaix, E. Nanofluidics, from bulk to interfaces. Chem. Soc. Rev. 39, 1073–1095 (2010).
Esfandiar, A. et al. Size effect in ion transport through angstrom-scale slits. Science 358, 511–513 (2017).
Shen, J., Liu, G. P., Han, Y. & Jin, W. Q. Artificial channels for confined mass transport at the sub-nanometre scale. Nat. Rev. Mater. 6, 294–312 (2021).
Tian, Y. S. et al. Promises and challenges of next-generation “beyond Li-ion” batteries for electric vehicles and grid decarbonization. Chem. Rev. 121, 1623–1669 (2021).
Zhao, Q., Stalin, S., Zhao, C.-Z. & Archer, L. A. Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater. 5, 229–252 (2020).
Iton, Z. W. B. & See, K. A. Multivalent ion conduction in inorganic solids. Chem. Mater. 34, 881–898 (2022).
Rodrigues, M.-T. F. et al. A materials perspective on Li-ion batteries at extreme temperatures. Nat. Energy 2, 17108 (2017).
Park, S. S., Tulchinsky, Y. & Dinca, M. Single-ion Li+, Na+, and Mg2+ solid electrolytes supported by a mesoporous anionic Cu-azolate metal-organic framework. J. Am. Chem. Soc. 139, 13260–13263 (2017).
Kamaya, N. et al. A lithium superionic conductor. Nat. Mater. 10, 682–686 (2011).
Kato, Y. et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 1, 16030 (2016).
Zhang, Z. Z. et al. New horizons for inorganic solid state ion conductors. Energy Environ. Sci. 11, 1945–1976 (2018).
Lu, J. H., Jaumaux, P., Wang, T. Y., Wang, C. Y. & Wang, G. X. Recent progress in quasi-solid and solid polymer electrolytes for multivalent metal-ion batteries. J. Mater. Chem. A 9, 24175–24194 (2021).
Banerjee, M., Gupta, A., Saha, S. K. & Chakravorty, D. 1-aza-15-crown-5 functionalized graphene oxide for 2D graphene-based Li+-ion conductor. Small 11, 3451–3457 (2015).
Li, M. T. et al. Graphene-analogues boron nitride nanosheets confining ionic liquids: a high-performance quasi-liquid solid electrolyte. Small 12, 3535–3542 (2016).
Qian, X. T. et al. CdPS3 nanosheets-based membrane with high proton conductivity enabled by Cd vacancies. Science 370, 596–600 (2020).
Chen, L. et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 550, 380–383 (2017).
Nicolosi, V., Chhowalla, M., Kanatzidis, M. G., Strano, M. S. & Coleman, J. N. Liquid exfoliation of layered materials. Science 340, 1420–1420 (2013).
Huang, K. et al. Cation-controlled wetting properties of vermiculite membranes and its promacise for fouling resistant oil-water separation. Nat. Commun. 11, 1097 (2020).
Xue, Y. H. et al. Atomic-scale ion transistor with ultrahigh diffusivity. Science 372, 501–503 (2021).
Saini, L., Nemala, S. S., Rathi, A., Kaushik, S. & Kalon, G. Selective transport of water molecules through interlayer spaces in graphite. Nat. Commun. 13, 498 (2022).
Liu, Z. X. et al. Voltage issue of aqueous rechargeable metal-ion batteries. Chem. Soc. Rev. 49, 180–232 (2020).
Wang, Z. F. et al. Hydrogel electrolytes for flexible aqueous energy storage devices. Adv. Funct. Mater. 28, 1804560 (2018).
Fan, L.-Z., He, H. C. & Nan, C.-W. Tailoring inorganic–polymer composites for the mass production of solid-state batteries. Nat. Rev. Mater. 6, 1003–1019 (2021).
He, X. F., Zhu, Y. Z. & Mo, Y. F. Origin of fast ion diffusion in super-ionic conductors. Nat. Commun. 8, 15893 (2017).
Kühne, M. et al. Ultrafast lithium diffusion in bilayer graphene. Nat. Nanotechnol. 12, 895–900 (2017).
Bao, W. Z. et al. Approaching the limits of transparency and conductivity in graphitic materials through lithium intercalation. Nat. Commun. 5, 4224 (2014).
Koltonow, A. R. & Huang, J. X. Two-dimensional nanofluidics. Science 351, 1395–1396 (2016).
Kondrat, S., Wu, P., Qiao, R. & Kornyshev, A. A. Accelerating charging dynamics in subnanometre pores. Nat. Mater. 13, 387–393 (2014).
Marcus, Y. Thermodynamics of solvation of ions. Part 5.—Gibbs free energy of hydration at 298.15 K. J. Chem. Soc. Faraday Trans. 87, 2995–2999 (1991).
Bernèche, S. & Roux, B. Energetics of ion conduction through the K+ channel. Nature 414, 73–77 (2001).
Zhang, Q. et al. Modulating electrolyte structure for ultralow temperature aqueous zinc batteries. Nat. Commun. 11, 4463 (2020).
Okazaki, Y., Taniuchi, T., Mogami, G., Matubayasi, N. & Suzuki, M. Comparative study on the properties of hydration water of Na- and K-halide ions by Raman OH/OD-stretching spectroscopy and dielectric relaxation data. J. Phys. Chem. A 118, 2922–2930 (2014).
Algara-Siller, G. et al. Square ice in graphene nanocapillaries. Nature 519, 443–445 (2015).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 52188101), the Key Research Program of Frontier Sciences of the Chinese Academy of Sciences (No. ZDBS-LY-JSC027), and the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB30000000). The authors thank X. T. Qian and Z. B. Liu for their help on experiments and valuable discussions.
Author information
Authors and Affiliations
Contributions
W.R. conceived and supervised the project. X.Y. performed the experiments. W.R. and X.Y. designed the experiments, analyzed the data, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Gopinadhan Kalon, Yahui Xue and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, X., Ren, W. 2D CdPS3-based versatile superionic conductors. Nat Commun 14, 3998 (2023). https://doi.org/10.1038/s41467-023-39725-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-39725-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.