Abstract
All antibodies approved for cancer therapy are monoclonal IgGs but the biology of IgE, supported by comparative preclinical data, offers the potential for enhanced effector cell potency. Here we report a Phase I dose escalation trial (NCT02546921) with the primary objective of exploring the safety and tolerability of MOv18 IgE, a chimeric first-in-class IgE antibody, in patients with tumours expressing the relevant antigen, folate receptor-alpha. The trial incorporated skin prick and basophil activation tests (BAT) to select patients at lowest risk of allergic toxicity. Secondary objectives were exploration of anti-tumour activity, recommended Phase II dose, and pharmacokinetics. Dose escalation ranged from 70 μg–12 mg. The most common toxicity of MOv18 IgE is transient urticaria. A single patient experienced anaphylaxis, likely explained by detection of circulating basophils at baseline that could be activated by MOv18 IgE. The BAT assay was used to avoid enrolling further patients with reactive basophils. The safety profile is tolerable and maximum tolerated dose has not been reached, with evidence of anti-tumour activity observed in a patient with ovarian cancer. These results demonstrate the potential of IgE therapy for cancer.
Similar content being viewed by others
Introduction
All monoclonal antibodies in clinical use for the treatment of cancer belong to the IgG class, the most prevalent immunoglobulin in human blood1. Circulating levels of IgE are much lower than IgG, and IgE has evolved to provide immune surveillance in tissues2,3,4. The mechanism of action of therapeutic antibodies is mediated in part by their engagement with cognate Fc receptors on specific immune effector cell populations5,6,7. The structure of the Fc domain of IgE differs from that of IgG, and it binds with very high affinity to FcɛRI receptors, expressed on cells including monocytes/macrophages, mast cells, basophils, and dendritic cells, resulting in long retention of antibody by these effector cells in the absence of immune complex formation8 (Fig. 1a). By contrast, the affinity of IgG for its Fc receptors, present on effector populations such as natural killer (NK) cells and monocytes/macrophages, is 100–10,000-fold lower9. IgE antibody drugs can mediate a more potent immune response to cancer cells than IgGs because of this higher affinity for Fc receptors, which in turn are expressed on an effector cell population distinct from those for IgG.
a The structurally distinct Fc domains of IgE and IgG underpin differences in their biological characteristics, supporting exploration of IgE therapies as potentially superior to IgG [refs. 8,9]. b (1) IgE bound to Fc receptors on macrophages is crosslinked by antigen expressed on tumour cells, leading to target cell cytotoxicity, release of proinflammatory mediators (e.g. TNFα) and macrophage repolarization. (2) TNFα upregulation in turn triggers MCP-1 production by monocytes and tumour cells, followed by (3) recruitment of further macrophages, mediated by MCP-1 [refs. 10,12,14].
Preclinical comparison of anti-tumour activity using an IgE or an IgG antibody, each specific for the same target antigen, has demonstrated that the efficacy of IgE is indeed superior, both in human tumour xenograft-bearing mouse models10,11,12,13 and in an immunocompetent rat model of metastatic cancer14,15. IgE induces antibody-dependent cell-mediated killing of cancer cells by both cytotoxic and phagocytic mechanisms10,11,12,16 mediated by monocytes and macrophages. These effects are coordinated by secreted mediators, such as TNFα, MCP-1 and IL-10 in tumours (Fig. 1b)14,17. We hypothesise that, in line with these studies using animal models, the higher affinity of IgE binding to Fc receptors on distinct effector-cell populations may result in superior clinical efficacy of IgE therapies compared to IgG. The first step in testing this hypothesis is establishing whether IgE drugs can be safely administered to humans.
The human folate receptor-alpha (FRα) was chosen as the target for clinical testing of IgE therapy because of its presence on the cell membrane (Fig. 2a) of a range of tumour types, and very limited expression on normal tissues18,19. IgG-based therapeutics specific for FRα have shown evidence of anti-tumour activity in trials20,21,22,23,24.
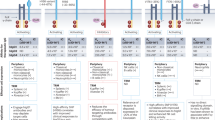
a Representative immunohistochemistry of paraffin-embedded ovarian cancers showing a range of FRα membrane expression on 10 to 95% of tumour cells. b FRα membrane expression for all enroled patients (mean ± SEM; dotted line represents the 5% expression threshold required for trial eligibility; n = 26). c The trial dosing schedule consisted of 6 weekly doses of MOv18 IgE, before first on-treatment tumour assessment. d Skin prick testing comprised histamine positive control [+], saline negative control [−] and MOv18 IgE solution. The result of this typical skin prick test was negative. e MOv18 IgE dose escalation, and number of patients treated in each cohort (*cohort 2 included 2 patients not evaluable for safety who did not receive intravenous dosing because of positive intradermal tests (see text); †a DLT occurred in these cohorts; ‡intra-patient dose escalation). Source data are provided as a Source Data file.
We have designed and conducted a Phase I trial of MOv18 IgE, a chimeric monoclonal IgE antibody specific for FRα, in patients with tumours expressing this antigen. Procedures prior to intravenous administration of MOv18 IgE were included to mitigate the possibility of unwanted IgE-mediated effects. First, skin prick testing with a solution of MOv18 IgE antibody was undertaken, with a rationale of detecting putative soluble factors, possibly originating from the target tumour, that might cross-link the antibody once bound to Fc receptors. This could result in mast cell and basophil degranulation and possible anaphylaxis. If present these factors would be expected to produce a wheal and flare reaction on skin prick testing, analogous to those seen in classical cutaneous allergy testing performed with solutions of antigens25. Secondly, pre-dose basophil activation tests (BAT) were performed as previously described26 on fresh whole blood from each patient using MOv18 IgE. This test was designed to model ex vivo the ability of MOv18 IgE to activate circulating cells, with the expectation that the BAT would function as an assay predictive of anaphylaxis. The safety profile of MOv18 IgE is tolerable, the most common toxicities being cutaneous, and there is evidence of anti-tumour activity.
Results
Dose escalation
Patients with any solid tumour expressing FRα using our immunohistochemical assay (Fig. 2a, b) were eligible, although all patients recruited to this trial had ovarian epithelial cancer (n = 21), tubal carcinoma (n = 3) or endometrial cancer (n = 2). A low prevalence of FRα expression was observed in other tumour types screened using our validated assay. 24 patients (median age 61) were treated with up to six weekly MOv18 IgE infusions (Fig. 2c) after intradermal or skin prick testing (Fig. 2d; see Methods) at escalating doses ranging from the starting dose of 70 μg to 12 mg (Fig. 2e). Patients potentially benefitting (stable disease or response), and without intolerable toxicity, could continue with three subsequent two-weekly maintenance doses. All patients had received prior systemic therapy, and in many cases were multiply pre-treated. The first patient developed symptomatic brain metastases after one 70 μg dose, and so was withdrawn and replaced. A dose-limiting toxicity (DLT) of grade 3 urticaria occurred in the second (250 μg) cohort, which was expanded. No further DLT was seen at this dose, but 5 patients in this cohort demonstrated a reaction to intradermal testing with MOv18 IgE prior to their first or second dose and were withdrawn. Intradermal testing was replaced by the skin prick test for subsequent recruitment, and 3 further patients safely received multiple 250 μg doses. A dose-limiting toxicity of anaphylaxis occurred in the third (500 μg) cohort, which was expanded to 6 patients without further DLT. Three patients were then treated at each of 750 μg, 1.5 mg, and 3 mg dose levels. Subsequently, based on a predictable safety profile in earlier cohorts, the protocol was amended to allow intra-patient dose escalation, and a further patient was treated at 6 mg and then 12 mg (cohorts 7(i) and 7(ii), respectively). No further DLTs occurred and a maximum tolerated dose has not been defined.
Safety
MOv18 IgE was generally well tolerated with the great majority of adverse events being low grade (≤2, NCI Common Terminology Criteria for Adverse Events version 4.0). The most common events were localised cutaneous toxicities including urticaria, pruritus and rash (Fig. 3a); cutaneous events appeared to be dose-related. Urticaria was often associated with pruritus, less prominent with repeat dosing, and in all but one case (described below) not associated with any systemic signs, symptoms or elevation of serum tryptase. Urticaria always resolved within hours of dosing, either spontaneously or with the administration of systemic steroids and antihistamines. Secondary prophylaxis with these supportive medications was allowed in patients experiencing urticaria with a previous dose.
a Adverse events (AEs) experienced by ≥10% of patients (as well as anaphylaxis in 1/26 (4%)) are grouped according to severity and by dose cohort (1–7(ii) from left to right for each AE). No related AEs occurred at the lowest dose (cohort 1), and there was no grade 4 or 5 toxicity at any dose. Acute hypotension responding promptly to intramuscular adrenaline was seen in 1 patient treated at 500 μg (cohort 3). b Serum tryptase was elevated in this patient at 2 and 6 h following the infusion, supporting a diagnosis of drug-related anaphylaxis to explain this adverse event (dotted line represents upper limit of normal for serum tryptase [14 ng/ml]). c Fold change (Log2) in median circulating cytokine levels post-dose (2, 6, 24 h and 7 days) relative to normalised baseline values for each patient (n = 26). d Marked upregulation of the basophil activation marker CD63 in response to ex vivo stimulation of patient blood using positive controls (1 = anti-FcεRI, 2 = fMLP, 3 = anti-IgE), but not to MOv18 or control IgEs. This BAT result (n = 26) remained negative when performed following the 1st (n = 24) and 3rd (n = 20) MOv18 IgE doses (mean ± SEM) for all trial subjects excluding the single patient who experienced anaphylaxis. Negative control IgE was not included in all assays. e By contrast, the baseline BAT was positive for the patient experiencing anaphylaxis at the time of their subsequent MOv18 IgE infusion (n = 1). Unlike all other patients, MOv18 IgE antibody produced a 17-fold increase in basophil activation in this individual’s blood at baseline (left panel, blue bars). The BAT for this patient became negative after the episode of anaphylaxis (right panel), likely as a result of basophil depletion. f Peripheral basophil counts fell immediately following MOv18 IgE infusion and anaphylaxis in this patient, but not in other trial subjects after their MOv18 IgE dosing (Supplementary Fig. 1), recovering by day 7 (n = 1). Source data are provided as a Source Data file.
One patient treated in the 500 μg cohort experienced an episode of transient hypotension during their first infusion, a few minutes following the onset of urticaria. Treatment was discontinued and the hypotension responded immediately to adrenaline and the administration of intravenous hydrocortisone, antihistamine and saline. The diagnosis of grade 3 anaphylaxis was confirmed by the detection of elevated serum tryptase following this episode (Fig. 3b), which was not seen after any of the episodes of isolated urticaria observed with multiple MOv18 IgE doses in other patients (not shown). Similar treatment-related changes in circulating cytokine concentrations were detected in all patients (median values for the trial population shown in Fig. 3c). Although the basophil activation test (BAT), using MOv18 IgE stimulation, had been negative for all other patients treated in the trial both before and after treatment (Fig. 3d), this patient’s BAT had been positive during screening prior to trial treatment (Fig. 3e). This systemic toxicity was not evident in any other patient, and because the mechanism was not considered to be related to dose, the protocol was amended with ethics committee approval to allow continued dose escalation, but only in patients with a negative baseline BAT. We hypothesised that a positive BAT at baseline predicts the possibility of systemic anaphylaxis developing as a result of MOv18 IgE therapy. The dose was subsequently safely escalated in all the remaining trial patients, each with a negative BAT, to 12 mg without repetition of anaphylaxis. No other DLTs were observed and MTD was not reached.
Pharmacodynamics
Skin prick testing was routinely performed before each intravenous dose. In general, the reaction to MOv18 IgE in this test was indistinguishable from the negative diluent control, although a wheal reaction of up to 3 mm was also considered negative (Fig. 2d). A positive cutaneous reaction to skin prick testing with MOv18 IgE was not observed in any of the patients in the study, including the patient who developed anaphylaxis following intravenous dosing. By contrast the performance of the BAT did distinguish the single patient who experienced anaphylaxis, exhibiting a positive BAT at baseline which became negative immediately following the event (Fig. 3e), then positive again by day 7 of follow-up (not shown), consistent with recovery of a functional circulating basophil population (Fig. 3f). Basophil depletion was not observed following infusion of MOv18 IgE in any other patient (Supplementary Fig. 1).
Free FRα protein was detected at one or more time points (baseline, prior to infusion 4 and at 28-day follow up) in the serum of eight patients, although in the remainder it was undetectable throughout (Supplementary Fig. 2a). Circulating anti-FRα antibodies were detected in only three patients, at the 28-day follow up, and in the remainder they were undetectable throughout (Supplementary Fig. 2b). One patient had detectable concentrations of both serum FRα and anti-FRα auto-antibodies at a single timepoint.
Analysis of circulating cytokine concentrations in all the treated patients revealed an increase in the mean fold-change serum concentration of IL-6 from baseline, which peaked 4–6 h after dosing and was detectable for up to 7 days (Fig. 3c). Mean fold-change serum concentrations of IFNγ and MCP-1 were similarly increased modestly within 24 h of dosing, while those of IL-10 showed a modest increase within 6 h, followed by a fall between 24 h and 7 days following dosing. Fold-change concentrations of circulating levels of TNFα and IL-8 were marginally decreased following dosing. No changes in IL-4 were observed (Fig. 3c).
Alpha-GAL IgE antibodies27,28 were measured in patient sera before, during and after MOv18 IgE treatment. At baseline, no patient had alpha-GAL antibody titres >0.1 kUA/L (threshold of positivity according to reference laboratory). Three patients developed anti-alpha-GAL antibodies (>0.1 kUA/L, patient values ranged from 0 to 0.99 kUA/L) but these were not associated with any clinical signs of anaphylaxis and were no longer measurable at the 28-day follow-up (Supplementary Fig. 3).
Anti-drug antibody responses
Samples for the ADA assay were collected from 26 patients. ADA detection was definitively confirmed, at 1 or 2 time points, in only 3 patients (Supplementary Fig. 4). There was no correlation between ADA titre and toxicity or clinical benefit.
Pharmacokinetics
Serum MOv18 IgE clearance profiles in each individual patient are shown in Fig. 4a. Systemic exposure, as measured by AUC(0–24h) and Cmax, was observed to increase in a dose-proportional manner (Fig. 4a, b). The mean terminal elimination half-life was 9.4 h (Fig. 4c), mean total body clearance was 2.81 L/h, and these parameters were consistent across the range of doses studied. In six of 24 patients who received at least one MOv18 IgE infusion, Cmax exceeded the baseline endogenous IgE level in that patient (endogenous IgE levels are shown in Supplementary Fig. 5).
a Linear plot of plasma drug concentrations for evaluable patients (n = 23) showing dose-proportional Cmax (inset; mean ± SEM; n = 23), which exceeded baseline endogenous IgE levels in 25% of patients. b Area under the curve (AUC(0–24h); mean ± SEM; n = 20) also increased with dose. c Half-life (mean ± SEM; n = 19) was consistent in the range of doses tested, with a mean t1/2 = 9.4 h across all cohorts. Some measurements were below level of quantification for MOv18 IgE. Source data are provided as a Source Data file.
Anti-tumour activity
Twenty of 24 patients, who had at least one on-treatment CT scan, were evaluable for efficacy (Fig. 5a). Six patients continued dosing into the maintenance period, with a best response of stable disease according to RECIST (Response Evaluation Criteria in Solid Tumours) criteria29. One patient with platinum-resistant mixed high grade serous and endometrioid ovarian carcinoma treated at 700 μg/week exhibited a 52% reduction of the serum CA125 tumour marker concentration in cycles 1 and 2 (Fig. 5b). This marker reduction was not sustained beyond 28 days, therefore not meeting formal Gynaecologic Cancer Intergroup criteria for response30. This patient’s on-treatment scan after 6 weeks revealed resolution of ascites and short-lived shrinkage of peritoneal tumour deposits (not amounting to RECIST partial response; Fig. 5c). The scan and tumour marker at 12 weeks demonstrated disease progression, following per protocol reduction of dosing frequency to two-weekly. This evidence of anti-tumour activity was seen in a patient who had derived little benefit from conventional chemotherapy. Their previous treatment course was notable for relapse within five months of adjuvant carboplatin and paclitaxel, followed by six cycles of weekly paclitaxel without response. At the time of trial enrolment this patient was aged 62 and had a performance status of zero. Membrane FRα expression was observed on 10% of their tumour cells (range for recruited patients 10–95%).
a Percentage change in CA125 circulating tumour marker for evaluable patients (n = 17; vertical dotted line indicates initiation of dosing; red line indicates a patient with marked CA125 reduction and tumour volume reduction; * and † = tumour marker progression >200%). b Marked but short-lived CA125 reduction following MOv18 IgE dosing in a patient with progression prior to treatment. CA125 relapse coincided with change from weekly to alternate-weekly dosing after 6 weeks. c Pelvic CT images for this patient at baseline and week 6 of treatment showing reduction of peritoneal tumour deposit (red oval). Source data are provided as a Source Data file.
Discussion
In this clinical trial of an IgE antibody for the treatment of cancer, a manageable safety profile, distinct from that of IgG drugs, was observed and preliminary evidence of efficacy demonstrated. Both anti-tumour activity and adverse events occurred at doses very much lower than typically observed for IgG antibodies. This reflects fundamental differences in Fc-receptor affinity and effector cell biology3,31, including the absence of an inhibitory IgE receptor, as well as lower circulating serum concentrations of endogenous IgE in humans. Serum concentrations of MOv18 IgE achieved during treatment exceeded those of endogenous IgE for several patients enrolled in this study. The plasma half-life of MOv18 IgE was substantially shorter than observed with IgG therapeutic antibodies, as might be expected given the rapid clearance of IgE, compared with antibodies of other isotypes, from the circulation of healthy subjects, regardless of atopic status32. This likely reflects avid binding of the IgE molecule to Fcε-receptors expressed on effector cells such as basophils and monocytes10,12,26. These cells readily traffic to tissue extravascular space or are already resident there.
Our finding of elevated fold increases in serum concentrations of IL-6, IFNγ, MCP-1 and IL-10 in treated patients is consistent with the mechanism of IgE-induced signalling by tumouricidal effector cells, such as monocytes and macrophages, described in animal surrogates (Fig. 1)14,17,33. Although the mean serum IL-6 concentration was increased in the 24-h period following MOv18 IgE treatment, we did not observe any of the IL-6-mediated toxicity associated with uncontrolled immune activation complicating some advanced therapies. The modest decrease in circulating TNFα in our patients may not fully reflect the changes in this cytokine in the tumour microenvironment. Insufficient pre- and post-treatment tumour biopsies were available for formal immunophenotyping in this trial. Serum IL-4, a cytokine characteristically implicated in allergy, did not change with MOv18 IgE treatment in either animal models or patients. This confirms the impression that the anti-tumour mechanism of IgE in this trial likely reflects its ability to activate cell-mediated immunity against tumour cells and parasitic pathogens, rather than involvement in a conventional Type 1 allergic reaction4,17,34,35,36.
In this trial we performed a basophil activation test (BAT) ex vivo on whole unfractionated blood from each patient just prior to dosing, with a rationale of addressing concerns that MOv18 IgE bound to endogenous Fcε receptors might be cross-linked by endogenous antibodies or other circulating factors. The BAT results remained negative in all patients with the exception of one who developed anaphylaxis on their first exposure to MOv18 IgE. The episode resolved rapidly and did not recur after withdrawal of drug. This patient demonstrated a positive BAT at baseline, prior to any exposure to MOv18 IgE. We hypothesise that, in this single patient, both anaphylaxis and the positive BAT at baseline reflected the presence of an endogenous circulating factor able to crosslink the MOv18 IgE antibodies bound to receptors on the surface of effector cells. One such candidate may be autoantibodies to the oligosaccharide galactose-α−1,3-galactose (alpha-GAL): subjects with an endogenous IgE response against this post-translational modification on a therapeutic antibody have been reported to develop Type 1 hypersensitivity reactions during treatment with some monoclonal antibodies27. Development of anti-alpha-GAL IgE antibodies following treatment with MOv18 IgE was detected in three patients in our trial but was not associated with any clinical sequelae. Furthermore, seropositivity to alpha-GAL was not detected, before or after treatment with MOv18 IgE, in the patient who experienced anaphylaxis (Supplementary Fig. 3). We also considered the possibility that release of FRα from tumour cells might have cross-linked MOv18 IgE bound to effector cells such as mast cells and basophils. However, serum concentrations of FRα and anti-FRα auto-antibodies were undetectable in this patient at all timepoints. Our previous ex vivo studies suggest that even large numbers of circulating tumour cells expressing FRα are not sufficient to cross-link IgE37.
Following this single case of anaphylaxis, the trial protocol was amended to exclude any further patients found to have a positive baseline BAT, and no further anaphylaxis was observed. In a separate observational study of blood samples drawn from a series of patients with ovarian cancer, we observed a positive BAT using MOv18 IgE in only one of 42 individuals26. The prevalence of positive baseline BAT therefore appears low in this population of patients with cancer, but screening for baseline BAT positivity may be useful in future to exclude these infrequent patients from IgE therapy and the associated risk of anaphylaxis.
The most common adverse events associated with MOv18 IgE were cutaneous, with some patients experiencing a diffuse patchy macular erythematous rash, with or without pruritus, but with urticaria the most frequent toxicity. Although the extent of this rash varied greatly between patients, it was tolerable and transient. Urticaria usually recurred with subsequent doses, but in general it was more prominent with first dose administration and became less obvious with subsequent doses. It is possible this could have been a result of the use of secondary prophylaxis with steroid and antihistamine. Urticaria was associated with symptoms or signs of anaphylaxis only in the single patient referred to above with a positive BAT at baseline. No elevation of the serum tryptase concentration, a diagnostic requirement in cases of anaphylaxis38, was observed in any of the remaining patients who experienced urticaria. Cutaneous reactions were not seen in animal models of IgE therapy15,39, and the mechanism accounting for the common occurrence of urticaria, in the absence of anaphylaxis, in humans receiving MOv18 IgE is currently unclear. Our own data, consistent with the literature40,41, suggest that FRα is not expressed in skin (Stavraka, personal communication), and although distribution to the skin of healthy subjects was claimed in a clinical study of a fluorescently labelled anti-FRα agent, this was attributed to drug aggregation42. MOv18 IgE does not bind to human skin ex vivo, and histological examination of biopsied urticarial skin from a patient in this trial did not demonstrate prominent infiltration by basophils (C Stavraka, personal communication).
FRα was chosen as the target for testing IgE therapy in a clinical trial. This tumour-associated antigen is present at very low levels in normal tissues43, but is expressed in a range of solid tumours18. Several IgG-based antibody therapies targeted to FRα have been tested clinically20,22,23,44, with the recent approval of mirvetuximab soravtansine further validating this target24. Our pre-clinical evidence supporting superior anti-tumour activity of FRα-targeting IgE suggests a route to improvement of earlier clinical results with anti-FRα IgG. Tumour types most commonly expressing FRα with the IHC used in this trial were epithelial ovarian/tubal and endometrial cancers. We observed preliminary evidence of MOv18 IgE anti-tumour activity in high grade serous ovarian carcinoma, with a fall in CA125 and tumour shrinkage in a patient with chemotherapy-resistant disease. If this is confirmed in subsequent clinical development, this antibody may join the growing immunotherapy armamentarium in FRα-positive cancers45. More broadly, these results offer the opportunity to generate a new class of IgE antibody cancer therapies specific for other antigens already validated as targets for IgG drugs.
Methods
Study design
This was an open-label, dose-escalating Phase I study of MOv18 IgE conducted at 4 sites in the United Kingdom. The study was undertaken under the sponsorship and management of the Cancer Research UK Centre for Drug Development, conducted in accordance with International Council for Harmonisation Good Clinical Practice guidelines and ethical principles of the Declaration of Helsinki, and approved by the UK Medicines and Healthcare products Regulatory Agency and National Health Service Health Research Authority (EudraCT number 2014-000070-19; ClinicalTrials.gov identifier NCT02546921, first registered 11 September 2015). Participants provided written informed consent prior to the conduct of any study procedures. Antibody was administered as an intravenous infusion weekly for a six-week treatment period. In the absence of disease progression or intolerable treatment-related toxicity, patients were offered the option to continue two-weekly dosing for a further maintenance period.
Screening tests at baseline included physical examination, computerised tomography (CT), standard tests of organ function, beta-tryptase, endogenous IgE level, tumour FRα expression, basophil activation test, and intradermal or skin-prick test. Safety assessments were repeated prior to each further dose throughout the study. All patients receiving at least 1 intravenous dose of MOv18 IgE were evaluable for safety. Anti-tumour activity was assessed using standard tumour markers where relevant (for example, CA125 in ovarian cancer cases), and CT according to RECIST version 1.1 at intervals of 6 weeks. Laboratory endpoints were analysed using SSPS version 29 and presented with Graphpad Prism version 9.5.
Patients
Recruitment and treatment took place between 23 February 2016 and 20 April 2021. Eligible patients had advanced or metastatic solid tumours not suitable for alternative standard treatment and were over 16 years of age. Immunohistochemical evidence of tumour FRα membrane expression was required (based on a criterion of ≥5% tumour cells with membrane positivity)46. Briefly, sections of formalin-fixed, paraffin embedded tissue were pre-treated using citrate-based heat-induced antigen retrieval (CC2) for 92 min, followed by staining with FRα antibody (NCL-L-FRα, Leica Novocastra) at a dilution of 1:500 using the Ventana Benchmark Ultra (Roche) automatic staining system. Positive (FRα expressing ovarian tumour tissue) and negative (normal ovarian tissue) controls were included. Of the 445 individuals assessed for tumour expression of FRα, 37% were deemed to be positive. Other inclusion criteria were: measurable or evaluable disease; resolution of any residual toxic effects, aside from sensory neuropathy, related to prior anti-cancer therapy to grade 1 or lower (as defined by the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0); no chemotherapy, radiotherapy, biological therapy, or hormone for at least four weeks, or five half-lives, prior to receiving the study treatment; an Eastern Cooperative Oncology Group (ECOG) performance score of 0 or 1; adequate bone marrow, liver, and kidney function. Patients with treated and stable brain metastases were allowed. Exclusion criteria included: a history of congenital or acquired immunosuppression, including an ongoing requirement for systemic steroid therapy; high risk or uncontrolled asthma; a history of anaphylaxis; elevated serum tryptase at baseline; a history of laryngeal oedema, known HIV, hepatitis B or C infection; pregnancy or breast-feeding; elevated risk in the event of anaphylaxis because of heart failure, uncontrolled infection, vascular disease, previous cerebrovascular accident, extensive lung metastases or pleural effusion; concurrent medication with drugs likely to inhibit or augment the effects of adrenaline, including beta-blockers, tricyclic antidepressants and monoamine oxidase inhibitors.
Drug administration
Drug was supplied (Cancer Research UK) and manufactured as single-use aseptic 1 mL fill in 2 mL glass vials containing a solution of MOv18 IgE at a concentration of 1 mg/mL at pH6.5 with 0.1 M sodium citrate, 30 g/L L-arginine, 50 g/L sucrose and 0.02% polysorbate 20 in water for injection. Stock was stored at 5 ± 3 °C, initially diluted to a concentration of 100 μg/mL in 0.9% sodium chloride, then infused over 90 min through a peripheral line in a total volume of 250 ml 0.9% saline. A shorter infusion time for subsequent doses of 60 min was allowed if no adverse reactions are encountered. Vital signs were monitored during the infusion and at regular intervals afterwards. Patients were hospitalised overnight following the first administration to facilitate safety monitoring procedures.
The protocol-defined treatment regimen was 6 doses of MOv18 IgE administered weekly, following which disease response was reassessed using CT. Patients tolerating treatment and appearing to benefit from treatment were offered the option of continuing into a maintenance phase of two-weekly dosing for a further 6 weeks (Fig. 2c).
Dose escalation
The planned escalation in successive flat dosing cohorts was 70 μg, 250 μg, 500 μg, 700 μg, 1.5 mg, 3 mg, 6 mg and 12 mg total protein. An accelerated dose escalation scheme, starting with single-patient cohorts, was planned up to cohort 5 (1.5 mg)47. An additional 2–5 patients could be added to each cohort in the event of emergent toxicity. Thereafter, three patients were enrolled per cohort, with an additional three patients added to a cohort if needed for toxicity. The starting dose of 70 μg was selected to be lower than endogenous IgE, and was based on the relatively low physiological IgE serum levels and high affinity for receptors on effector cells. It also reflected partial occupancy of Fc receptors by endogenous IgE, meaning that anti-tumour IgE could engage and activate immune cells at concentrations much lower than the equivalent IgG. Each patient was assessed for safety and dose-limiting toxicities (DLTs). DLT was defined as any grade 3/4 non-haematological adverse event (with exceptions in the case of incomplete supportive medication for diarrhoea or vomiting, grade 3 fatigue, or biochemical abnormalities deemed clinically non-significant) occurring in the first 3 weeks of MOv18 IgE administration. Haematological DLTs were defined as grade 4 neutropenia or thrombocytopenia that was prolonged, or grade 3/4 neutropenia associated with fever or infection.
Cutaneous testing and basophil activation test
Skin prick testing (SPT) with a solution of MOv18 IgE antibody was undertaken in patients prior to each intravenous administration. Positive histamine and negative saline controls were included, and the presence of a wheal reaction in response to the positive control was required for a test to be considered valid (Fig. 2d). Concomitant dosing with antihistamines was not allowed within 4 half-lives before SPT. For early patients recruited to the trial, intradermal testing was used instead of SPT [https://www.bsaci.org/Guidelines/SOPs], but subsequently SPT was preferred due to serial positive results with intradermal testing, which did not appear to discriminate those at risk.
Similarly, basophil activation tests (BAT) were performed as previously described26 on fresh whole blood using aliquots of the trial supply of MOv18 IgE, and a laboratory standard solution. Briefly, basophils in unfractionated whole blood samples were incubated with positive controls anti-FcεRI, fMLP (each as part of Buhlmann FlowCAST kit FK-CCR-U), and anti-IgE (Dako Aligent A0094), or MOv18 and control IgE antibodies (non-FRα-reactive IgE antibodies) for 30 min at 37 °C. Staining antibodies (Buhlmann FlowCAST kit FK-CCR-U) were used for CCR3 (to identify basophils) and CD63 (as a marker of activation)48. Flow gating strategy is shown in Supplementary Fig. 6. Data were analysed as the fold change in percentage CD63 expression relative to the patient’s background. BAT was performed at baseline and after administration of the first and third intravenous doses to each patient, as well as immediately following any systemic adverse event.
Other pharmacodynamic assays
No features of anaphylaxis or other manifestation of allergic toxicity were observed in preclinical animal models, but in addition to the risk mitigation steps described above, serial measurements of serum tryptase were included in this trial. Tryptase is released by degranulation of activated effector cells, and elevation is characteristic of anaphylaxis38. The protocol mandated tryptase measurement at baseline, at the end of each infusion, and following any infusion-related event, primarily to distinguish cytokine release from anaphylaxis. It was also measured following the onset of urticaria, which can occur as a feature of anaphylaxis49.
Patient specimens were also collected for pharmacodynamic analysis. Serum samples were collected pre- and post-dose for multiplex analysis of a cytokine panel (Randox) relevant to IgE biology15,17 and to the proposed mechanism of anti-tumour activity of MOv18 IgE14,50. Where possible, serial tumour biopsies were collected for analysis of changes in the tumour microenvironment.
Anti-drug antibody (ADA) samples were drawn at baseline and 2 and 5 weeks following initiation of weekly dosing. Additional samples were collected 28 days after the last dose was administered and following any suspected infusion reaction. Serum samples were stored frozen at −80 °C prior to analysis using a bridging ELISA assay with MOV18 IgE to capture, and biotinylated MOv18 IgE followed by streptavidin-HRP (BD 554066) to detect the presence of ADAs. A confirmatory assay was performed on all positive samples, pre-incubating with drug to distinguish specific responses from false positives.
Further blood samples were drawn at baseline, 3 weeks following initiation of weekly dosing and 28 days after the last dose, as well as following any observed adverse events. Serum was separated and stored at −80°C prior to evaluation of circulating soluble FRα and anti-FRα antibodies by ELISA, as previously described37. Briefly, FRα in patient sera was captured by mouse anti-human FRα IgG1 (R&D Systems MAB5646), and detected by biotinylated polyclonal goat anti-human FRα antibody (R&D Systems BAF5646) followed by streptavidin-HRP (Pierce 21130). Similarly, anti-FRα antibodies were captured by recombinant FRα (BioTechne 5646-FR) and detected by anti-IgG1-HRP antibody (Jackson Immuno Research 109-036-098). Levels were quantified by recombinant FRα (BioTechne 5646-FR) and anti-FRα IgG1 (in house) standard curves, with lower levels of quantification (LLOQ) of 6.25 ng/ml and 3.125 ng/ml in serum, respectively.
Pharmacokinetic sampling and assays
Pharmacokinetic blood samples were drawn immediately pre-dose and at 0.5, 2, 4, 6, and 24 h, then 7 days, following the first dose, and 28 and 70 days after the final dose. Serum was separated from blood and stored at −70°C prior to analysis for MOv18 IgE concentrations by indirect ELISA, using a fully validated assay. Briefly, MOv18 IgE antibodies were captured by recombinant FRα (BioTechne 5646-FR) and detected by an anti-IgE-HRP detection antibody (Sigma A9667). Levels were quantified by a MOv18 IgE standard curve with a limit of quantification of 2.5 ng/ml and a dynamic range of 2.5–200 ng/mL in serum. The following pharmacokinetic parameters were evaluated: terminal half-life calculated from the terminal slope of the log concentration–time curve (t½), maximum concentration (Cmax), time of peak serum concentration (Tmax), and area under the serum concentration-time curve (AUC).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The summary study data are available within this Article, Supplementary Information and Source Data file. The full clinical and laboratory data set that support the findings of this trial are held by Cancer Research Horizons, a wholly owned subsidiary of the trial sponsor Cancer Research UK. Tabulated de-identified patient data (including demographics and adverse event information) will be shared on request from the date of publication and will be retained for 15 years. Access requests are subject to review and approval by Epsilogen (info@epsilogen.com). The study protocol is also available on request from the sponsor (horizons@cancer.org.uk). Source data are provided with this paper.
References
Kretschmer, A., Schwanbeck, R., Valerius, T. & Rosner, T. Antibody isotypes for tumor immunotherapy. Transfus. Med. Hemother 44, 320–326 (2017).
Mukai, K., Tsai, M., Starkl, P., Marichal, T. & Galli, S. J. IgE and mast cells in host defense against parasites and venoms. Semin. Immunopathol. 38, 581–603 (2016).
Sutton, B.J., Davies, A.M., Bax, H.J. & Karagiannis, S.N. IgE antibodies: from structure to function and clinical translation. Antibodies (Basel) 8 (2019).
Hagan, P., Blumenthal, U. J., Dunn, D., Simpson, A. J. & Wilkins, H. A. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature 349, 243–245 (1991).
Hsiao, H. C., Fan, X., Jordan, R. E., Zhang, N. & An, Z. Proteolytic single hinge cleavage of pertuzumab impairs its Fc effector function and antitumor activity in vitro and in vivo. Breast Cancer Res. 20, 43 (2018).
Arce Vargas, F. et al. Fc effector function contributes to the activity of human anti-CTLA-4 antibodies. Cancer Cell 33, 649–663.e644 (2018).
Weiner, G. J. Building better monoclonal antibody-based therapeutics. Nat. Rev. Cancer 15, 361–370 (2015).
Gould, H. J. et al. The biology of IgE and the basis of allergic disease. Annu. Rev. Immunol. 21, 579–628 (2003).
Hogarth, P. M. & Pietersz, G. A. Fc receptor-targeted therapies for the treatment of inflammation, cancer and beyond. Nat. Rev. Drug Discov. 11, 311–331 (2012).
Karagiannis, S. N. et al. Role of IgE receptors in IgE antibody-dependent cytotoxicity and phagocytosis of ovarian tumor cells by human monocytic cells. Cancer Immunol. Immunother. 57, 247–263 (2008).
Karagiannis, S. N. et al. IgE-antibody-dependent immunotherapy of solid tumors: cytotoxic and phagocytic mechanisms of eradication of ovarian cancer cells. J. Immunol. 179, 2832–2843 (2007).
Karagiannis, S. N. et al. Activity of human monocytes in IgE antibody-dependent surveillance and killing of ovarian tumor cells. Eur. J. Immunol. 33, 1030–1040 (2003).
Gould, H. J. et al. Comparison of IgE and IgG antibody-dependent cytotoxicity in vitro and in a SCID mouse xenograft model of ovarian carcinoma. Eur. J. Immunol. 29, 3527–3537 (1999).
Josephs, D. H. et al. Anti-folate receptor-alpha IgE but not IgG recruits macrophages to attack tumors via TNFalpha/MCP-1 signaling. Cancer Res. 77, 1127–1141 (2017).
Josephs, D. H. et al. An immunologically relevant rodent model demonstrates safety of therapy using a tumour-specific IgE. Allergy 73, 2328–2341 (2018).
Karagiannis, P. et al. Characterisation of an engineered trastuzumab IgE antibody and effector cell mechanisms targeting HER2/neu-positive tumour cells. Cancer Immunol. Immunother. 58, 915–930 (2009).
Pellizzari, G. et al. IgE re-programs alternatively-activated human macrophages towards pro-inflammatory anti-tumoural states. EBioMedicine 43, 67–81 (2019).
Cheung, A. et al. Targeting folate receptor alpha for cancer treatment. Oncotarget 7, 52553–52574 (2016).
Cheung, A. et al. Anti-folate receptor alpha-directed antibody therapies restrict the growth of triple-negative breast cancer. Clin. Cancer Res. 24, 5098–5111 (2018).
Moore, K. N. et al. Safety and activity findings from a phase Ib escalation study of mirvetuximab soravtansine, a folate receptor alpha (FRalpha)-targeting antibody-drug conjugate (ADC), in combination with carboplatin in patients with platinum-sensitive ovarian cancer. Gynecol. Oncol. 151, 46–52 (2018).
Moore, K. N. et al. Safety and activity of mirvetuximab soravtansine (IMGN853), a folate receptor alpha-targeting antibody-drug conjugate, in platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: a phase I expansion study. J. Clin. Oncol. 35, 1112–1118 (2017).
Vergote, I. et al. A randomized, double-blind, placebo-controlled, phase III study to assess efficacy and safety of weekly farletuzumab in combination with carboplatin and taxane in patients with ovarian cancer in first platinum-sensitive relapse. J. Clin. Oncol. 34, 2271–2278 (2016).
Molthoff, C. F. et al. Escalating protein doses of chimeric monoclonal antibody MOv18 immunoglobulin G in ovarian carcinoma patients: a phase I study. Cancer 80, 2712–2720 (1997).
American Association for Cancer Research. FDA gives nod to mirvetuximab soravtansine. Cancer Discov. 13, 8 (2023).
Bousquet, J. et al. Practical guide to skin prick tests in allergy to aeroallergens. Allergy 67, 18–24 (2012).
Bax, H. J. et al. Basophil activation test in cancer patient blood evaluating potential hypersensitivity to an anti-tumor IgE therapeutic candidate. Allergy 75, 2069–2073 (2020).
Chung, C. H. et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N. Engl. J. Med. 358, 1109–1117 (2008).
Gonzalez-Quintela, A. et al. IgE antibodies to alpha-gal in the general adult population: relationship with tick bites, atopy, and cat ownership. Clin. Exp. Allergy 44, 1061–1068 (2014).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Rustin, G. J. et al. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG). Int. J. Gynecol. Cancer 21, 419–423 (2011).
Karagiannis, S. N., Josephs, D. H., Bax, H. J. & Spicer, J. F. Therapeutic IgE antibodies: harnessing a macrophage-mediated immune surveillance mechanism against cancer. Cancer Res. 77, 2779–2783 (2017).
Lawrence, M. G. et al. Half-life of IgE in serum and skin: consequences for anti-IgE therapy in patients with allergic disease. J. Allergy Clin. Immunol. 139, 422–428.e424 (2017).
Nakamura, M. et al. IgE activates monocytes from cancer patients to acquire a pro-inflammatory phenotype. Cancers (Basel) 12 (2020).
Soulat, D. & Bogdan, C. Function of macrophage and parasite phosphatases in Leishmaniasis. Front. Immunol. 8, 1838 (2017).
Capron, M. & Capron, A. Immunoglobulin E and effector cells in schistosomiasis. Science 264, 1876–1877 (1994).
Nakamura, M. et al. Immune mediator expression signatures are associated with improved outcome in ovarian carcinoma. Oncoimmunology 8, e1593811 (2019).
Rudman, S. M. et al. Harnessing engineered antibodies of the IgE class to combat malignancy: initial assessment of FcεRI-mediated basophil activation by a tumour-specific IgE antibody to evaluate the risk of type I hypersensitivity. Clin. Exp. Allergy 41, 1400–1413 (2011).
Passia, E. & Jandus, P. Using baseline and peak serum tryptase levels to diagnose anaphylaxis: a review. Clin. Rev. Allergy Immunol. 58, 366–376 (2020).
Williams, I. P. et al. In vivo safety profile of a CSPG4-directed IgE antibody in an immunocompetent rat model. MAbs 12, 1685349 (2020).
Parker, N. et al. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal. Biochem. 338, 284–293 (2005).
Weitman, S. D. et al. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 52, 3396–3401 (1992).
Hoogstins, C. E. et al. A novel tumor-specific agent for intraoperative near-infrared fluorescence imaging: a translational study in healthy volunteers and patients with ovarian cancer. Clin. Cancer Res. 22, 2929–2938 (2016).
O’Shannessy, D. J., Somers, E. B., Wang, L. C., Wang, H. & Hsu, R. Expression of folate receptors alpha and beta in normal and cancerous gynecologic tissues: correlation of expression of the beta isoform with macrophage markers. J. Ovarian Res. 8, 29 (2015).
Scaranti, M., Cojocaru, E., Banerjee, S. & Banerji, U. Exploiting the folate receptor alpha in oncology. Nat. Rev. Clin. Oncol. 17, 349–359 (2020).
Kandalaft, L. E., Odunsi, K. & Coukos, G. Immunotherapy in ovarian cancer: are we there yet? J. Clin. Oncol. 37, 2460–2471 (2019).
Smith, A. E. et al. A novel monoclonal antibody for detection of folate receptor alpha in paraffin-embedded tissues. Hybrid. (Larchmt.) 26, 281–288 (2007).
Simon, R. et al. Accelerated titration designs for phase I clinical trials in oncology. J. Natl Cancer Inst. 89, 1138–1147 (1997).
Bax, H. J. et al. Basophils from cancer patients respond to immune stimuli and predict clinical outcome. Cells 9 (2020).
Williams, K. W. & Sharma, H. P. Anaphylaxis and urticaria. Immunol. Allergy Clin. North Am. 35, 199–219 (2015).
Josephs, D. H., Spicer, J. F., Karagiannis, P., Gould, H. J. & Karagiannis, S. N. IgE immunotherapy: a novel concept with promise for the treatment of cancer. MAbs 6, 54–72 (2014).
Acknowledgements
The trial was funded and sponsored by Cancer Research UK, who collaborated with the academic authors in designing the trial, collated the clinical data, and reviewed the manuscript. The authors acknowledge additional financial support from the UK Department of Health and Cancer Research UK via Experimental Cancer Medicine Centre and NIHR Biomedical Research Centre grants to King’s Health Partners/Guy’s & St Thomas’ NHS Foundation Trust (JS), the University of Cambridge/Cambridge University Hospitals NHS Foundation Trust (BB), University College London/UCL Hospital NHS Trust (RM), and Institute of Cancer Research/Royal Marsden Hospital (UB). We acknowledge the Immune Monitoring Core Facility team at Guy’s and St Thomas’ NHS Foundation Trust (IS-BRC-1215-20006) for flow cytometry facilities, and the Cancer Research UK King’s Health Partners Centre at King’s College London (C604/A25135). The authors would like to thank the patients, their families, the clinical research teams, as well as Sharmistha Ghosh, Amy Pope and Natalie Woodman for their contribution to this study.
Author information
Authors and Affiliations
Contributions
S.K., H.G., M.F., S.Can., D.J. and J.S. developed MOv18 IgE. J.S. (chief clinical investigator), S.K. (chief scientific investigator), C.C., C.B., P.J. and S.M. wrote the trial protocol. S.Car. and C.Sel. manufactured MOv18 IgE. J.S., S.K., H.B. and J.C. wrote a first draft of the manuscript, and all authors reviewed and approved the final submission. J.S., B.B., A.M., U.B., R.K., R.M., G.N., V.K., I.F., G.D. and C.Sta. recruited patients, collected clinical data and provided clinical care. C.C. and S.T. provided clinical allergy advice. S.M., P.J. and C.B. managed the trial and database. J.C., G.P., M.N., K.I., A.K., C.Sta. and H.B. developed and performed pharmacodynamic assays. G.V. developed and delivered pharmacokinetic assays and data. S.P. and C.G. supervised tissue collection and the FRα immunohistochemical assay.
Corresponding author
Ethics declarations
Competing interests
J.S. and S.K. are co-founders of Epsilogen Ltd. H.B. is presently employed, and J.C. formerly employed, through a fund from Epsilogen Ltd. S.K., H.B., H.G., D.J, G.P. and J.S. hold patents on anti-tumour IgE antibodies. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Spicer, J., Basu, B., Montes, A. et al. Safety and anti-tumour activity of the IgE antibody MOv18 in patients with advanced solid tumours expressing folate receptor-alpha: a phase I trial. Nat Commun 14, 4180 (2023). https://doi.org/10.1038/s41467-023-39679-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-39679-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.