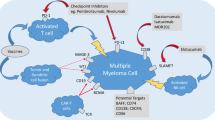
Abstract
Multiple myeloma is a genetically complex and heterogenous malignancy with a 5-year survival rate of approximately 60%. Despite advances in therapy, patients experience cycles of remission and relapse, with each successive line of therapy associated with poorer outcomes; therefore, therapies with different mechanisms of action against new myeloma antigens are needed. G protein–coupled receptor class C group 5 member D (GPRC5D) has emerged as a novel therapeutic target for the treatment of multiple myeloma. We review the biology and target validation of GPRC5D, and clinical data from early phase trials of GPRC5D-targeting bispecific antibodies, talquetamab and forimtamig, and chimeric antigen receptor T cell (CAR-T) therapies, MCARH109, OriCAR-017, and BMS-986393. In addition to adverse events (AEs) associated with T-cell–redirection therapies irrespective of target, a consistent pattern of dermatologic and oral AEs has been reported across several trials of GPRC5D-targeting bispecific antibodies, as well as rare cerebellar events with CAR-T therapy. Additional studies are needed to understand the underlying mechanisms involved in the development of skin- and oral-related toxicities. We review the strategies that have been used to manage these GPRC5D-related toxicities. Preliminary efficacy data showed overall response rates for GPRC5D-targeting T-cell–redirecting therapies were ≥64%; most responders achieved a very good partial response or better. Pharmacokinetics/pharmacodynamics showed that these therapies led to cytokine release and T-cell activation. In conclusion, results from early phase trials of GPRC5D-targeting T-cell–redirecting agents have shown promising efficacy and manageable safety profiles, including lower infection rates compared with B-cell maturation antigen- and Fc receptor-like protein 5-targeting bispecific antibodies. Further clinical trials, including those investigating GPRC5D-targeting T-cell–redirecting agents in combination with other anti-myeloma therapies and with different treatment modalities, may help to elucidate the future optimal treatment regimen and sequence for patients with multiple myeloma and improve survival outcomes.

Video Summary
Similar content being viewed by others
Introduction
Multiple myeloma (MM) is a genetically complex and heterogenous malignancy that is associated with substantial morbidity and mortality [1,2,3]. It accounts for 15%–20% of all hematologic malignancies and has a 5-year survival rate of 60% [4,5,6]. MM can affect multiple organ systems, leading to fatigue, pain, breathlessness, and mobility issues, as well as neurological effects (including numbness/tingling), renal impairment, bone disease, blood disorders, and frequent infections [7,8,9,10,11,12,13]. The symptom burden is associated with poor health-related quality of life, including depression, anxiety, poor function, and lower social satisfaction [14].
Despite advances in therapy, including B-cell maturation antigen (BCMA)-targeting antibody-drug conjugates, bispecific antibodies, and chimeric antigen receptor T-cell (CAR-T) therapies, patients experience cycles of remission and relapse; with each new line of therapy (LOT), the risk of discontinuation increases due to poor efficacy, accumulating toxicities, and increasingly prevalent comorbidities [15,16,17]. New therapies with different mechanisms of action against new targets are needed across LOT to improve patient outcomes. G protein–coupled receptor class C group 5 member D (GPRC5D) is a novel therapeutic target for the treatment of MM. Here, we describe the biology and target validation of GPRC5D and review data from GPRC5D-targeting T-cell–redirecting agents in clinical development.
Biology of GPRC5D
GPRC5D, located on human chromosome 12p13, is an orphan G-protein coupled receptor, meaning that its ligand is unknown (Fig. 1) and due to the lack of in vivo models, its signaling mechanism and function in normal tissue and MM are not yet established [18,19,20,21]. Due to the short extracellular domains, the exposed epitopes of GPRC5D for T-cell–redirecting agents are likely closer to the plasma membrane, facilitating tighter immunological synapses between T cells and target cells that may drive greater cytotoxicity [20, 22]. As a seven-pass transmembrane receptor protein with a short extracellular N-terminal domain, GPRC5D is unlikely to be shed from target cells into the serum, which may reduce the risk of decreased efficacy related to target antigen shedding [20, 23].
GPRC5D expression in normal tissue
Although there is a relative paucity of high-quality immunohistochemistry reagents for the detection of GPRC5D protein in normal (non-diseased) and tumor tissues, GPRC5D expression profiling can be accomplished by augmenting immunohistochemical data with complementary assays, such as in situ hybridization (ISH), RNA-sequencing, and flow cytometry [19, 20, 24, 25]. GPRC5D protein has been detected in immune cells and in epithelial structures of the skin and tongue [24]. In the immune cell compartment, GPRC5D protein is predominantly expressed in cells with a plasma cell phenotype [20, 21, 26] and has little to no expression in normal B cells, T cells, natural killer cells, monocytes, granulocytes, and bone marrow progenitors [21, 25,26,27], unlike CD38 and BCMA, which have a broader expression profile. In epithelium, GPRC5D expression has been detected in hair follicles (during the mid- and late-anagen and catagen phases only), in eccrine glands, in the skin (hair follicle–specific keratin), and at the base of filiform papillae on the tongue [19, 21, 24, 25, 28]. Expression of GPRC5D is suspected to occur in the nailbed in humans because GPRC5D messenger RNA (mRNA) expression has been reported in the nailbeds of mice [19]. GPRC5D mRNA expression in other healthy tissues is shown in Fig. 2A, with no GPRC5D expression detected in the human testis by immunohistochemistry (IHC) [25].
The first panel (A) shows mRNA expression levels of GPRC5D across a range of healthy tissues. The second panel (B) shows mRNA expression levels of GPRC5D in multiple myeloma compared with expression levels observed across a range of hematological and solid tumor malignancies. ALL acute lymphoblastic leukemia, AML acute myeloid leukemia, CML chronic myelogenous leukemia, DLBCL diffuse large B- cellly mphoma, NSC non-small cell. Reproduced from Smith EL, et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci Transl Med. 2019;11(485):eaau7746.
Low levels of GPRC5D mRNA have been detected in the motor neurons of the inferior olivary nucleus of the brainstem in the central nervous system by ISH, but without correlative protein detection by IHC [24, 29]. No GPRC5D mRNA or protein expression has been detected in the cerebral cortex, basal ganglia, midbrain, or cerebellum by ISH or IHC [24]. At present, no data are available to describe potential racial differences in GPRC5D expression.
GPRC5D as target for MM treatment
Successful T-cell–redirection therapy is dependent on eliminating malignant cells expressing a target that has minimal to no expression in other tissues [20]. GPRC5D is highly expressed in MM cells and is abundant in the bone marrow from patients with MM and smoldering MM [20, 25, 27, 30]. Additionally, GPRC5D mRNA expression is also higher in MM cells versus other hematologic cancers (Fig. 2B) [25]. The selective expression in MM cells suggests that GPRC5D is an ideal target for immune effector cell–mediated therapy to treat MM [25, 26]. While GPRC5D and BCMA have similar expression on CD138+ cells, the expression patterns are independent of each other, offering distinct clinical targets [25]. GPRC5D expression is also unaffected by BCMA loss, which has been associated with disease relapse following treatment with BCMA-targeting therapies and may support combining GPRC5D-targeting and BCMA-targeting T-cell–redirecting agents to address the heterogeneity of MM [20, 31].
Increased GPRC5D mRNA expression from bone marrow or MM cells is associated with a high number of genetic aberrations and high-risk disease [21, 26, 27]; however, this may be due to the proportions of malignant plasma cells, which have elevated levels of GPRC5D expression, in bone marrow samples. GPRC5D is also associated with International Staging System (ISS) stage and β2-macroglobulin [27]. Increased GPRC5D mRNA expression in bone marrow has been associated with poor prognosis and disease outcomes, including shorter progression-free survival and overall survival [25, 27]. However, many of these observations may be due to an overall increase in tumor burden and a higher proportion of features associated with high-risk disease, as a causative relationship between GPRC5D and malignant plasma cell transformation or high-risk disease has not been demonstrated. Furthermore, genome and RNA sequencing in patients with MM showed that a significant number of patients have aberrations in genes encoding immunotherapy targets, including GPRC5D. However, while 15% of patients had heterozygous deletion of GPRC5D, these aberrations did not impact gene expression [32]. A recent analysis reported four cases of relapse with biallelic mutations of GPRC5D following GPRC5D-targeting bispecific antibody therapy, including two cases with convergent evolution where multiple subclones lost GPRC5D through somatic events [33]. GPRC5D loss has also been observed with GPRC5D-targeting CAR-T therapy [29]. Biallelic genetic inactivation of GPRC5D has also been described elsewhere in the literature as a mechanism of resistance, along with long-range epigenetic silencing of its promoter and enhancer regions [34].
GPRC5D-targeted therapies in clinical development for the treatment of MM
Two GPRC5D×CD3 bispecific antibodies and 3 GPRC5D-targeted CAR-T therapies are in development for the treatment of patients with MM (Table 1).
T-cell–redirecting bispecific antibodies
Bispecific antibodies have been engineered to overcome some of the limitations of conventional monoclonal antibodies and enhance therapeutic efficacy [35]. There are two antigen-binding sites on T-cell–redirecting bispecific antibodies: CD3 on the surface of T cells and a target antigen that is expressed on tumor cells [35]. Binding to both the T-cell surface receptor and tumor-specific antigen brings the T cells into proximity with tumor cells, leading to synapse formation and induction of T-cell activation and subsequent killing of malignant cells [36]. T-cell–redirecting antibodies may overcome some forms of tumor-mediated evasion from the immune system because they do not require antigen presentation by major histocompatibility complex molecules, which are often downregulated or lost in tumor cells, to carry out effector functions [36].
Bispecific antibodies can have different constructs, including single-chain variable fragments (scFv), which do not include an Fc domain, and full-size antibody constructs generated through methods, such as Fab-arm exchange [37, 38]. Some of the scFv-based antibodies (e.g., BiTEs®) are relatively small molecules (~25 kDa) with an antigen-binding site generated by fusing immunoglobulin G (IgG) heavy- and light-chain variable regions through a flexible polypeptide linker [36, 39]. The lack of an Fc domain is thought to prevent activation of off-target effector functions, including antibody-dependent cell-mediated cytotoxicity, complement-dependent cytotoxicity, and antibody-dependent cellular phagocytosis; however, modifications may be needed to extend the half-life in vivo [38]. In full-length IgG-like antibodies, the IgG portion resembles a natural IgG molecule, with two antigen-binding regions joined via an Fc stem (Fig. 3A) [38]. Inclusion of the Fc domain contributes to a more stable antibody with longer serum half-life than scFv antibodies [38, 40]. With an Fc domain, full-size antibodies may retain effector functions; however, the Fc domain can be functionally “silenced” to prevent nonspecific effector functions. Talquetamab and forimtamig both have silenced Fc domains.
The first panel (A) shows a generalized structure ofGPRC5D-targeting bispecific antibodies with the Fc region and CD3 and antigen-binding regions depicted. The second panel (B) shows the structure of CAR-T therapies with the target scFv and spacer regions and costimulatory and CD3 signaling domains depicted.
Structure, mechanism of action, and preclinical evidence of T-cell–redirecting bispecific antibodies
Talquetamab
Talquetamab is an off-the-shelf, full-sized, humanized, T-cell–redirecting IgG4 bispecific antibody targeting both GPRC5D and CD3 receptors [20, 21, 41, 42]. Talquetamab is the first GPRC5D-targeting agent approved for the treatment of patients with triple-class exposed relapsed/refractory MM [43, 44].
Preclinically, talquetamab promoted efficient T-cell activation and T-cell–mediated cytotoxicity across three different GPRC5D positive (+) cell lines (H929, MM.1 R, and OPM2), with no effect on GPRC5D-negative cell lines [20]. Additionally, talquetamab induced cell lysis in primary MM cells from bone marrow aspirates in newly diagnosed, daratumumab-naive relapsed/refractory, and daratumumab-refractory patients with MM [21]. This cell lysis was induced regardless of resistance to prior therapies or the presence of high-risk cytogenetic abnormalities [21].
Forimtamig
Forimtamig is a T-cell–redirecting bispecific antibody that binds to CD3 on T cells and GPRC5D on plasma cells [28, 45]. It has a unique 2:1 (GPRC5D:CD3) configuration for hypothetical increased potency versus a 1:1 configuration [28, 45, 46]. Dual binding induces T-cell–directed killing of plasma cells [28, 45].
Preclinically, forimtamig exhibited cytotoxicity against all GPRC5D + MM cell lines against which it was tested [47]. In an autologous ex vivo model of MM samples using total bone marrow aspirates from newly diagnosed patients with MM, forimtamig increased cytotoxicity potency. Forimtamig also inhibited growth of established NCI-H929 xenograft tumors in humanized mice after repeat dosing and eradicated tumors at doses of 0.1–10.0 mg/kg.
CAR-T therapies
CAR-T cells have been described as a breakthrough in cancer immunotherapy, revolutionizing the treatment of B-cell malignancies and improving prognosis in patients with relapsed/refractory MM [48,49,50]. CAR-T therapy uses a patient’s own T cells, engineered to target tumor cells through expression of CARs comprising an scFv, a CD3ζ T-cell receptor molecule, and a costimulatory domain (usually CD28 or 4-1BB) (Fig. 3B) [51].
CAR-T therapies are typically administered as a single dose [51]. GPRC5D-targeting CAR-T therapies in clinical development are MCARH109, OriCAR-017, and BMS-986393 (Table 1).
Structure, mechanism of action, and preclinical evidence of CAR-T therapies
MCARH109, OriCAR-017, and BMS-986393
MCARH109 is a second-generation CAR-T therapy with a human B-cell–derived anti-GPRC5D single-chain variable fragment, a 4-1BB costimulatory domain, and CD3 signaling domain [29]. OriCAR-017 is an autologous, second-generation, GPRC5D-targeting CAR-T therapy [52, 53]. The proprietary signal activation domain element Ori is hypothesized to improve CAR-T expansion and durability [53]. BMS-986393 is a GPRC5D-targeting autologous CAR-T therapy that has the same structure as MCARH109 [54].
The in vitro and in vivo antitumor activity of GPRC5D CAR-T cells has been shown in a preclinical study of a CAR-T therapy incorporating a human-derived GPRC5D-targeted scFv clone 109 (GPRC5D[109]) using MM cell lines [25]. GPRC5D(109), which is a precursor to MCARH109 [29], eradicated primary MM cells obtained via bone marrow aspirate and other MM cell lines with a range of GPRC5D mRNA expression profiles [25].
Clinical data
In total, ten clinical trials of GPRC5D-targeting T-cell–redirecting agents are either completed or ongoing (Table 1). Of these, five trials have published results, which are summarized below: MonumenTAL-1 (NCT03399799/NCT04634552), NCT04557150, NCT04555551, POLARIS (NCT05016778), and NCT04674813 (Tables 1, 2).
Safety
Talquetamab
In MonumenTAL-1, patients had a median of five prior LOT, and 29% and 23% of patients treated with the subcutaneous (SC) recommended phase 2 doses (RP2Ds) of 0.4 mg/kg weekly (QW; [n = 143]) and 0.8 mg/kg biweekly (Q2W; [n = 145]), respectively, were penta-drug refractory [42]. In the 0.4 mg/kg QW and 0.8 mg/kg Q2W cohorts, 31% and 29% had high-risk cytogenetics, respectively [42].
A summary of safety results across trials of GPRC5D-targeting T-cell–redirecting agents, including from the MonumenTAL-1 trial [42], is shown in Table 3. In MonumenTAL-1, there were low rates of discontinuation due to adverse events (AEs) observed with 0.4 mg/kg QW and 0.8 mg/kg Q2W dosing (5% and 6%, respectively); 8% and 14% had dose delays due to AEs, and 15% and 6% had dose reductions due to AEs. No patients died due to drug-related AEs [42].
The most common grade 3/4 AEs were hematologic: anemia, neutropenia, lymphopenia, and thrombocytopenia [42]. Cytopenias were mostly confined to the first few cycles. The most common non-hematologic AEs were cytokine release syndrome (CRS), skin-related events, nail-related events, and dysgeusia. Dermatologic AEs were mostly grade 1/2. Grade 3/4 rashes occurred in two patients (1%) at 0.4 mg/kg QW and eight patients (6%) at 0.8 mg/kg Q2W, and one patient (1%) had a grade 3/4 skin-related AE in the 0.8 mg/kg Q2W cohort. Oral-related AEs included dysgeusia, dry mouth, and dysphagia, which were mostly grade 1/2, except for three (2%) patients in the 0.8 mg/kg Q2W cohort who had grade 3 dysphagia. Any-grade weight loss was experienced by 40% of patients in the 0.4 mg/kg QW cohort and 32% of patients in the 0.8 mg/kg Q2W cohort [42].
CRS events were mostly grade 1/2 and were largely confined to step-up doses and the first full dose. Median time to onset of CRS was 48 h, with a median duration of 48 h in both cohorts [42]. In total, 74% and 69% of patients in each cohort, respectively, received supportive measures for CRS, most commonly tocilizumab (35% and 37%, respectively). Immune effector cell–associated neurotoxicity syndrome (ICANS), which was assessed in phase 2 only, occurred in 11% and 10% of patients treated with each dose, respectively, and were mostly grade 1/2. Across both doses, 7%–8% of patients received supportive measures for ICANS, including tocilizumab and corticosteroids. Most patients (86% and 79% in each cohort, respectively) recovered from ICANS [42].
Grade 3/4 infections occurred in 17% of patients treated with 0.4 mg/kg QW and in 12% of patients treated with 0.8 mg/kg Q2W [42]. Opportunistic infections occurred in 4% of patients in the 0.4 mg/kg QW cohort and in 3% patients in the 0.8 mg/kg Q2W cohort. COVID-19 occurred in 9% of patients in the 0.4 mg/kg QW cohort and 11% in the 0.8 mg/kg Q2W cohort, which led to death in two patients. Intravenous (IV) immunoglobulin support was low with 0.4 mg/kg QW (13%) and 0.8 mg/kg Q2W (10%) dosing [42].
Forimtamig
In the phase 1 trial of forimtamig, 51 patients received IV forimtamig 18–10,000 µg and 57 patients received SC forimtamig 1200−7200 µg, preceded by step-up doses [45]. Patients had a median of five prior LOT in the IV cohort and four prior LOT in the SC cohort; 36% and 42% of patients were penta-drug refractory in each cohort, respectively. High-risk cytogenetics were observed in 47% of patients in both cohorts [45].
In the IV and SC cohorts, respectively, six (12%) and two (4%) patients had AEs leading to dose reductions; three (6%) and five (9%) patients had an AE leading to forimtamig discontinuation [45]. In the IV cohort, three patients died due to AEs (Escherichia coli sepsis, malignant neoplasm, and pneumonia aspiration; none considered related to treatment), and two patients died due to AEs in the SC cohort (COVID-19, considered unrelated to treatment, and acute respiratory failure, considered related to treatment). CRS was a commonly reported AE, was generally low grade, confined to the first cycle, and had a median time to onset of 5 h in the IV cohort and 24 h in the SC cohort; most CRS events resolved (98%). Twenty-seven (53%) and 14 (26%) patients required corticosteroids in the IV and SC cohorts, respectively, to manage CRS; 20 (39%) and 14 (26%) were treated with tocilizumab, respectively. Single vasopressors were administered to one patient in each cohort. Central nervous system toxicity consistent with ICANS was observed in five patients (10%) in the IV cohort (headache [n = 3], including one grade ≥3 event; confusion [n = 1]; and muscular weakness [n = 2]) and seven (12%) patients in the SC cohort (headache [n = 1]; confusion [n = 2]; insomnia [n = 2]; encephalopathy [n = 1]; ICANS [n = 1], which was grade ≥3; and syncope [n = 1], which was grade ≥3). Hematologic AEs were common in both cohorts (Table 3). AEs related to GPRC5D expression on non-myeloma cells were mostly grade 1/2, including skin-related AEs, mucosal AEs, and hair and nail changes [45].
MCARH109
The phase 1 trial of MCARH109 enrolled 17 patients, many of whom with high-risk features [29]. The median number of prior LOT was six, and ten (59%) patients received prior BCMA-targeting therapies, including eight (47%) who received prior BCMA CAR-T therapy. In total, 76% had high-risk cytogenetics [29].
All patients had ≥1 AE that emerged or worsened after MCARH109 infusion (Table 3) [29]. Dose-limiting toxicities were observed in patients who received the 450 × 106 CAR-T cells dose (one patient had three grade 4 events: CRS, ICANS, and macrophage activation syndrome; two patients had grade 3 cerebellar disorder possibly related to MCARH109) [29]. Grade 3/4 neutropenia, thrombocytopenia, and anemia occurred in 100%, 65%, and 41% of patients, respectively. Non-hematologic grade 3/4 events were uncommon. Two patients (12%) had grade 3 infections (bacterial infection and parvovirus infection).
Time-limited on-target, off-tumor toxic effects related to GPRC5D expression in the skin and keratinized tissue manifested as grade 1 nail changes, including nail loss in 11 patients (65%) (at all dose levels), grade 1 rashes, and grade 1 dysgeusia or dry mouth. The median time from MCARH109 infusion to nail changes was 3.3 months, and nail changes resolved in 10/11 patients (91%) without intervention. Rash was treated with topical glucocorticoids or observation alone, and symptoms resolved; dysgeusia resolved in both patients without intervention [29].
OriCAR-017
In the phase 1 POLARIS trial, OriCAR-017 was administered to ten patients: nine in the dose-escalation phase (n = 3 each to 1 × 106, 3 × 106, and 6 × 106 CAR-T cells per kg) and one in the dose-expansion phase (3 × 106 CAR-T cells per kg) [52]. In total, 60% of patients had high-risk cytogenetics, 40% had baseline extramedullary lesions, and 80% had ISS stage II/III disease; median number of previous LOT was 5.5, and all patients were refractory to both proteasome inhibitors and immunomodulatory drugs. In total, 50% of patients had received prior anti-BCMA CAR-T therapy [52].
The most common grade 3/4 AEs were neutropenia, thrombocytopenia, leukopenia, and anemia (Table 3) [52]. On-target, off-tumor AEs related to GPRC5D were grade 1 nail disorders, grade 1/2 pruritus, and grade 2 dry skin. Nail disorders and dry skin resolved without intervention, and pruritus resolved with antipruritic treatment [52].
All patients experienced CRS events (all grade 1/2), which was grade 1 in nine (90%) patients [52]. All CRS cases were rapidly relieved with intervention with tocilizumab (n = 4), glucocorticoids (n = 1), or tocilizumab plus glucocorticoids (n = 3). Median time to CRS onset was 48 h, which progressively became shorter with increasing infusion doses, and median duration was 144 h (6 days). No neurological toxicities were reported. No dose-limiting toxicities were observed during the dose-escalation phase, and no deaths due to AEs were reported [52].
BMS-986393
The phase 1, multicenter, open-label trial of BMS-986393 enrolled 33 patients who received doses of 25 (n = 6), 75 (n = 9), 150 (n = 11), 300 (n = 6), and 450 (n = 1) × 106 CAR-T cells [54]. In total, 49% (n = 16) had high-risk cytogenetics and 46% (n = 15) had baseline extramedullary lesions. In total, 55% (n = 18) had received prior BCMA-targeting therapies, 39% (n = 13) of whom received prior BCMA-targeting CAR-T therapy [54].
Treatment-emergent AEs were experienced by 29 patients (88%), which were grade 3/4 in 24 (73%) [54]. There were two dose-limiting toxicities in the groups that received 25 × 106 and 75 × 106 CAR-T cells: grade 4 neutropenia and/or thrombocytopenia. There were no deaths related to treatment [54].
The most common treatment-emergent AEs were predominantly hematologic: neutropenia, thrombocytopenia, and anemia [54] (Table 3). On-target, off-tumor AEs included skin-related AEs, dysgeusia/taste disorders, nail disorders, and dysphagia, which were all grade 1/2, and did not require management in most patients (79%). CRS was a commonly reported AE and was mostly grade 1/2. The median onset to CRS was day 3 and median duration was 4 days. Only two patients (6%) had ICANS, with both events being grade 1/2. A total of ten patients (30%) had headache. There were no cerebellar events [54].
GPRC5D-related AEs and management
A unique pattern of AEs has been reported in several trials of GPRC5D-targeting T-cell–redirecting therapies in MM (Table 3) [29, 42, 45, 52, 54]. Overall, most AEs were low grade and clinically manageable with appropriate dose modifications and supportive care. Given the novelty of these therapies, guidance on clinical management is required to ensure patients maintain quality of life and adherence to treatment. Consensus on the grouping of GPRC5D-specific AEs is required, given the differences observed across the five trials (Table 3).
Cross-trial comparisons are challenging in the context of the five reviewed agents, owing to differences in the grouping of some AEs and the differences in dosing for the bispecific antibodies as well as between CAR-T therapies and bispecific antibodies (a single infusion versus continuous dosing). Most skin-, nail-, and taste-related AEs were low grade, rarely required dose modifications, and were manageable [29, 41, 42, 45, 52, 54]. Rashes were responsive to emollients, and topical and oral corticosteroids in MonumenTAL-1, and topical glucocorticoids in the trial of MCARH109; in both trials, rashes did not result in study drug discontinuation [29, 41]. Supportive care for rash in MonumenTAL-1 enabled patients to continue to receive talquetamab without dose modification [41]. Most cases of dry mouth and dysphagia were grade 1/2 [29, 42, 45, 54]. Dysgeusia has a maximum severity of grade 2 per Common Terminology Criteria for Adverse Events guidelines (grade 2 defined as altered taste with change in diet [e.g., oral supplements]; noxious or unpleasant taste; loss of taste). Dysgeusia was a commonly reported AE but appeared to have a higher incidence with bispecific antibodies compared with CAR-T therapies [29, 42, 54]. Dysgeusia was managed with supportive care and, at times, dose adjustments [41, 42, 55]. It is unknown whether dysgeusia is a direct on-target, off-tumor effect because GPRC5D immunoreactivity in salivary glands is limited to resident plasma cells, and while GPRC5D is also expressed on filiform papillae, they are not responsible for taste [25, 41]. However, the larger class of family C metabotropic G-protein coupled receptors include taste receptors [56], which may explain the taste-associated AEs experienced with GPRC5D-targeting T-cell–redirecting therapies. Physicians should be mindful of the potential impact of oral toxicities on weight management and ensure that patients receive adequate nutritional support; weight-based medications, such as hypotensive and hypoglycemic drugs, may require dose adjustment. Regular dental check-ups should be encouraged. The impact of oral toxicities on quality of life has not been assessed, as commonly used questionnaires do not evaluate these toxicities; as such, assessing the impact of oral toxicities on quality of life is a question of high clinical interest. Nail-related AEs were reported frequently, were low grade, likely related to on-target activity, and while often resolved without intervention, were sometimes managed with emollients, nail hardeners, vitamin E oil, as well as hydration, biotin, and protective nail coverings based on investigator experience from the MonumenTAL-1 trial [29, 41, 42, 45, 52]. Differences in observed rates of GPRC5D-related AEs between bispecific antibodies and CAR-T therapies may be due to continuous dosing versus single-infusion dosing, different epitopes, or differences in drug distribution. Overall, the pattern of AEs did not always correspond with the location of keratinized tissue. Additional studies are ongoing to understand the underlying mechanisms involved in skin- and oral-related toxicities and to define management strategies for these AEs. Based on the experiences of patients treated with talquetamab, GPRC5D-targeting T-cell–redirecting therapies result in sustained clinically meaningful improvements in quality of life, despite the occurrence of these AEs throughout the course of treatment [57].
T-cell redirection–related AEs and management
CRS was commonly reported for all GPRC5D-targeting T-cell–redirecting therapies [29, 42, 45, 52, 54]. In trials of talquetamab and forimtamig, CRS mitigation strategies were incorporated into trial designs. For MonumenTAL-1, pre-treatment with a glucocorticoid, antihistamine, and paracetamol was required prior to all step-up doses and the initial full dose, as well as hospitalization for initial treatment doses [41, 42]. For forimtamig, CRS mitigation strategies included step-up dosing, use of corticosteroids pre-medication during cycle 1, and hospitalization for cycle 1 doses [28, 45]. Common supportive measures for CRS included tocilizumab, corticosteroids, and rarely vasopressors [29, 42, 45, 52]. ICANS occurred in approximately 10% of patients in both MonumenTAL-1 and the forimtamig trial [42, 45]; no patients in the OriCAR-017 trial had neurological toxicities. In the MCARH109 trial, one patient experienced ICANS and two patients had a grade 3 cerebellar disorder [29, 52]. Further investigation is required to better understand the presence of rare cerebellar events with CAR-T therapies, as they have not been reported with bispecific antibodies [29]; however, this observation may be due to differences in drug distribution between the two therapy types.
Infections have been reported with the use of T-cell–redirecting bispecific antibodies; however, infections were reported less frequently in trials of GPRC5D-targeting T-cell–redirecting bispecific antibodies compared with trials of BCMA- and Fc receptor-like protein 5–targeting bispecific antibodies [58,59,60,61], which may be due to the more limited expression profile of GPRC5D in the immune compartment compared with the expression profiles of these other target antigens [20, 21, 25,26,27]. Additionally, the lower expression of GPRC5D on normal plasma cells compared with malignant plasma cells may preserve normal plasma cells during treatment [21, 59]. This is underpinned by data showing that GPRC5D-targeting bispecific antibodies do not lead to a reduction in CD19 + B-cell levels over time based on experience with talquetamab [59]. Additionally, there was no decrease in IgG over time with talquetamab; this may mean that IV immunoglobulin is required less frequently with GPRC5D-targeting T-cell–redirecting bispecific antibodies compared with bispecific antibodies targeting other antigens [59].
Efficacy
Talquetamab
The key efficacy results of the MonumenTAL-1 trial, as well as from the trials of the other reviewed therapies, are summarized in Table 4 [42]. Overall response rate (ORR) was 74% (106/143) for 0.4 mg/kg QW and 73% (106/145) for 0.8 mg/kg Q2W. Median time to first confirmed response was 1.2 months for the 0.4 mg/kg QW cohort and 1.3 months for the 0.8 mg/kg Q2W cohort. Most responders had a very good partial response (VGPR) or better (59% and 57%, respectively). In patients who received prior T-cell–redirection therapies, ORR was 63% (72% in patients with prior CAR-T therapy; 44% in patients with prior bispecific antibody therapy). ORR in other key patient subgroups (0.4 mg/kg QW and 0.8 mg/kg Q2W, respectively) were 73% and 71% in triple-class refractory patients, and 71% in both groups in penta-drug refractory patients. ORR was consistent across other subgroups, including number of prior therapies, refractoriness to prior therapy, prior belantamab exposure, and baseline cytogenetic risk, except among patients with baseline plasmacytomas. Median duration of response was 9.3 and 13.0 months in the two cohorts, respectively, and 12.7 months in patients who received prior T-cell–redirection therapies [42].
Forimtamig
In the IV cohort, ORR was 71%, and 59% of patients achieved ≥VGPR; ORR in the SC cohort was 64%, with 53% of patients achieving a ≥VGPR [45]. Of the ten and 11 patients with prior anti-BCMA exposure in the IV and SC cohorts, respectively, five (50%) and six (55%) had a response. Median time to first response was 1.4 and 1.6 months in each cohort, respectively. Median DOR was 10.8 months in the IV cohort and 12.5 months in the SC cohort. At data cut-off, responses were still ongoing in 23/35 (66%) patients in the IV cohort and in 25/35 (71%) patients in the SC cohort [45].
MCARH109
In the overall population (n = 17), 71% of patients had a response [29]. Of these, 12 (71%) patients had a partial response (PR) or better, ten (59%) had VGPR or better, and six (35%) had a complete response (CR)/stringent CR (sCR) [29]. A ≥PR was observed in 7/10 patients (70%) who received prior BCMA-targeting therapies and received MCARH109 across all four dose levels, and in 3/6 patients (50%) treated at doses of 25 × 106 to 150 × 106 CAR-T cells [29].
OriCAR-017
In the overall population (n = 10), ORR was achieved by 100% of patients, including six patients (60%) with sCR and four patients (40%) with VGPR [52]. Median time to best response was 3.1 months and median time to ≥CR was 4.1 months. Of the five patients who relapsed following prior BCMA-targeting CAR-T therapy, two had sCR and three had VGPR [52].
BMS-986393
In the overall efficacy population (N = 19), 17 patients (90%) achieved an ORR [54]. Of these, six patients achieved sCR, three patients achieved a CR, and one patient achieved a VGPR [54].
Pharmacokinetics, pharmacodynamics, and immunogenicity
Data for talquetamab RP2Ds and forimtamig showed that both were associated with consistent T-cell activation and cytokine production, including interleukin-6 [42, 62]. Forimtamig also showed bone marrow infiltration and MM cell depletion early after treatment [62]. Preliminary data with forimtamig suggest that both SC and IV formulations led to similar safety and efficacy [45], although SC administration induced delayed and lower cytokine release compared with IV infusion [45]. In comparison, SC formulation of talquetamab, which is considered more convenient for patients and healthcare providers [63, 64], had a favorable pharmacokinetic (PK) profile compared with the IV formulation [42]. PK profiles were comparable across both talquetamab RP2Ds, with mean concentration-time profiles that were comparable between QW and Q2W dosing and maintained at or above the maximum EC90 values identified in an ex vivo cytotoxicity assay [42]. Anti-drug antibodies were detected in 20% of patients, which did not appear to impact safety, efficacy, or PK [42]. Immunogenicity data are not yet available with forimtamig.
CAR-T cell expansion was observed with MCARH109, OriCAR-017, and BMS-986393, which was dose dependent [29, 52, 54]. CAR-T cells were durable over time, with 90% of patients showing detectable OriCAR-017 at 1 month, 70% at 3 months, and 50% at 6 months [52]. Persistent MCARH109 was detected in the peripheral blood at 4 and 24 weeks in 100% of patients and at 52 weeks in 50% of patients [29]. BMS-986393 reduced soluble BCMA levels across all doses [54].
Future directions
Additional trials are currently underway investigating the safety and efficacy of combining GPRC5D-targeting T-cell–redirecting agents with other anti-myeloma agents in patients with relapsed/refractory MM. These include trials evaluating the use of talquetamab in combination with teclistamab, daratumumab, pomalidomide, an anti-programmed cell-death protein-1 antibody, carfilzomib, and lenalidomide. MCARH109 is being evaluated in combination with MCARH125, a BCMA-targeting CAR-T therapy. The relatively low rates of high-grade cytopenia and severe infections, including infections leading to death, observed with these therapies and non-overlapping AE profiles with conventional MM agents may make GPRC5D-targeting bispecific antibodies versatile combination partners. With regard to the GPRC5D-associated AEs, further research is needed to assess the effectiveness of dose modification strategies, including reduced dose frequency and fixed duration dosing, as well as other mitigation measures, to more optimally balance on-target, off-tumor toxicity with the promising efficacy observed with these therapies. In addition to these trials, CAR–natural killer, bispecific–natural killer cell engager, and antibody-drug conjugate therapies may also offer advancements in treatment outcomes [65,66,67,68].
Additional trials are needed to determine the sequence in which GPRC5D-targeting T-cell–redirecting agents should be placed in the MM treatment paradigm. Based on the MonumenTAL-1 trial, efficacy outcomes in patients with prior T-cell redirection treatment were not as promising as in patients without prior T-cell redirection exposure; however, efficacy data with talquetamab in combination with either daratumumab or teclistamab in patients with prior exposure to T-cell redirection therapy is promising [31, 69]. Immune correlative analyses will be important to understand the impact of GPRC5D-targeting T-cell–redirecting therapies on immune system health, as will understanding mechanisms of resistance [33, 34]. As GPRC5D mRNA is expressed in samples from patients with smoldering MM, GPRC5D-targeting T-cell–redirecting therapies may provide clinical benefit in patients with pre-malignant disease, although the safety profile should be considered in relation to disease state and outcomes. Investigation into the use of GPRC5D-targeting T-cell–redirecting agents in the treatment of other plasma cell diseases is needed.
Conclusions
GPRC5D is a validated target for MM therapies. In early phase trials, GPRC5D-targeting T-cell–redirecting agents have shown promising efficacy and manageable safety profiles, which need to be confirmed in phase 3 trials. GPRC5D-targeting T-cell–redirecting therapies, as monotherapy or in combination with other anti-myeloma agents, will expand the number of treatment options available for patients with MM. These therapies may provide options for patients who may need treatment with a novel mechanism of action that preserves BCMA-targeting therapy for later LOT, have experienced suboptimal response or antigen loss with other agents, or for patients who exhibit clonal heterogeneity [18, 26]. However, the optimal treatment sequence for patients with MM will need to be elucidated.
Data availability
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparancy. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Change history
06 March 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41408-024-01018-6
References
Cowan AJ, Allen C, Barac A, Basaleem H, Bensenor I, Curado MP, et al. Global burden of multiple myeloma: a systematic analysis for the global burden of disease study 2016. JAMA Oncol. 2018;4:1221–7. https://doi.org/10.1001/jamaoncol.2018.2128
de Mel S, Lim SH, Tung ML, Chng WJ. Implications of heterogeneity in multiple myeloma. Biomed Res Int. 2014;2014:232546 https://doi.org/10.1155/2014/232546
Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–60. https://doi.org/10.1056/NEJMra1011442
Cancer stat facts: myeloma. 2023. https://seer.cancer.gov/statfacts/html/mulmy.html.
Globocan 2020 world fact sheet: world. 2020. https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf
He J, Schmerold L, Van Rampelbergh R, Qiu L, Potluri R, Dasgupta A, et al. Treatment pattern and outcomes in newly diagnosed multiple myeloma patients who did not receive autologous stem cell transplantation: a real-world observational study: treatment pattern and outcomes in patients with multiple myeloma. Adv Ther. 2021;38:640–59. https://doi.org/10.1007/s12325-020-01546-0
Cowan AJ, Green DJ, Kwok M, Lee S, Coffey DG, Holmberg LA, et al. Diagnosis and management of multiple myeloma: a review. JAMA. 2022;327:464–77. https://doi.org/10.1001/jama.2022.0003
Dimopoulos MA, Sonneveld P, Leung N, Merlini G, Ludwig H, Kastritis E, et al. International myeloma working group recommendations for the diagnosis and management of myeloma-related renal impairment. J Clin Oncol. 2016;34:1544–57. https://doi.org/10.1200/jco.2015.65.0044
Kamal M, Wang XS, Shi Q, Zyczynski TM, Davis C, Williams LA, et al. Symptom burden and its functional impact in patients with “symptomatic” relapsed or refractory multiple myeloma. Support Care Cancer. 2021;29:467–75. https://doi.org/10.1007/s00520-020-05493-y
Ramsenthaler C, Kane P, Gao W, Siegert RJ, Edmonds PM, Schey SA, et al. Prevalence of symptoms in patients with multiple myeloma: a systematic review and meta-analysis. Eur J Haematol. 2016;97:416–29. https://doi.org/10.1111/ejh.12790
Ramsenthaler C, Osborne TR, Gao W, Siegert RJ, Edmonds PM, Schey SA, et al. The impact of disease-related symptoms and palliative care concerns on health-related quality of life in multiple myeloma: a multi-centre study. BMC Cancer. 2016;16:427 https://doi.org/10.1186/s12885-016-2410-2
Schluterman KO, Fassas AB, Van Hemert RL, Harik SI. Multiple myeloma invasion of the central nervous system. Arch Neurol. 2004;61:1423–9. https://doi.org/10.1001/archneur.61.9.1423
Terpos E, Ntanasis-Stathopoulos I, Gavriatopoulou M, Dimopoulos MA. Pathogenesis of bone disease in multiple myeloma: from bench to bedside. Blood Cancer J. 2018;8:7 https://doi.org/10.1038/s41408-017-0037-4
Zaleta AK, Miller MF, Olson JS, Yuen EYN, LeBlanc TW, Cole CE, et al. Symptom burden, perceived control, and quality of life among patients living with multiple myeloma. J Natl Compr Canc Netw. 2020;18:1087–95. https://doi.org/10.6004/jnccn.2020.7561
Fonseca R, Usmani SZ, Mehra M, Slavcev M, He J, Cote S, et al. Frontline treatment patterns and attrition rates by subsequent lines of therapy in patients with newly diagnosed multiple myeloma. BMC Cancer. 2020;20:1087 https://doi.org/10.1186/s12885-020-07503-y
Paul B, Rodriguez C, Usmani SZ. BCMA-targeted biologic therapies: the next standard of care in multiple myeloma therapy. Drugs. 2022;82:613–31. https://doi.org/10.1007/s40265-022-01697-0
Rajkumar SV. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematol. 2020;95:548–67. https://doi.org/10.1002/ajh.25791
Bräuner-Osborne H, Jensen AA, Sheppard PO, Brodin B, Krogsgaard-Larsen P, O’Hara P. Cloning and characterization of a human orphan family C G-protein coupled receptor GPRC5D. Biochim Biophys Acta. 2001;1518:237–48. https://doi.org/10.1016/s0167-4781(01)00197-x
Inoue S, Nambu T, Shimomura T. The RAIG family member, GPRC5D, is associated with hard-keratinized structures. J Investig Dermatol. 2004;122:565–73. https://doi.org/10.1046/j.0022-202X.2004.12628.x
Pillarisetti K, Edavettal S, Mendonça M, Li Y, Tornetta M, Babich A, et al. A T-cell-redirecting bispecific G-protein-coupled receptor class 5 member D x CD3 antibody to treat multiple myeloma. Blood. 2020;135:1232–43. https://doi.org/10.1182/blood.2019003342
Verkleij CPM, Broekmans MEC, van Duin M, Frerichs KA, Kuiper R, de Jonge AV, et al. Preclinical activity and determinants of response of the GPRC5DxCD3 bispecific antibody talquetamab in multiple myeloma. Blood Adv. 2021;5:2196–215. https://doi.org/10.1182/bloodadvances.2020003805
Li J, Stagg NJ, Johnston J, Harris MJ, Menzies SA, DiCara D, et al. Membrane-proximal epitope facilitates efficient T cell synapse formation by anti-FcRH5/CD3 and is a requirement for myeloma cell killing. Cancer Cell. 2017;31:383–95. https://doi.org/10.1016/j.ccell.2017.02.001
Hipp S, Tai YT, Blanset D, Deegen P, Wahl J, Thomas O, et al. A novel BCMA/CD3 bispecific T-cell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo. Leukemia. 2017;31:1743–51. https://doi.org/10.1038/leu.2016.388
Goldsmith RB, Cornax I, Ma JY, Yao X, Peng P, Carreira V. Normal human tissue expression of G protein–coupled receptor class C group 5 member D (GPRC5D), a promising novel target for multiple myeloma, is restricted to plasma cells and hard keratinized tissues. Poster presented at IMW; September 8-11, 2021; Vienna, Austria.
Smith EL, Harrington K, Staehr M, Masakayan R, Jones J, Long TJ, et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci Transl Med. 2019;11:eaau7746 https://doi.org/10.1126/scitranslmed.aau7746
Kodama T, Kochi Y, Nakai W, Mizuno H, Baba T, Habu K, et al. Anti-GPRC5D/CD3 bispecific T-cell-redirecting antibody for the treatment of multiple myeloma. Mol Cancer Ther. 2019;18:1555–64. https://doi.org/10.1158/1535-7163.Mct-18-1216
Atamaniuk J, Gleiss A, Porpaczy E, Kainz B, Grunt TW, Raderer M, et al. Overexpression of G protein-coupled receptor 5D in the bone marrow is associated with poor prognosis in patients with multiple myeloma. Eur J Clin Investig. 2012;42:953–60. https://doi.org/10.1111/j.1365-2362.2012.02679.x
Hasselbalch Riley C, Hutchings M, Yoon S, Koh Y, Manier S, Facon T, et al. RG6234, a novel GPRC5D T-Cell engaging bispecific antibody, induces rapid responses in patients with relapsed/refractory multiple myeloma: preliminary results from a first-in-human trial. Oral presentation at EHA; June 9–12, 2022; Vienna, Austria.
Mailankody S, Devlin SM, Landa J, Nath K, Diamonte C, Carstens EJ, et al. GPRC5D-targeted CAR T cells for myeloma. N Engl J Med. 2022;387:1196–206. https://doi.org/10.1056/NEJMoa2209900
Frigyesi I, Adolfsson J, Ali M, Christophersen MK, Johnsson E, Turesson I, et al. Robust isolation of malignant plasma cells in multiple myeloma. Blood. 2014;123:1336–40. https://doi.org/10.1182/blood-2013-09-529800
Cohen YC, Morillo D, Gatt M, Sebag M, Kim K, Min CK, et al. First results from the RedirecTT-1 study with teclistamab + talquetamab simultaneously targeting BCMA and GPRC5D in patients with relapsed/refractory multiple myeloma Oral presentation at ASCO; June 2–6, 2023; Chicago, IL.
Truger MS, Duell J, Zhou X, Heimeshoff L, Ruckdeschel A, John M, et al. Single- and double-hit events in genes encoding immune targets before and after T cell-engaging antibody therapy in MM. Blood Adv. 2021;5:3794–8. https://doi.org/10.1182/bloodadvances.2021004418
Lee H, Ahn S, Maity R, Leblay N, Ziccheddu B, Truger M, et al. Mechanisms of antigen escape from BCMA- or GPRC5D-targeted immunotherapies in multiple myeloma. Nat Med. 2023;29:2295–306. https://doi.org/10.1038/s41591-023-02491-5
Derrien J, Gastineau S, Frigout A, Giordano N, Cherkaoui M, Gaborit V, et al. Acquired resistance to a GPRC5D-directed T-cell engager in multiple myeloma is mediated by genetic or epigenetic target inactivation. Nat Cancer. Published online September 1, 2023. https://doi.org/10.1038/s43018-023-00625-9.
Sedykh SE, Prinz VV, Buneva VN, Nevinsky GA. Bispecific antibodies: design, therapy, perspectives. Drug Des Devel Ther. 2018;12:195–208. https://doi.org/10.2147/dddt.S151282
Strohl WR, Naso M. Bispecific T-cell redirection versus chimeric antigen receptor (CAR)-T cells as approaches to kill cancer cells. Antibodies. 2019;8:41 https://doi.org/10.3390/antib8030041
Shah N, Chari A, Scott E, Mezzi K, Usmani SZ. B-cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches. Leukemia. 2020;34:985–1005. https://doi.org/10.1038/s41375-020-0734-z
Lejeune M, Köse MC, Duray E, Einsele H, Beguin Y, Caers J. Bispecific, T-cell-recruiting antibodies in B-cell malignancies. Front Immunol. 2020;11:762 https://doi.org/10.3389/fimmu.2020.00762
Wang Q, Chen Y, Park J, Liu X, Hu Y, Wang T, et al. Design and production of bispecific antibodies. Antibodies. 2019;8:43 https://doi.org/10.3390/antib8030043
Engelberts PJ, Hiemstra IH, de Jong B, Schuurhuis DH, Meesters J, Beltran Hernandez I, et al. DuoBody-CD3xCD20 induces potent T-cell-mediated killing of malignant B cells in preclinical models and provides opportunities for subcutaneous dosing. EBioMedicine. 2020;52:102625 https://doi.org/10.1016/j.ebiom.2019.102625
Chari A, Minnema MC, Berdeja JG, Oriol A, van de Donk N, Rodríguez-Otero P, et al. Talquetamab, a T-cell-redirecting GPRC5D bispecific antibody for multiple myeloma. N Engl J Med. 2022;387:2232–44. https://doi.org/10.1056/NEJMoa2204591
Chari A, Touzeau C, Schinke C, Minnema MC, Berdeja JG, Oriol A, et al. Talquetamab, a G protein-coupled receptor family C group 5 member D × CD3 bispecific antibody, in patients with relapsed/refractory multiple myeloma: phase 1/2 results from MonumenTAL-1. Oral presentation at ASH; December 10-13, 2022; New Orleans, LA.
U.S. Food and Drug Administration. TALVEY™ (talquetamab-tgvs) prescribing information. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761342s000lbl.pdf.
European Medicines Agency. Summary of opinion on Talvey (talquetamab). 2023. https://www.ema.europa.eu/en/documents/smop-initial/chmp-summary-positive-opinion-talvey_en.pdf.
Carlo-Stella C, Mazza R, Manier S, Facon T, Yoon S, Koh Y, et al. Forimtamig (RG6234), a GPRC5DxCD3 T cell engaging bispecific antibody, is highly active in patients with relapsed/refractory multiple myeloma: updated intravenous and first subcutaneous results from a Phase I dose escalation study. Oral presentation at ASH; December 10–13, 2022; New Orleans, LA.
Bacac M, Colombetti S, Herter S, Sam J, Perro M, Chen S, et al. CD20-TCB with obinutuzumab pretreatment as next-generation treatment of hematologic malignancies. Clin Cancer Res. 2018;24:4785–97. https://doi.org/10.1158/1078-0432.Ccr-18-0455
Eckmann J, Fauti T, Zabaleta A, Blanco L, Kassem S, Carrié N, et al. RG6234: a novel 2:1 GPRC5D T cell bispecific antibody exhibits best in class potential for the treatment of multiple myeloma as a monotherapy and in combination. Blood. 2022;140:2091–2. https://doi.org/10.1182/blood-2022-157485
Yang J, Zhou W, Li D, Niu T, Wang W. BCMA-targeting chimeric antigen receptor T-cell therapy for multiple myeloma. Cancer Lett. 2023;553:215949 https://doi.org/10.1016/j.canlet.2022.215949
Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398:314–24. https://doi.org/10.1016/s0140-6736(21)00933-8
Munshi NC, Anderson LD Jr., Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384:705–16. https://doi.org/10.1056/NEJMoa2024850
Larson RC, Maus MV. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat Rev Cancer. 2021;21:145–61. https://doi.org/10.1038/s41568-020-00323-z
Zhang M, Wei G, Zhou L, Zhou J, Chen S, Zhang W, et al. GPRC5D CAR T cells (OriCAR-017) in patients with relapsed or refractory multiple myeloma (POLARIS): a first-in-human, single-centre, single-arm, phase 1 trial. Lancet Haematol. 2023;10:e107–e116. https://doi.org/10.1016/s2352-3026(22)00372-6
Oricell website. 2022. https://www.oricell.com/en/about/news/143.html.
Bal S, Kocoglu MH, Nadeem O, Htut M, Gregory T, Anderson LD, Jr., et al. Clinical activity of BMS-986393 (CC-95266), a G protein-coupled receptor class C group 5 member D (GPRC5D)-targeted chimeric antigen receptor (CAR) T cell therapy, in patients with relapsed and/or refractory (R/R) multiple myeloma (MM): first results from a phase 1, multicenter, open-label study. Oral presentation at ASH; December 10–13, 2022; New Orleans, LA.
van de Donk NW, Bahlis N, Mateos M, Weisel K, Dholaria B, Garfall AL, et al. Novel combination immunotherapy for the treatment of relapsed/refractory multiple myeloma: updated phase 1b results for talquetamab (a GPRC5D x CD3 bispecific antibody) in combination with daratumumab. Oral presentation at EHA; June 9–12, 2022; Vienna, Austria.
Rajkumar P, Cha B, Yin J, Arend LJ, Păunescu TG, Hirabayashi Y, et al. Identifying the localization and exploring a functional role for GPRC5C in the kidney. FASEB J. 2018;32:2046–59. https://doi.org/10.1096/fj.201700610RR
Touzeau C, Chari A, Schinke C, Minnema MC, Berdeja JG, Oriol A, et al. Health-related quality of life in patients with relapsed/refractory multiple myeloma treated with talquetamab, a G protein-coupled receptor family C group 5 member D x CD3 bispecific antibody: patient-reported outcomes from MonumenTAL-1. Oral presentation at ASH; December 10–13, 2022; New Orleans, LA.
Moreau P, Garfall AL, van de Donk N, Nahi H, San-Miguel JF, Oriol A, et al. Teclistamab in relapsed or refractory multiple myeloma. N Engl J Med. 2022;387:495–505. https://doi.org/10.1056/NEJMoa2203478
Rodríguez-Otero P, Schinke C, Chari A, Lipe B, Lavi N, Rasche R, et al. Analysis of infections and parameters of humoral immunity in patients with relapsed/refractory multiple myeloma treated with talquetamab monotherapy in MonumenTAL-1. Poster presented at ASCO; June 2–6, 2023; Chicago, IL.
Sim BZ, Longhitano A, Er J, Harrison SJ, Slavin MA, Teh BW. Infectious complications of bispecific antibody therapy in patients with multiple myeloma. Blood Cancer J. 2023;13:34 https://doi.org/10.1038/s41408-023-00808-8
Usmani SZ, Garfall AL, van de Donk N, Nahi H, San-Miguel JF, Oriol A, et al. Teclistamab, a B-cell maturation antigen × CD3 bispecific antibody, in patients with relapsed or refractory multiple myeloma (MajesTEC-1): a multicentre, open-label, single-arm, phase 1 study. Lancet. 2021;398:665–74. https://doi.org/10.1016/s0140-6736(21)01338-6
Dekhtiarenko I, Lelios I, Attig J, Sleiman N, Lazzaro D, Clausen I, et al. T-cell activation and myeloma cell killing confirm the mode of action of RG6234, a novel GPRC5D T-cell engaging bispecific antibody, in patients with relapsed/refractory multiple myeloma. Oral presentation at EHA; June 9–12, 2022; Vienna, Austria.
Bittner B, Richter W, Schmidt J. Subcutaneous administration of biotherapeutics: an overview of current challenges and opportunities. BioDrugs. 2018;32:425–40. https://doi.org/10.1007/s40259-018-0295-0
Sánchez-Félix M, Burke M, Chen HH, Patterson C, Mittal S. Predicting bioavailability of monoclonal antibodies after subcutaneous administration: Open innovation challenge. Adv Drug Deliv Rev. 2020;167:66–77. https://doi.org/10.1016/j.addr.2020.05.009
Cho SF, Xing L, Anderson KC, Tai YT. Promising antigens for the new frontier of targeted immunotherapy in multiple myeloma. Cancers. 2021;13:6136 https://doi.org/10.3390/cancers13236136
Nogami A, Sasaki K. Therapeutic advances in immunotherapies for hematological malignancies. Int J Mol Sci. 2022;23:11526 https://doi.org/10.3390/ijms231911526
Sasaki M, Suzuki K, Abe Y, Ito S, Nishiwaki K, Handa H, et al. Efficacy and safety of ixazomib plus lenalidomide and dexamethasone following injectable proteasome inhibitor-based therapy in patients with relapsed/refractory multiple myeloma. Poster presented at IMS; August 25–27, 2022; Los Angeles, CA.
Huang W, Luo J, Li Y, Fei D, Qin X, Li R. Abstract 6020: Preclinical activity of LM-305 targeting G-protein-coupled receptor class 5 member D (GPRC5D) antibody drug conjugate for the treatment of multiple myeloma. Cancer Res. 2022;82:6020 https://doi.org/10.1158/1538-7445.Am2022-6020
Dholaria B, Weisel K, Mateos MV, Goldschmidt H, Martin TG, Morillo D, et al. Talquetamab + daratumumab in patients with relapsed/refractory multiple myeloma: updated TRIMM-2 results. Oral presentation at ASCO; June 2–6, 2023; Chicago, IL.
Acknowledgements
Editorial and medical writing support was provided by Claire Line, PhD, and Craig Turner, MSc, of Eloquent Scientific Solutions, and was funded by Janssen Global Services, LLC.
Author information
Authors and Affiliations
Contributions
All authors participated in drafting or revising the manuscript, and all approved the final version for submission. All authors accept full responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Competing interests
PRO reports a consulting or advisory role with AbbVie, BMS, GlaxoSmithKline, Janssen, Pfizer, and Sanofi; is on the speakers’ bureau for BMS, GlaxoSmithKline, Janssen, and Sanofi; has received travel, accommodations, and expenses from Pfizer; and has received honoraria from AbbVie, Celgene, GlaxoSmithKline, H3 Biomedicine, Janssen, Pfizer, and Sanofi. NWCJvdD has received research funding from Amgen, BMS, Celgene, Cellectis, Janssen, and Novartis; and reports a consulting or advisory role with AbbVie, Adaptive Biotechnologies, Amgen, Bayer, BMS, Celgene, Janssen, Novartis, Pfizer, Roche, Servier, and Takeda. KP is an employee of Janssen Oncology and report stock and other ownership interests in Johnson & Johnson/Janssen. IC is an employee of Janssen Oncology and reports stock and other ownership interests in Johnson & Johnson/Janssen. DV is an employee of Janssen Oncology and reports stock and other ownership interests in Johnson & Johnson/Janssen. KG is an employee of Janssen Oncology and reports stock and other ownership interests in Johnson & Johnson/Janssen. BH is an employee of Janssen Oncology and reports stock and other ownership interests in Johnson & Johnson/Janssen. JT is an employee of Johnson & Johnson and reports stock and other ownership in Johnson & Johnson; and has received research funding from Janssen Research & Development. TR is an employee of Janssen Oncology and reports stock and other ownership interests in Johnson & Johnson/Janssen. TM is an employee of Janssen Research & Development and reports stock and other ownership interests in Johnson & Johnson. CH is an employee of Janssen Research & Development; reports stock and other ownership interests in Janssen Research & Development; and reports patents, royalties, and other intellectual property in Janssen Research & Development. CK is an employee of Janssen Research & Development; reports stock and other ownership interests in Janssen Research & Development; and has received travel, accommodation, and expenses from Janssen Research & Development. PM has served in a consulting/advisory role and has received honoraria from AbbVie, Amgen, Celgene, GlaxoSmithKline, Janssen, Oncopeptides, and Sanofi. NB has served in a consulting/advisory role for AbbVie, Amgen, Bristol Myers Squibb, Forus, Genentech, GlaxoSmithKline, Janssen, Pfizer, Sanofi, and Takeda; has received honoraria from AbbVie, Amgen, Bristol Myers Squibb, Forus, Janssen, Pfizer, and Sanofi; has received research funding from Pfizer; and has served as a member of an IRC and/or steering committee for AbbVie, GlaxoSmithKline, and Janssen. AC reports a consulting or advisory role for AbbVie, Antengene, BMS, Genentech, Genzyme, GlaxoSmithKline, Janssen Oncology, Karyopharm Therapeutics, OncoPeptides, Seagen, Secura Bio, Shattuck Bio, Shattuck Labs, and Takeda; and has received research funding from Amgen, Celgene, Janssen, Pharmacyclics, Seagen, and Takeda.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rodriguez-Otero, P., van de Donk, N.W.C.J., Pillarisetti, K. et al. GPRC5D as a novel target for the treatment of multiple myeloma: a narrative review. Blood Cancer J. 14, 24 (2024). https://doi.org/10.1038/s41408-023-00966-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-023-00966-9