Abstract
Bromodomain and extraterminal proteins (BET) play key roles in regulation of gene expression, and may play a role in cancer-cell proliferation, survival, and oncogenic progression. CC-90010-ST-001 (NCT03220347) is an open-label phase I study of trotabresib, an oral BET inhibitor, in heavily pretreated patients with advanced solid tumors and relapsed/refractory diffuse large B-cell lymphoma (DLBCL). Primary endpoints were the safety, tolerability, maximum tolerated dose, and RP2D of trotabresib. Secondary endpoints were clinical benefit rate (complete response [CR] + partial response [PR] + stable disease [SD] of ≥4 months’ duration), objective response rate (CR + PR), duration of response or SD, progression-free survival, overall survival, and the pharmacokinetics (PK) of trotabresib. In addition, part C assessed the effects of food on the PK of trotabresib as a secondary endpoint. The dose escalation (part A) showed that trotabresib was well tolerated, had single-agent activity, and determined the recommended phase 2 dose (RP2D) and schedule for the expansion study. Here, we report long-term follow-up results from part A (N = 69) and data from patients treated with the RP2D of 45 mg/day 4 days on/24 days off or an alternate RP2D of 30 mg/day 3 days on/11 days off in the dose-expansion cohorts (parts B [N = 25] and C [N = 41]). Treatment-related adverse events (TRAEs) are reported in almost all patients. The most common severe TRAEs are hematological. Toxicities are generally manageable, allowing some patients to remain on treatment for ≥2 years, with two patients receiving ≥3 years of treatment. Trotabresib monotherapy shows antitumor activity, with an ORR of 13.0% (95% CI, 2.8–33.6) in patients with R/R DLBCL (part B) and an ORR of 0.0% (95% CI, 0.0–8.6) and a CBR of 31.7% (95% CI, 18.1–48.1) in patients with advanced solid tumors (part C). These results support further investigation of trotabresib in combination with other anticancer agents.
Similar content being viewed by others
Introduction
Bromodomain and extraterminal proteins (BET) are epigenetic readers that bind to acetylated lysine residues on histones in chromatin1,2,3,4,5,6,7. The BET family comprises four proteins: BRD2, BRD3, BRD4, and BRDT1,2. BRD2, BRD3, and BRD4 are ubiquitously expressed, while BRDT expression in healthy tissue is restricted to the testes2,8,9. BET proteins play key roles in the regulation of gene expression, primarily through recruitment of the Mediator complex and positive transcription elongation factor b (p-TEFb) to acetylated histones. Aberrant acetylation of histones surrounding proto-oncogenes during oncogenesis and tumor progression suggests that BET proteins may play a role in cancer cell proliferation, survival, and oncogenic progression, providing a rationale for using BET inhibitors as anticancer drugs5,6,7,10,11,12,13,14,15,16,17. BET proteins, particularly BRD4, have been identified as having an oncogenic role in lymphomas and various solid tumors via effects on MYC and other transcription factors and signaling modulators12,17,18,19. MYC overexpression or rearrangement has been shown to be associated with poor prognosis in patients with diffuse large B-cell lymphoma (DLBCL)20.
Trotabresib (CC-90010; BMS-986378) is an orally administered, potent, reversible inhibitor of BET family members. CC-90010-ST-001 is a phase I, multicenter, open-label study to assess the safety, tolerability, pharmacokinetics (PK), and preliminary efficacy of trotabresib in patients with advanced or unresectable solid tumors or relapsed/refractory (R/R) DLBCL. The dose escalation (part A) of this trial evaluated a wide range of doses and treatment schedules, with the intention of optimizing the treatment-free interval in each treatment cycle to improve the tolerability of the dose selected for further evaluation in the expansion cohorts18. The recommended phase II dose (RP2D) was 45 mg/day 4 days on/24 days off, with 30 mg/day 3 days on/11 days off selected as an alternative RP2D18, based on the observed good tolerability, favorable PK, encouraging pharmacodynamics, and antitumor activity18. At the time of analysis, eight patients in part A remained on treatment with an ongoing response or stable disease (SD), of whom five subsequently remained on treatment for ≥24 cycles18,21.
Here, we report long-term follow-up of safety and efficacy from the dose escalation (part A) and, for the first time, present results from the dose-expansion cohorts (parts B and C), including evaluation of food effects on trotabresib safety and PK.
Results
Patients and treatment
Patients were enrolled at 11 study sites in France, Italy, Japan, and Spain. Patient enrollment in part A took place between July 31, 2017 and November 12, 2018, with a total of 69 patients enrolled and treated, of whom 67 had advanced or unresectable solid tumors and two had R/R DLBCL. Patient enrollment in part B began on June 18, 2019, and was ongoing at the time of the data cutoff; the last patient enrolled at the time of the data cutoff was enrolled on January 11, 2021. In total, 23 patients with R/R DLBCL were enrolled and treated in part B, of whom 19 received trotabresib 45 mg/day 4 days on/24 days off and four received an alternate RP2D dose and schedule of trotabresib 30 mg/day 3 days on/11 days off. The alternate RP2D was investigated further because it had comparable tolerability to 45 mg/day 4 days on/24 days off, delivered the same cumulative dose over a 28-day treatment cycle, and was potentially more suitable for the treatment of patients with aggressive tumors such as R/R DLBCL, due to more constant exposure and target engagement. Patient enrollment in Part C took place between October 21, 2019 and July 27, 2020, with a total of 41 patients with advanced solid tumors enrolled to evaluate the effects of food on the PK and safety profile of trotabresib 45 mg/day 4 days on/24 days off.
Details of patient disposition are shown in Fig. 1. At the time of preparing this report (June 16, 2022), one patient in part A, one patient in part B, and one patient in part C remained on treatment. Analysis of overall safety and efficacy was performed using a data cutoff of July 15, 2021, for parts A, B, and C; food effect during treatment cycles 1 and 2 in part C was evaluated using data with a cutoff of January 29, 2021.
Patient characteristics
Baseline patient demographics and characteristics are shown in Table 1. Patients in part A and patients with R/R DLBCL in part B had received a median (range) of 4 (1–9) prior treatment regimens, and patients in part C had received a median of 2 (1–8) prior treatment regimens. Compared with patients in part A and part C (median age 57 and 58 years, respectively), patients with R/R DLBCL in part B were older (median age 66 years), and a higher proportion had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 1 (70% vs 52% in part A and 37% in part C). Two patients with R/R DLBCL (9%) had double-hit lymphoma, and no patients had received prior chimeric antigen receptor T-cell therapy. Detailed DLBCL-specific patient and tumor characteristics are shown in Supplementary Table 1.
Safety
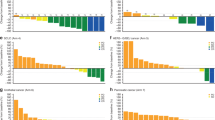
Safety and tolerability were the primary endpoints of the study. Safety results for part A at the most recent follow-up were consistent with the preliminary analysis reported previously18. In patients with R/R DLBCL in part B, any-grade and grade 3/4 TRAEs were reported in 23 (100%) and 21 (91%) patients, respectively (Fig. 2). The most common grade 3/4 TRAEs were hematologic, including thrombocytopenia (18 patients [78%]), anemia (six patients [26%]), and neutropenia (six patients [26%]). Serious TRAEs were reported in seven (30%) patients. Two (9%) patients with R/R DLBCL in part B died due to TEAEs that were not considered to be related to the study treatment. In part C, any-grade TRAEs were reported in 40 (98%) patients, and grade 3/4 TRAEs were reported in 24 (59%) patients. The most common grade 3/4 TRAE was thrombocytopenia, reported in seven (17%) patients. A TEAE leading to death was reported in one patient in part C, but was not considered to be related to the study treatment.
Grade 3 essential hypertension, hypokalemia, lipase increased, liver function test abnormal, presyncope, skin hemorrhage, and grade 4 blood creatinine phosphokinase increased, diabetes mellitus, and inappropriate antidiuretic hormone secretion were reported in one patient each in the overall part A population. Grade 3 abdominal infection, blood bilirubin increased, pneumonia and grade 4 blood creatinine increased, febrile neutropenia, leukopenia, and lymphopenia were reported in one patient each in the part B R/R DLBCL population. Grade 3 gamma-glutamyl transferase increased, hyperamylasemia, and syncope were reported in one patient each in the part C population. Source data are provided as a source data file. aNo grade 5 TRAEs were reported during the study. bNineteen patients received trotabresib 45 mg/day 4 days on/24 days off and four patients received trotabresib 30 mg/day 3 days on/11 days off. ALT alanine aminotransferase, DLBCL diffuse large B-cell lymphoma; R/R relapsed/refractory, TRAE treatment-related adverse event.
Details of TRAEs and all-cause TEAEs occurring at any-grade and grade 3–5 severity are shown in Supplementary Tables 2 and 3, respectively. A summary of dose modifications and treatment discontinuation due to adverse events is shown in Supplementary Table 4.
Efficacy
Preliminary efficacy was a secondary endpoint of the study. Response rates in part A were unchanged from those previously reported18. One patient with grade 2 diffuse astrocytoma had a durable complete response (CR) lasting 19 cycles before disease progression, and one patient with endometrial cancer had a confirmed partial response (PR) for 8 cycles. The objective response rate (ORR; CR or PR) was 2.9% (95% CI, 0.4–10.1) and the clinical benefit rate (CBR; CR, PR, or SD ≥4 months) was 17.4% (95% CI, 9.3–28.4). A total of five patients with various tumor types remained on treatment for ~2 years or longer (≥24 cycles) with sustained SD. One patient with thymic cancer remained on treatment for 3.1 years (38 cycles) before discontinuing due to disease progression, and treatment was ongoing in one patient with salivary gland cancer at cycle 53 as of June 16, 2022, with a total treatment duration of 4.0 years (Supplementary Fig. 1).
Of the 23 patients with R/R DLBCL treated in part B, 15 (65.2%) were evaluable for response (Fig. 3). Two patients (8.7%) had CR, one patient had PR (4.3%), and two patients had SD (8.7%), with one patient (4.3%) having SD ≥4 months, resulting in an ORR of 13.0% (95% CI, 2.8–33.6) and a CBR of 17.4% (95% CI, 5.0–38.8). PD was reported in 10 (43.5%) patients. One patient with Ann Arbor stage IVa transformed DLBCL with germinal center B-cell molecular subtype, positive BCL2 translocation, and negative MYC and BCL6 translocation remained on treatment at cycle 17 as of June 16, 2022, with a total treatment duration of 1.6 years. Of note, the patient had an initial PR at cycle 2 that subsequently deepened to CR at cycle 4 (Fig. 4b).
Source data are provided as a source data file. aConfirmed best response is presented for part C, defined as a consecutive response of the same or better that is at least 4 weeks apart, regardless of tumor type. bORR = CR + PR. cCBR = CR + PR + SD ≥4 months. CBR clinical benefit rate, CR complete response, DLBCL diffuse large B-cell lymphoma, ORR objective response rate, PR partial response, R/R relapsed/refractory, SD stable disease.
a Patients with R/R DLBCL in part B (n = 23) and b part C (n = 41). A swim plot showing the duration of treatment for patients in part A is shown in Supplementary Fig. 1. Source data are provided as a source data file. CR complete response, DLBCL diffuse large B-cell lymphoma, NE not evaluable, R/R relapsed/refractory, SD stable disease, PD progressive disease, PR partial response.
Of the 41 patients treated in part C, a total of 34 (82.9%) were evaluable for response (Fig. 3). No confirmed CRs or PRs were reported at the time of data cutoff (ORR 0.0% [95% CI, 0.0–8.6]). A total of 26 (45.5%) patients had a best response of SD, of whom 13 had SD of ≥4 months’ duration, resulting in a CBR of 31.7% (95%CI, 18.1–48.1). PD was reported in 8 (19.5%) patients. As of June 16, 2022, treatment was ongoing in a patient with treatment-refractory high-grade astrocytoma, with a duration of 2.5 years. A further seven patients with various tumor types remained on treatment for ≥1 year with sustained SD (Fig. 4c).
Imaging results showing representative responses to trotabresib in a patient with grade 2 astrocytoma enrolled in part A, a patient with germinal center immunophenotype R/R DLBCL enrolled in part B, and a patient with high-grade astrocytoma enrolled in part C are presented in Fig. 5a–c, respectively.
a MRI evaluation of a patient with grade 2 astrocytoma. The patient had a complete response on trotabresib (red arrows), with the disappearance of both enhancing and non-enhancing areas compared with baseline. The patient discontinued after 19 cycles of treatment due to disease progression. b FDG-PET/CT evaluation of a patient with germinal center B-cell immunophenotype R/R DLBCL who had a 57% reduction in target lesion size after two cycles of trotabresib (blue arrow). This was accompanied by the appearance of a new metabolic lesion (red arrow), and treatment was discontinued after four cycles of trotabresib due to disease progression. c MRI evaluation of a patient with refractory high-grade astrocytoma. The patient had a minor response on trotabresib with a 38% reduction in tumor size (red arrows), and the patient remained on study treatment at cycle 34 with a continued response as of June 16, 2022. CT computed tomography, DLBCL diffuse large B-cell lymphoma, FDG fluorodeoxyglucose, MRI magnetic resonance imaging, PET positron emission tomography, R/R relapsed/refractory. The two left-most images in panel a are reprinted from Annals of Oncology 31(6), Moreno V, et al. Phase I study of CC-90010, a reversible, oral BET inhibitor in patients with advanced solid tumors and relapsed/refractory non-Hodgkin’s lymphoma, Page 780–788, Copyright 2020, with permission from Elsevier.
Median overall survival (OS) and median progression-free survival (PFS) in the treated patient population are shown in Table 2. In part A, median OS and PFS were 189 days (95% CI, 161–258) and 57 days (95% CI, 52–81), respectively. In part B, median OS and PFS were 159 days (95% CI, 101 to not estimable [NE]) and 54 days (95% CI, 49–65), respectively. In part C, the median OS was 508 days (95% CI, 239–NE), and the median PFS was 114 days (95% CI, 84–239).
Pharmacokinetics and food effects
Food effects on the PK and safety profile of trotabresib were secondary endpoints of the study. A total of 15 patients completed both the fasted and fed treatment periods in part C and were included in the food effect–evaluable population for analysis of the effects of food on trotabresib PK (Table 3). Trotabresib showed generally comparable PK and interindividual variability in fed and fasted patients. Although peak plasma trotabresib concentration (Cmax) was higher and time-to-peak plasma trotabresib concentration (tmax) was shorter in fasted patients compared with fed patients, area under the plasma trotabresib concentration–time curve (AUC) from 0 to infinity and terminal half-life (t1/2) were similar in fasted and fed patients.
Details of TRAEs reported in patients in part C following fasted (n = 39) or fed (n = 35) administration of trotabresib during cycles 1 and 2 are shown in Supplementary Table 5. The incidence of TRAEs appeared generally similar in patients treated in both the fed and fasted state. Any-grade TRAEs were reported in 37 of 39 (95%) patients treated in the fasted state and 32 of 35 (91%) patients treated in the fed state, and grade 3/4 TRAEs were reported in 13 of 39 (33%) patients treated in the fasted state and seven of 35 (20%) patients treated in the fed state.
Pharmacodynamics
The pharmacodynamic effects of trotabresib in peripheral blood were assessed as an exploratory endpoint. Blood C-C motif chemokine receptor 1 (CCR1) mRNA levels were used as a pharmacodynamic marker of BET inhibition. A minimum of 50% reduction in levels of blood CCR1 mRNA has previously been shown to be associated with clinical response to a BET inhibitor in patients with R/R lymphoma22. Mean CCR1 mRNA levels were reduced to <50% of baseline at 4 h after the first and last dose of trotabresib 45 mg/day 4 days on/24 days off in part B (Fig. 6a), and reductions were sustained during the first 96 h of treatment (Fig. 6b). CCR1 modulation with 45 mg/day 4 days on/24 days off was similar in parts A and B18.
a Four hours after the first (left) and last dose (right) in part B, and b from 0–192 h in patients receiving trotabresib 45 mg/day 4 days on/24 days off. For both figures, colored data points indicate individual patient values for the percentage of baseline CCR1 expression and black lines and error bars indicate mean ± SD. Source data are provided as a source data file. aData not shown for one patient with a percentage of baseline CCR1 expression of 1110.93; SD for this time point was 260.36. CCR1 C-C motif chemokine receptor 1, mRNA messenger RNA.
Discussion
Effective new treatments for patients with advanced malignancies are urgently needed, particularly for those patients who have tumor types with limited treatment options or whose tumors are refractory to currently available therapies. BET inhibitors have a mechanism of action that differs from existing marketed drugs, and have demonstrated anticancer activity across a broad range of tumor types in preclinical models and patients with various solid and hematological malignancies10,23,24,25,26,27,28,29,30,31. However, clinical trials of first-generation BET inhibitors have generally shown high rates of treatment-related toxicities, particularly dose-limiting hematological and gastrointestinal adverse events, likely due to toxicities arising from the involvement of BET proteins in multiple processes required for cellular homeostasis and the specific features of these agents10,23,24,25,26,27,28,29,30,31. As first-generation BET inhibitors have typically had poor oral bioavailability and short half-lives, ranging from approximately 2 to 20 h, continuous daily dosing or schedules with relatively short treatment-free intervals (≤7 days) have been needed to produce antitumor activity10,23,24,25,26,27,28,29,30,31. Such treatment schedules may have contributed to the increased toxicity due to patients not having sufficient time off treatment to recover.
Trotabresib is a next-generation BET inhibitor with properties that provide potent and selective inhibition of BRD family proteins, as well as a PK profile that allowed evaluation of a range of dosing schedules in part A. Preliminary results of this study were encouraging, with trotabresib showing high oral bioavailability and a comparatively long t1/2 of ~60 h18. The biweekly and monthly dosing schedules selected for further evaluation showed antitumor activity and incorporated a relatively long treatment-free interval that was found to improve tolerability compared with schedules with more frequent dosing18.
Longer follow-up of the dose escalation (part A) and data from parts B and C confirmed that trotabresib was generally very well tolerated in heavily pretreated patients with advanced, unresectable solid tumors and R/R DLBCL. Most TRAEs were mild or moderate and were easily manageable with dose modifications and/or treatment interruption. The most frequent grade 3/4 TRAE was thrombocytopenia, which is a class effect of BET inhibitors32. The higher incidence of severe thrombocytopenia reported in patients with R/R DLBCL in part B compared with patients with advanced solid tumors in part C could be related to patients' underlying disease, poorer ECOG PS, older age, and extent of previous treatment. Importantly, thrombocytopenia was reversible with appropriate management.
Biomarker analysis using blood levels of CCR1 mRNA, a pharmacodynamic marker of BET inhibition evaluated in previous clinical trials22,33,34, indicated target engagement and dose/schedule dependency. Reduction of CCR1 expression to ≤50% of baseline levels has been associated with plasma BET inhibitor concentrations and selected response metrics in lymphoma in a previous study22. Analysis of blood CCR1 mRNA levels during trotabresib treatment in the current study showed a mean reduction of >50% after the first and last dose with trotabresib 45 mg/day 4 days on/24 days off in part B, with sustained inhibition of CCR1 below baseline during the first 96 h of treatment, providing further support to the observed antitumor activity and dose/schedule selection.
The preliminary efficacy of trotabresib monotherapy in patients with heavily pretreated advanced solid tumors and R/R DLBCL was supportive of antitumor activity, with one CR and one PR reported in part A (ORR 2.9%; 95% CI, 0.4–10.1), two CRs and one PR in part B (ORR 13.0; 95% CI, 2.8–33.6), and one minor response per Response Assessment in Neuro-Oncology (RANO) criteria in part C (ORR 0.0; 95% CI, 0.0–8.6). The longer follow-up of patients enrolled in part A allowed further characterization of responses in this cohort, with the patient with a CR having sustained response for 19 cycles. A total of five patients in part A remained on treatment for ≥24 cycles, including a patient who received 38 cycles of treatment and a patient who remains on treatment at cycle 53, corresponding to treatment durations of 3 and 4 years, respectively. One patient with high-grade astrocytoma in part C has a minor antitumoral response that is ongoing at cycle 34, and a further seven patients with advanced solid tumors in part C completed ≥1 year of treatment. These data suggest that trotabresib monotherapy may provide long-lasting disease control in some patients, and demonstrate the long-term efficacy and good tolerability of intermittent dosing of trotabresib even in patients with advanced, progressive disease. Importantly, the durable CR in a patient with progressive diffuse astrocytoma and the minor radiographic response in a patient with high-grade astrocytoma, a tumor type with typically poor outcomes and limited effective treatment options, illustrate the ability of trotabresib to penetrate brain tumor tissue35. Penetration of brain tumor tissue is a key challenge in the treatment of gliomas and other central nervous system tumors36,37. Preclinical studies in a wide variety of solid tumors and hematological malignancies have also shown that BET inhibition may be synergistic with a range of anticancer agents, including various targeted therapies and cytotoxic agents16,38,39,40,41,42,43,44,45,46,47,48.
Evaluation of the effects of food on trotabresib PK showed generally comparable exposure and plasma concentrations between fed and fasted patients, with a similar t½ to that previously reported for patients in part A18. Interindividual variability, characterized by the geometric coefficient of variation, was generally similar in fed and fasted patients. These data suggest that food does not markedly alter trotabresib PK, making it an attractive candidate for combination with various anticancer agents that require fed or fasted administration.
As this exploratory phase I first-in-human trial was designed to evaluate trotabresib in patients with advanced cancers for whom no standard therapy was available, as well as to guide dose selection for subsequent studies, it is subject to a number of limitations. Thus, its results should be interpreted with care in the absence of an adequately powered randomized controlled trial.
In summary, the results from this study demonstrated a tolerable safety profile during long-term treatment, a long t½, favorable pharmacodynamics, and antitumor activity with trotabresib monotherapy in heavily pretreated patients with advanced solid tumors and R/R DLBCL. Prolonged follow-up of part A, with treatment durations of up to 3 years in some patients, and data from parts B and C confirm the good tolerability of trotabresib when administered using monthly and biweekly intermittent dosing regimens. The extended treatment-free interval included in these regimens is enabled by the long t½ of trotabresib. Importantly, evaluation of the effects of food on trotabresib treatment found generally similar PK in fed and fasted patients, providing flexibility for dosing.
The results reported here demonstrate the preliminary anticancer properties of trotabresib monotherapy across a range of heavily pretreated advanced solid tumors and in R/R DLBCL, supporting further investigation of its use in combination with other anticancer agents. Based on the responses observed in this study, a clinical trial has been initiated to investigate trotabresib in combination with temozolomide in patients with newly diagnosed glioblastoma (NCT04324840)35,49,50,51.
Methods
Study design
The CC-90010-ST-001 trial comprises three parts (Fig. 7). Part A was a dose-escalation study to determine the RP2D of trotabresib monotherapy. Trotabresib was administered at doses ranging from 15 to 55 mg and schedules ranging from 3 days on/4 days off to 4 days on/24 days off, as previously reported18. Part B is an ongoing dose expansion evaluating the safety and efficacy of trotabresib in patients with R/R DLBCL or basal cell carcinoma (BCC). Two trotabresib dosing schedules are being evaluated in patients with R/R DLBCL: the RP2D of trotabresib of 45 mg/day 4 days on/24 days off and an alternative dosing regimen of trotabresib 30 mg 3 days on/11 days off18. Part C is an ongoing dose expansion evaluating the safety and efficacy of trotabresib 45 mg/day 4 days on/24 days off in patients with advanced solid tumors, as well as the impact of food on trotabresib PK. The trial is registered on ClinicalTrials.gov (NCT03220347).
Dose-escalation decisions in part A were guided by a Bayesian logistic regression model with overdose control52. The number of patients treated at each dose level and schedule was based on empirical considerations, with a maximum of six patients typically treated in each group. For the part B R/R DLBCL expansion cohort, enrollment was based on the probability of making a no-go decision when the true efficacy was truly below the target level. For instance, if the no-go criteria is Pr (ORR <26%) >80%, enrollment of 25 patients would provide a 73% chance to make a no-go decision when the true ORR is 14% (based on the posterior probability of beta-binomial distribution with prior beta [0.35, 1]). Planned enrollment for the part C food effect cohort was also driven by empirical experience, with the enrolled sample size believed to produce sufficient precision for PK parameter assessment based on preliminary PK results from part A.
The study was conducted in accordance with the Declaration of Helsinki and in adherence to Good Clinical Practice. The protocol was reviewed and approved by the Institutional Review Board or Independent Ethics Committee of each site before initiation of the study, and all patients provided written informed consent. The protocol was approved by the Institutional Review Board or Independent Ethics Committee of Institut Gustave Roussy, Villejuif, France; Institut Bergonie Centre Regional de Lutte Contre Le Cancer de Bordeaux et Sud Ouest, Bordeaux, France; Vall d’Hebron University Hospital, Vall d’Hebron Institute of Oncology (VHIO), Barcelona, Spain; Hospital Universitario Fundación Jimenez Diaz, Madrid, Spain; Hospital Universitario 12 de Octubre, Madrid, Spain; Istituto Nazionale Tumori, Fondazione G. Pascale, IRCCS, Naples, Italy; Instituto Clinico Humanitas, Rozzano, Milano, Italy; Instituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori, Meldola, Italy; National Cancer Center Hospital East, Tokyo, Japan; Aichi Cancer Center Hospital, Nagoya, Japan; and The Cancer Institute Hospital of Japanese Foundation for Cancer Research, Tokyo, Japan.
Patient selection
Patients were enrolled from July 31, 2017 to January 11, 2021. The study included patients aged ≥18 years with an ECOG PS of 0 or 1. Part A included patients with histologically or cytologically confirmed advanced or unresectable solid tumors or R/R advanced NHL that had progressed on standard anticancer therapy or for which no conventional therapy was available.
Part B included patients with histologically or cytologically confirmed R/R DLBCL that had progressed following two or more previous lines of therapy, including autologous stem cell transplant, or that had progressed after at least one previous line of therapy and where the patient was not eligible for or had declined autologous stem cell transplant. Patients with transformed lymphoma following chemotherapy for follicular lymphoma who had received at least two standard treatment regimens for DLBCL were also eligible. Patients must have had a lack of response after chimeric antigen receptor (CAR) T-cell therapy (if available), been ineligible for CAR T-cell therapy, or declined CAR T-cell therapy. Part B also included a cohort of patients with histologically or cytologically confirmed advanced BCC, nuclear protein in testis (NUT) midline carcinoma, advanced salivary gland carcinoma, or advanced endometrial carcinoma and disease progression on, or inability to tolerate, standard therapy, or for whom no standard therapy exists. Patient enrollment in this cohort was subsequently restricted to patients with advanced BCC only in a protocol amendment.
Part C included patients with histologically or cytologically confirmed advanced solid tumors and disease progression on, or inability to tolerate standard therapy, or for whom no standard therapy exists.
Patients with solid tumors had at least one site of measurable disease; patients with NHL had bidimensionally measurable disease on cross-sectional imaging, with at least one lesion >1.5 cm in diameter.
Endpoints
The primary endpoints of the study were the safety and tolerability of trotabresib, as well as the MTD and/or RP2D of trotabresib. Safety was assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0353.
Secondary objectives were the preliminary efficacy of trotabresib in terms of clinical benefit rate (CR + PR + SD of ≥4 months’ duration), ORR (CR + PR), duration of response or SD, PFS, OS, and the PK of trotabresib. In addition, part C assessed the effects of food on the PK and the safety of trotabresib as a secondary objective. The pharmacodynamics of trotabresib were assessed as an exploratory objective.
Response was assessed using Response Evaluation Criteria in Solid Tumors (RECIST) v1.154 in patients with solid tumors, International Working Group criteria in patients with DLBCL55, and Response Assessment in Neuro-Oncology (RANO) criteria56 in patients with CNS tumors (astrocytoma and glioblastoma). For patients with BCC, the response was assessed using a combination of radiological assessment of target lesions per RECIST v1.1, digital clinical photography assessed per World Health Organization (WHO) criteria57, and punch biopsies to confirm CR. PK parameters assessed included Cmax, tmax, AUC, t½, apparent clearance (CL/F), and apparent volume of distribution (Vz/F).
Pharmacokinetic and pharmacodynamic assays
Serial blood samples for PK and pharmacodynamic analyses were collected for each dosing schedule after the first and last doses in cycle 1. Additional blood samples for assessment of the food effect on trotabresib PK in part C were collected during cycle 2. The food effect–evaluable population comprised all patients with adequate PK data to allow calculation of trotabresib AUC from time 0 (day 1) to day 22 from both the fed and fasted treatment periods and who had completed a 4-h post-dose fast during the fasted cycle or consumed the entire high-fat, high-calorie meal during the fed cycle and had received all trotabresib doses during both treatment periods.
Changes in peripheral CCR1 mRNA levels, expression of which is modulated following BET inhibition33, were assessed using QuantiGene technology (Thermo Fisher Scientific) and normalized to the expression of a control gene, peptidylprolyl isomerase B (PPIB). The data were presented as a percentage of pre-dose mRNA levels.
Statistical analyses
Bayesian logistic regression model based on dose-limiting toxicity was used to provide initial guidance for dose recommendations in the dose-escalation phase. The final decision was reached by considering the totality of safety, PK, and PD data. For binary efficacy endpoints, two-sided 95% Clopper–Pearson exact confidence intervals were provided. For time-to-event endpoints (PFS and OS), the median and 95% CI were estimated using the Kaplan–Meier method.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Anonymized participant-level data cannot be shared due to an increased risk of patient reidentification. Bristol Myers Squibb will consider requests to share anonymized clinical trial data from interventional trials in patients that have completed on or after January 1, 2008. In addition, primary results from these trials must have been published in peer-reviewed journals and the medicines or indications approved in the United States, EU, and other designated markets. Requests to access clinical trial data will be considered case by case and may be submitted to Celgene, a Bristol Myers Squibb Company, using the inquiry form at https://vivli.org/ourmember/bristol-myers-squibb/. Requests for clinical data are initially reviewed by internal Bristol Myers Squibb personnel to ensure alignment with the scope of the data-sharing policy and to check the current or expected availability of the data sets and are then evaluated by an Independent Review Committee to ensure that qualifying requests have a consistent, complete, and fair assessment. Sharing is also subject to the protection of patient privacy and respect for the patient’s informed consent. and must include a description of the research proposal. Information from eligible trials that may be considered for disclosure upon request includes deidentified study-level clinical data, Clinical Study Reports, Statistical Analysis Plans, and Protocols. The study protocol is available as Supplementary Note 1 in the Supplementary Information file. The remaining data were available within the Article, Supplementary Information, or Source Data file. Source data are provided with this paper.
References
LeRoy, G., Rickards, B. & Flint, S. J. The double bromodomain proteins Brd2 and Brd3 couple histone acetylation to transcription. Mol. Cell 30, 51–60 (2008).
Doroshow, D. B., Eder, J. P. & LoRusso, P. M. BET inhibitors: a novel epigenetic approach. Ann. Oncol. 28, 1776–1787 (2017).
Shi, J. & Vakoc, C. R. The mechanisms behind the therapeutic activity of BET bromodomain inhibition. Mol. Cell 54, 728–736 (2014).
Filippakopoulos, P. et al. Selective inhibition of BET bromodomains. Nature 468, 1067–1073 (2010).
Wu, X. et al. Inhibition of BRD4 suppresses cell proliferation and induces apoptosis in renal cell carcinoma. Cell Physiol. Biochem. 41, 1947–1956 (2017).
Tan, Y. et al. Inhibition of BRD4 suppresses tumor growth in prostate cancer via the enhancement of FOXO1 expression. Int. J. Oncol. 53, 2503–2517 (2018).
Shafran, J. S., Andrieu, G. P., Györffy, B. & Denis, G. V. BRD4 regulates metastatic potential of castration-resistant prostate cancer through AHNAK. Mol. Cancer Res. 17, 1627–1638 (2019).
Pivot-Pajot, C. et al. Acetylation-dependent chromatin reorganization by BRDT, a testis-specific bromodomain-containing protein. Mol. Cell Biol. 23, 5354–5365 (2003).
The Human Protein Atlas. BRDT. https://www.proteinatlas.org/ENSG00000137948-BRDT/pathology (2022).
Shorstova, T., Foulkes, W. D. & Witcher, M. Achieving clinical success with BET inhibitors as anti-cancer agents. Br. J. Cancer 124, 1478–1490 (2021).
Chapuy, B. et al. Discovery and characterization of super-enhancer-associated dependencies in diffuse large B cell lymphoma. Cancer Cell 24, 777–790 (2013).
Delmore, J. E. et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 146, 904–917 (2011).
Mochizuki, K. et al. The bromodomain protein Brd4 stimulates G1 gene transcription and promotes progression to S phase. J. Biol. Chem. 283, 9040–9048 (2008).
Sinha, A., Faller, D. V. & Denis, G. V. Bromodomain analysis of Brd2-dependent transcriptional activation of cyclin A. Biochem J. 387, 257–269 (2005).
Yang, Z., He, N. & Zhou, Q. Brd4 recruits P-TEFb to chromosomes at late mitosis to promote G1 gene expression and cell cycle progression. Mol. Cell Biol. 28, 967–976 (2008).
Boi, M. et al. The BET bromodomain inhibitor OTX015 affects pathogenetic pathways in preclinical B-cell tumor models and synergizes with targeted drugs. Clin. Cancer Res. 21, 1628–1638 (2015).
Lovén, J. et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 153, 320–334 (2013).
Moreno, V. et al. Phase I study of CC-90010, a reversible, oral BET inhibitor in patients with advanced solid tumors and relapsed/refractory non-Hodgkin’s lymphoma. Ann. Oncol. 31, 780–788 (2020).
Mertz, J. A. et al. Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc. Natl Acad. Sci. USA 108, 16669–16674 (2011).
Meriranta, L. et al. Molecular background delineates outcome of double protein expressor diffuse large B-cell lymphoma. Blood Adv. 4, 3742–3753 (2020).
Moreno, V. et al. 16O Phase I study of CC-90010, a reversible, oral BET inhibitor in patients (Pts) with advanced solid tumors (STs) and relapsed/refractory non-Hodgkin lymphoma (R/R NHL). Ann. Oncol. 31, S5 (2020).
Abramson, J. S. et al. BET inhibitor CPI-0610 is well tolerated and induces responses in diffuse large B-cell lymphoma and follicular lymphoma: preliminary analysis of an ongoing phase 1 study. Blood 126, 1491 (2015).
Blum, K. A. et al. A phase I study of CPI-0610, a bromodomain and extra terminal protein (BET) inhibitor in patients with relapsed or refractory lymphoma. Ann. Oncol. 29, iii7 (2018).
Lewin, J. et al. Phase Ib trial with birabresib, a small-molecule inhibitor of bromodomain and extraterminal proteins, in patients with selected advanced solid tumors. J. Clin. Oncol. 36, 3007–3014 (2018).
Piha-Paul, S. A. et al. Phase 1 study of molibresib (GSK525762), a bromodomain and extra-terminal domain protein inhibitor, in NUT carcinoma and other solid tumors. JNCI Cancer Spectr. 4, pkz093 (2020).
Piha-Paul, S. A. et al. First-in-human study of mivebresib (ABBV-075), an oral pan-inhibitor of bromodomain and extra terminal proteins, in patients with relapsed/refractory solid tumors. Clin. Cancer Res. 25, 6309–6319 (2019).
Falchook, G. et al. Development of 2 bromodomain and extraterminal inhibitors with distinct pharmacokinetic and pharmacodynamic profiles for the treatment of advanced malignancies. Clin. Cancer Res. 26, 1247–1257 (2020).
Amorim, S. et al. Bromodomain inhibitor OTX015 in patients with lymphoma or multiple myeloma: a dose-escalation, open-label, pharmacokinetic, phase 1 study. Lancet Haematol. 3, e196–e204 (2016).
Berthon, C. et al. Bromodomain inhibitor OTX015 in patients with acute leukaemia: a dose-escalation, phase 1 study. Lancet Haematol. 3, e186–e195 (2016).
Postel-Vinay, S. et al. First-in-human phase I study of the bromodomain and extraterminal motif inhibitor BAY 1238097: emerging pharmacokinetic/pharmacodynamic relationship and early termination due to unexpected toxicity. Eur. J. Cancer 109, 103–110 (2019).
Stathis, A. et al. Clinical response of carcinomas harboring the BRD4-NUT oncoprotein to the targeted bromodomain inhibitor OTX015/MK-8628. Cancer Disco. 6, 492–500 (2016).
Alqahtani, A. et al. Bromodomain and extra-terminal motif inhibitors: a review of preclinical and clinical advances in cancer therapy. Future Sci. OA 5, FSO372 (2019).
Stathis, A. & Bertoni, F. BET proteins as targets for anticancer treatment. Cancer Disco. 8, 24–36 (2018).
Tsujikawa, L. et al. Preclinical development and clinical validation of a whole blood pharmacodynamic marker assay for the BET bromodomain inhibitor ZEN-3694 in metastatic castration-resistant prostate cancer (mCRPC) patients. Cancer Res. 77, Abstract LB–038 (2017).
Moreno, V. et al. Trotabresib, an oral potent bromodomain and extraterminal inhibitor, in patients with high-grade gliomas: a phase I, “window-of-opportunity” study. Neuro Oncol. https://doi.org/10.1093/neuonc/noac263 (2022).
Kan, L. K. et al. Potential biomarkers and challenges in glioma diagnosis, therapy and prognosis. BMJ Neurol. Open 2, e000069 (2020).
Taylor, O. G., Brzozowski, J. S. & Skelding, K. A. Glioblastoma multiforme: an overview of emerging therapeutic targets. Front. Oncol. 9, 963 (2019).
Muralidharan, S. V. et al. BET bromodomain inhibitors synergize with ATR inhibitors to induce DNA damage, apoptosis, senescence-associated secretory pathway and ER stress in Myc-induced lymphoma cells. Oncogene 35, 4689–4697 (2016).
Fiorentino, F. P. et al. BET-inhibitor I-BET762 and PARP-inhibitor talazoparib synergy in small cell lung cancer cells. Int. J. Mol. Sci. 21, 9595 (2020).
Gusyatiner, O. et al. BET inhibitors repress expression of interferon-stimulated genes and synergize with HDAC inhibitors in glioblastoma. Neuro Oncol. 23, 1680–1692 (2021).
Kang, S. K. et al. Transcriptome analysis of iBET-151, a BET inhibitor alone and in combination with paclitaxel in gastric cancer cells. Genomics Inf. 18, e37 (2020).
Kanojia, D. et al. BET inhibition increases βIII-tubulin expression and sensitizes metastatic breast cancer in the brain to vinorelbine. Sci. Transl. Med. 12, eaax2879 (2020).
Lei, L. et al. The bromodomain and extra-terminal domain inhibitor JQ1 synergistically sensitizes human colorectal cancer cells to topoisomerase I inhibitors through repression of Mre11-mediated DNA repair pathway. Invest. New Drugs 39, 362–376 (2021).
Miller, A. L. et al. The BET inhibitor JQ1 augments the antitumor efficacy of gemcitabine in preclinical models of pancreatic cancer. Cancers 13, 3470 (2021).
Ramsey, H. E. et al. BET inhibition enhances the antileukemic activity of low-dose venetoclax in acute myeloid leukemia. Clin. Cancer Res. 27, 598–607 (2021).
Shigeta, S. et al. Targeting BET proteins BRD2 and BRD3 in combination with PI3K-AKT inhibition as a therapeutic strategy for ovarian clear cell carcinoma. Mol. Cancer Ther. 20, 691–703 (2021).
Zhou, X. et al. BET inhibitors combined with chemotherapy synergistically inhibit the growth of NSCLC cells. Oncol. Rep. 45, 70 (2021).
Bagratuni, T. et al. JQ1 inhibits tumour growth in combination with cisplatin and suppresses JAK/STAT signalling pathway in ovarian cancer. Eur. J. Cancer 126, 125–135 (2020).
ClinicalTrials.gov. A study of CC-90010 in combination with temozolomide with or without radiation therapy in subjects with newly diagnosed glioblastoma. https://clinicaltrials.gov/ct2/show/NCT04324840 (2020).
Clinicaltrials.gov. CNS penetration, PK and PD of preoperative CC-90010 in progressive/recurrent diffuse astrocytoma, anaplastic astrocytoma and glioblastoma. https://clinicaltrials.gov/ct2/show/NCT04047303 (2021).
Vieito, M. et al. Trotabresib (CC90010) in combination with adjuvant temozolomide or concomitant temozolomide plus radiotherapy in patients with newly diagnosed glioblastoma. Neuro-Oncol. Adv. 4, vdac146 (2022).
Neuenschwander, B., Branson, M. & Gsponer, T. Critical aspects of the Bayesian approach to phase I cancer trials. Stat. Med. 27, 2420–2439 (2008).
National Institutes of Health & National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. (U.S. Department of Health and Human Services, 2009).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Cheson, B. D. et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J. Clin. Oncol. 32, 3059–3068 (2014).
Wen, P. Y. et al. Updated response assessment criteria for high-grade gliomas: response assessment in Neuro-Oncology Working Group. J. Clin. Oncol. 28, 1963–1972 (2010).
Miller, A. B., Hoogstraten, B., Staquet, M. & Winkler, A. Reporting results of cancer treatment. Cancer 47, 207–214 (1981).
Acknowledgements
The authors thank the patients and their families who made this study possible and the clinical study teams who participated in the study. The authors also thank Olga Ferrero, the primary research nurse for the study, and Esther Chan for assistance with pharmacodynamic analyses. This study was sponsored by Celgene, a Bristol Myers Squibb Company. The study sponsor was involved in the study design, analysis of data, and writing the manuscript. Medical writing and editorial assistance were provided by Bernard Kerr, PGDipSci, and Agata Shodeke, of Spark, funded by Bristol Myers Squibb.
Author information
Authors and Affiliations
Contributions
R.S., B.A., X.W., Z.N., V.M., and I.B. contributed to the study design. V.M., M.V., J.M.S., V.G., T.H.-G., B.D., O.S., C.C.-S., J.-M.M., A.I., M.M., C.C., A.P., and I.B. contributed to patient recruitment. B.H. contributed to PK data analysis and interpretation. I.A. and E.F. contributed to CCR1 data analysis and interpretation. All authors contributed to data analysis and interpretation and to the drafting and critical review of the manuscript.
Corresponding author
Ethics declarations
Competing interests
V.M. has received consulting fees from Bristol Myers Squibb, Basilea, Bayer, Janssen, Nanobiotix, Pieris Pharmaceuticals, Regeneron/Sanofi, and Roche, and honoraria from Bristol Myers Squibb and Bayer. M.V. has received personal fees from Roche, EMD Serono, and TFS. J.M.S. has received personal fees and non-financial support from Celgene (a Bristol Myers Squibb Company), AbbVie, and Astellas, non-financial support from Ipsen, and research grants from Pfizer and IDP Pharma. J.-M.M. has received research grants from Bristol Myers Squibb, AstraZeneca, Boehringer Ingelheim, GSK, INCA, Janssen Cilag, Merck, Novartis, Pfizer, Roche, and Sanofi, non-financial support from Bristol Myers Squibb, AstraZeneca, Bayer, Boehringer Ingelheim, GSK, MedImmune, Merck, NH TherAguix, Pfizer, and Roche, and other support from Bristol Myers Squibb, AstraZeneca, Boehringer Ingelheim, Eli Lilly Oncology, F. Hoffmann-La Roche Ltd, Merck, Merck Sharp & Dohme, Pfizer, and Regeneron. A.I. has received consulting fees from Epizyme, Lilly, Merck Sharp & Dohme, Novartis, Pharmamar, and Roche, and received research grants from Bristol Myers Squibb, AstraZeneca, Bayer, Chugai, Merck Sharp & Dohme, Novartis, Pharmamar, Pfizer, and Roche. M.M. has received honoraria from AstraZeneca and Sanofi. C.C. has received consulting fees for acting in an advisory role from Bristol Myers Squibb, Regeneron, Novartis, and Takeda. A.P. has received honoraria from Bristol Myers Squibb, F. Hoffmann-La Roche Ltd, Helsinn Healthcare, Janssen, Celgene, Gilead Sciences, Incyte, Servier, Merck Sharp & Dohme, and Takeda. I.B. has received personal fees from Bristol Myers Squibb, AstraZeneca, Merck Serono, Merck Sharp & Dohme, Orion Pharma, Rakuten Aspyrian, Roche, and research funding from Bristol Myers Squibb, AstraZeneca, Merck Serono, Merck Sharp & Dohme, Orion Pharma, Rakuten Aspyrian, Roche, Celgene, GlaxoSmithKline, Incyte, Janssen, Kura, Novartis, Pfizer, Shattuck Labs, and VCN Biosciences. V.G., T.H.-G., B.D., O.S., and C.C.-S. have no conflicts of interest to disclose. R.S., B.A., and Z.N. are employed by the Centre for Innovation and Translational Research Europe, a Bristol Myers Squibb Company. E.F., B.H., and X.W. are employed by Bristol Myers Squibb. B.H., E.F., R.S., X.W., and Z.N. hold Bristol Myers Squibb stock. I.A. was employed by Bristol Myers Squibb at the time of the study.
Peer review
Peer review information
Nature Communications thanks Frank Lieberman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moreno, V., Vieito, M., Sepulveda, J.M. et al. BET inhibitor trotabresib in heavily pretreated patients with solid tumors and diffuse large B-cell lymphomas. Nat Commun 14, 1359 (2023). https://doi.org/10.1038/s41467-023-36976-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-36976-1
This article is cited by
-
The progress of novel strategies on immune-based therapy in relapsed or refractory diffuse large B-cell lymphoma
Experimental Hematology & Oncology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.