Abstract
Genome-wide association studies have discovered hundreds of associations between common genotypes and kidney function but cannot comprehensively investigate rare coding variants. Here, we apply a genotype imputation approach to whole exome sequencing data from the UK Biobank to increase sample size from 166,891 to 408,511. We detect 158 rare variants and 105 genes significantly associated with one or more of five kidney function traits, including genes not previously linked to kidney disease in humans. The imputation-powered findings derive support from clinical record-based kidney disease information, such as for a previously unreported splice allele in PKD2, and from functional studies of a previously unreported frameshift allele in CLDN10. This cost-efficient approach boosts statistical power to detect and characterize both known and novel disease susceptibility variants and genes, can be generalized to larger future studies, and generates a comprehensive resource (https://ckdgen-ukbb.gm.eurac.edu/) to direct experimental and clinical studies of kidney disease.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) is a major public health concern affecting ~10% of the global adult population1. Previous genetic studies have either focused on identifying rare, pathogenic variants among patients with suspected monogenic forms of kidney disease, or on using genome-wide association studies (GWAS) in large population-based studies to identify common susceptibility variants for CKD or the most commonly used markers of kidney function and damage, namely, the estimated glomerular filtration rate (eGFR)2,3,4,5,6 and the urinary albumin-to-creatinine ratio (UACR)7,8,9. Initial studies of the coding exome or the whole genome to detect rare pathogenic variants and risk genes for kidney dysfunction had limited power10,11. These limitations can now be addressed through the availability of whole-exome sequencing (WES) data from the UK Biobank12, and the possibility of imputing the samples not sequenced but array genotyped at the time this study started.
As the direct measurement of kidney function is not feasible in population-based studies and clinical routine, the glomerular filtration rate (GFR) is typically estimated from biomarker measurements and demographic information. The most commonly used biomarker for GFR estimation is serum creatinine, a metabolite predominantly produced in skeletal muscle. More recently developed GFR estimating equations also include cystatin C, a protein produced ubiquitously and hence less dependent on muscle mass. Serum urea and urate levels, metabolites mainly produced in the liver, are also strongly reflective of kidney function. Conversely, the UACR reflects damage to the glomerular filter rather than filtration function, by quantifying the leakage of albumin into the urine. Although creatinine and cystatin C have been studied as two of thousands of outcomes in several phenome-wide screens of the coding exome in the UK Biobank13,14,15,16,17, a dedicated effort to assess associations with kidney function and disease by leveraging different biomarkers and ICD-based CKD classifications has not been performed. Moreover, the contribution of rare exonic variants independently from common kidney function variants has received little attention. Lastly, previous efforts have stopped short of experimental validation of causal genes or variants.
Here, we use WES-powered imputation in the large white British ancestry sample of the UK Biobank to increase sample size for studies of the coding genome from 166,891 to 408,511. We discover rare variants and genes associated with measures of kidney function and disease, namely creatinine- and cystatin C-based eGFR (“eGFRcrea” and “eGFRcys”, respectively), serum urea (“urea”), serum urate (“urate”), and the UACR in the general population. We validate our findings using the full set of exome sequences from the UK Biobank that was released over the course of this study, characterize our findings across different kidney phenotypes and clinical definitions of CKD, identify cell types and tissues in which the genes are highly expressed, explore the relationship of identified genes across the phenome, and experimentally validate a previously unreported splice allele in CLDN10 as proof-of-principle for imputation-powered discovery. This comprehensive approach enabled the replication of many known kidney disease-causing variants and genes that serve as positive controls, and the discovery of variants and genes not previously connected to kidney function and disease that represent promising candidates for experimental follow-up. The results are made publicly available as a resource to the scientific community.
Results
WES-powered Imputation
Using available WES data of 166,891 samples as a backbone, we imputed genotypes at 7,575,566 variants for an additional 241,620 individuals without WES data, resulting in a total sample size of 408,511 individuals for exome-wide association studies (ExWAS; Methods).
Initial imputation performance was evaluated using 10,000 samples with WES data set aside from the imputation reference panel and imputing their genotypes, allowing determination of imputation accuracy for 2,191,400 imputed variants with a minor allele count (MAC) of ≥1. The imputation accuracy at these variants measured as the average squared correlation (R2) between sequenced hard call genotypes and imputed allele dosages showed an overall median of 0.9971 (Q1 = 0.8787, Q3 = 1.0000; mean = 0.8375, sd = 0.3119, Fig. 1a). Very rare variants with MAC 2–5 achieved a median R2 of 0.9999 (n = 499,179; Q1 = 0.4995, Q3 = 1.0000; mean = 0.7362, sd = 0.4145).
a Boxplots of the squared correlation (R2) of sequenced genotypes with imputed dosages. Variants were binned based on their MAC (2–10) or MAF (≥0.0001) in the 156 K reference panel, resulting in a mean of 157 K (27–630 K) variants per bin. The boxes represent the first to the third quartile, the horizontal line the median, and the whiskers extend to 1.5 times the interquartile range. b Mean genotype concordance (number of matching genotypes/total number of genotypes) of the hard call imputed genotypes with sequenced data for homozygous reference (HomRef), homozygous alternative (HomAlt), and heterozygous (Het) calls. Variants were binned based on their MAC in the 156 K reference panel, resulting in a mean of 1.5 × 109 (3 × 108–6 × 109) hard calls per bin. c Mean squared correlation of sequenced genotypes and imputed dosages using a reference panel of 156 K individuals (this study) and a reference panel of 50 K individuals (Barton et al.). Variants were binned based on the MAC or MAF in their respective reference panel.
The concordance (number of matching genotypes/total number of genotypes) of homozygous reference, heterozygous, and homozygous alternative genotypes based on sequenced and imputed data in the validation sample was 0.9998, 0.9778, and 0.9877, respectively (Fig. 1b). Very rare variants with a MAC of 2–5 in the reference panel achieved a concordance of 0.99996, 0.5573, and 0.9569 for homozygous reference, heterozygous, and homozygous alternative calls, respectively. In this low MAC range, the vast majority (>99.9%) of genotypes are (correctly classified) homozygous reference, and true heterozygotes are more frequently imputed as homozygous reference, leading to the relatively low concordance in this genotype class. The true positive rate (TPR) and true negative rate (TNR), computed by defining heterozygous and homozygous alternative genotypes (i.e., carriers of a given variant) as “positive” and homozygous reference genotype (i.e., non-carriers) as “negative”, were 0.9817 and 0.9998, respectively. The false negative rate (carriers incorrectly imputed as non-carriers) was 0.0101, and the false positive rate (non-carriers incorrectly imputed as carriers) was 0.0002.
Together, these comparisons show that study specific WES-imputation enables the estimation of unobserved genotypes with high quality even for very rare variants, providing a solid foundation for subsequent association studies. The imputation quality of our study based on an imputation reference panel of ~167 K individuals was considerably higher than the imputation quality achieved in a previous imputation effort that used a reference panel of ~50 K individuals (Fig. 1c)16.
ExWAS of kidney function traits identifies 158 rare variants
Results from ExWAS of the five continuous kidney markers and 7.5 million variants from 408,511 individuals of European ancestry showed no signs of unaccounted stratification (inflation factor λː 1.00–1.04, Supplementary Fig. 1; Methods). We identified 174 associations at rare variants of minor allele frequency (MAF) < 1% and MAC ≥ 5 that were significantly associated with at least one kidney phenotype (p < 6.8 × 10−9; Supplementary Fig. 2, Methods). These associations corresponded to 158 unique variants, of which 112 were imputed (median imputation quality rsq = 0.92, range 0.31–1.00) and 46 were directly genotyped. Thirty-three variants were associated with eGFRcrea (Supplementary Data 1a), 44 with eGFRcys (Supplementary Data 1b), 11 with UACR (Supplementary Data 1c), 82 with urate (Supplementary Data 1d), and four with urea (Supplementary Data 1e). Over 60% (106/174) of these associations were not significant (n = 102) or could not be tested due to MAC < 5 (n = 4) when we performed a sensitivity ExWAS that used only the ~167 K samples with WES, which demonstrates the imputation-based increase in statistical power.
Of all 174 associations, 158 were phenotype-specific (Supplementary Data 1; Methods) and are described in the subsequent paragraphs. Between 30% (eGFRcys) and 62% (urate) of ExWAS variants mapped into GWAS loci (index SNP ± 500 kb; Methods). To assess whether any of the ExWAS results were driven by common (MAF > 1%) variants identified by previous GWAS of the respective trait, ExWAS were repeated while adjusting for the known common variants: effect estimates were largely unchanged as shown in Supplementary Fig. 3 (R2 estimates from regression analyses comparing unadjusted versus adjusted effect estimates were 0.99 for eGFRcrea, 0.97 for UACR, and 0.98 for urate; Methods), indicating that most of the rare variants tested in this ExWAS are statistically independent of known common GWAS variants. Notable exceptions were observed for urate, where effect sizes of rare variant associations in SLC2A9, the GWAS locus with the largest effect on urate18, were attenuated by more than 50% upon common variant adjustment.
Rare variants associated with kidney function (eGFRcrea, eGFRcys, urea)
To distinguish variants that are likely related to biomarker metabolism from those that are truly relevant for kidney function, we assessed the overlap and direction of effects across the three kidney function markers eGFRcrea, eGFRcys, and urea (Supplementary Data 1). We prioritized 32 kidney function genes that showed (i) a significant association with one or both GFR estimates, (ii) direction-consistent effects, (iii) at least nominal significance (p < 0.05) for the respective other GFR estimate, and (iv) inverse associations with urea as well as with an ICD-10 based definition of CKD (Table 1; Supplementary Data 2; Methods). Thirteen (41%) of these prioritized genes are known to contain rare variants that cause monogenic diseases with a kidney phenotype: CLDN10, CUBN, G6PC1, HNF4A, LRP2, NPHS1, PKD2, PKHD1, SLC12A1, SLC34A1, SLC34A3, SLC6A19, and SLC7A919. In fact, some of the identified variants are known pathogenic mutations for these conditions (e.g., p.Ser192Leu (rs199690076) for SLC34A3, p.Asp173Asn (rs121434346) for SLC6A19). Noteworthy candidates supported by kidney phenotypes in model organisms but not yet established as human kidney disease genes include EPB41L5 and FNIP1 (Table 1; Supplementary Data 2). In addition, several genes, such as MITF, have been linked to kidney cancers but not to reduced kidney function or CKD.
The value of investigating multiple kidney function markers was illustrated by the SLC22A2 frameshift variant p.Phe24ThrfsTer4 (rs8177505), which was strongly associated with eGFRcrea (p = 1.9 × 10−61), but showed little or no association with eGFRcys (p = 4.1 × 10−3) or urea (p = 0.38). SLC22A2 encodes an organic cation transporter of creatinine, among other substrates. It is, therefore, likely that this variant reflects transport-related changes in creatinine rather than a mechanism that results in reduced GFR.
Of particular interest was a previously unreported variant, p.Phe472*fs (a one-base deletion chr4:88046737:TC:T) in PKD2 with MAC = 8 (eGFRcys p = 5.8 × 10−10). PKD2 is a major CKD gene whose mutations cause autosomal-dominant polycystic kidney disease (ADPKD), the most frequent monogenic kidney disease. The detected PKD2 variant is absent from the GnomAD and ClinVar databases, but we found its association with CKD highly significant (OR > 1000, p = 3.7 × 10−15). The eight carriers in the UK Biobank were not closely related when considering kinship coefficient pairs of <0.0625 estimated genome-wide using common alleles. Upon inspection of inpatient hospital records (codes Q61.3 and/or Q61.2; Methods), we found that all eight carriers had clinical diagnoses of polycystic kidney disease (Fig. 2a), thereby validating the unbiased, imputation-powered association findings. Five of the carriers had CKD-related ICD codes, including one person whose kidney disease had progressed to kidney failure (Fig. 2a). eGFRcrea was clearly lower in carriers compared to non-carriers (Fig. 2b). The variant was directly sequenced in three carriers, and imputed in five (rsq = 0.99), demonstrating the effectiveness of the imputation approach and the value of combining complementary biomarker and ICD code-based information.
a Kidney ICD10 diagnosis by the carrier, color-coded (red—cystic kidney disease codes, blue—CKD codes). Carrier age was annotated in the columns. The median age was 60 (range 41–67). b Boxplot of eGFRcrea for the non-carriers of the p.Phe472*fs (4:88046737:TC:T) frameshift variant (left) and of the carriers (right); eGFRcrea of carriers ranged from 25 to 108 ml/min/1.73 m2 (mean 65, SD 28). The boxes represent the first to the third quartile, the horizontal line the median, and the whiskers extend to 1.5 times the interquartile range.
Rare variants associated with kidney damage (UACR) and serum urate
The 11 ExWAS variants found associated with UACR map into the five genes IGFLR1, COL4A3, COL4A4, CUBN, and NPHS1. The high linkage disequilibrium (LD) between the variants in IGFLR1 and NPHS1 (Supplementary Data 1) suggests that the association with IGFLR1 results from a shared haplotype with the neighboring NPHS1, a known cause of monogenic proteinuric kidney disease. Among the 82 urate-associated variants, 16 mapped into a region on chromosome 4 that includes SLC2A9, and another 30 variants mapped into an extended region on chromosome 11 that includes SLC22A12. Consistent with the physiology of the urate-reabsorption mediating transporters encoded by these genes, the urate-associated variants had a negative effect on urate levels and showed strong protection from gout. Known disease causing variants in genes associated with UACR and urate, as well as putative novel causative alleles, are described in detail in the Supplementary Results and Supplementary Fig. 4.
Genes associated with kidney traits from aggregate variant testing
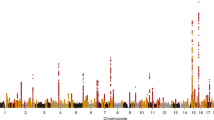
We implemented gene-level burden tests for all five phenotypes over 18,727 genes to increase power to detect associations with rare variants of MAC ≥ 1 that may cluster in individual genes. Two alternative clustering methods (masks) were applied: (a) high confidence loss-of-function and missense variants, including protein truncating and other damaging variants (ptv_dmg, Methods) and (b) missense variants assumed to be deleterious by several in silico prediction tools including the CADD score (dmg_cadd). We identified 83 significant gene-trait associations for 57 unique genes (p < 6.7 × 10−7; Fig. 3, Table 2, Table 3, Supplementary Fig. 5, Methods). Thirty-one genes were associated with eGFRcrea, 22 with eGFRcys, 2 with UACR, 21 with urate, and 7 with urea (Supplementary Fig. 6). Together with the single variant results, associations were identified in a total of 105 genes. Detailed test statistics including the direction of effect for all phenotypes and masks are given in Supplementary Data 3. Effect directions were consistent with clinical expectations across related phenotypes, with the effects on eGFRcrea and eGFRcys being in the same direction, and the effects on eGFR and urea in opposite directions (Fig. 3).
Circular heatmap for genes significantly associated with at least one phenotype of interest. Genes are depicted in the radials with one band per phenotype and divided by chromosome. Coloring according to effect size and direction. Significant gene-phenotype pairs (p < 6.8 × 10−7) are marked with a small black box. Effect size color is only shown for nominally significant (p < 0.05) gene-phenotype associations. Binary trait effect sizes are scaled by 10% (range: −2 to 2). Two-sided p-values were obtained from linear regression models of mask variant risk allele dosage on phenotypes.
All variants contributing to any of the identified gene-trait pairs are provided in Supplementary Data 4, allowing for the identification of variant consequence (e.g., truncating) and putative mechanism of action (gain- vs. loss-of-function). To assess whether genes were identified as a result of aggregation of multiple variants or mostly driven by the contribution of a single variant, we examined and categorized the 57 unique genes into three groups, using the trait for which the gene achieved the smallest p-value (Supplementary Fig. 7, Methods): (1) “multi-variant signal”, where ≥two variants were needed to achieve significance (n = 16; example: SLC34A1 with eGFRcys); (2) “multi-variant signal with one variant sufficient to achieve significance” (n = 34; example: SLC7A9 with eGFRcrea); (3) “not a multi-variant signal” (n = 7; example: SLC25A45 and eGFRcrea).
Genes associated with kidney function (eGFRcrea, eGFRcys, urea)
We compiled a list of 32 genes that were significantly associated with eGFRcrea or eGFRcys either via GBT or ExWAS analyses, and which showed a direction-consistent and at least nominally significant (p < 0.05) association with the respective other GFR estimate, along with a direction consistent association with urea and CKD (Table 1; Supplementary Data 2). Thirteen of the 32 genes (41%) are listed in the OMIM catalog as genes that, when mutated, can cause monogenic diseases of the kidney, 19 (59%) mapped into a known eGFR GWAS locus, and 16 (50%) showed a corresponding kidney phenotype when genetically manipulated in mice. Of note, seven of the 32 genes (22%) were not identified by ExWAS, but only through aggregate variant testing.
There were two genes that have not yet been described as causative for monogenic kidney diseases in humans, but for which genetically manipulated mice show a kidney disease phenotype, namely ARHGEF16 and FNIP1. Little is known about Rho Guanine Nucleotide Exchange Factor 16, encoded by ARHGEF16. Phenotypic characterization of genetically manipulated mice in the Mouse Genome Informatics resource showed enlarged kidneys and abnormal kidney morphology (MGI: 2446219). Fnip1/Fnip2 double knockout mice develop polycystic kidneys and renal cancer20, while Fnip1 disruption is sufficient for renal cyst formation21
Genes associated with kidney damage (UACR) and serum urate
The two genes identified through aggregate variant testing as associated with UACR, CUBN (p = 1.3 × 10−63) and COL4A4 (p = 1.1 × 10−30), have well-known roles in monogenic diseases that feature proteinuria22,23 (Table 1; Supplementary Data 2).
For serum urate, aggregate variant testing identified significant associations with 21 genes, six of which were not identified through ExWAS for this trait (SLC7A9, CLDN10, XDH, INSRR, RORC, and PAAF1; Table 3). XDH encodes for xanthine dehydrogenase, a central enzyme in purine metabolism, of which uric acid is the end-product. Rare mutations are a known cause of autosomal recessive xanthinuria type I (MIM #278300): affected individuals show very low serum urate levels. Accordingly, we observed a negative association with serum urate (beta = −0.04, p = 2.0 × 10−11) and a protective effect on gout (gout OR = 0.8, beta = −0.22, p = 0.16), consistent with the effectiveness of XDH inhibitors for urate-lowering treatment. The association with PAAF1, encoding a protein involved in proteasome assembly regulation, and serum urate has not been reported previously. Interestingly, PAAF1 was described as an interactor of ALDH16A1, another locus identified here24. The exceptionally large effects of rare, damaging variants in SLC22A12 and SLC2A9, two genes encoding for the major transporters responsible for urate reabsorption in the kidney, on serum urate and gout have been described in previous WES studies of serum urate10 and were confirmed in this study.
Validation of imputation-based signals using sequencing data and comparison with published UK Biobank WES study
Over the course of this study, WES data from the full UK Biobank became available. We therefore validated all discovered significant ExWAS and GBT associations by repeating the respective analysis using sequence rather than imputed information for the same set of 408,511 individuals (Methods). For the ExWAS associations, we observed excellent concordance of the association p-values between imputed and sequenced variants (Pearson correlation coefficient of 0.996, Fig. 4a). Statistical significance was reached for 159/174 sequenced variants (Supplementary Data 1). The 15 variants that did not pass the significance threshold showed association p-values closely above the threshold (median: 2.5 × 10−8, IQR: 1.0 × 10−8−6.1 × 10−8), and the association effect sizes of imputed compared to sequenced variants were highly correlated across the range of minor allele frequencies (Pearson coefficient 0.994, Fig. 4b). Secondly, we also observed excellent agreement of association p-values originating from the 123 significant GBT associations (Pearson correlation 0.999, Fig. 4c). We found that 112/123 genes were statistically significantly associated. Again, variants that did not reach statistical significance had p-values closely above the threshold (median: 1.4 × 10−6; IQR: 1.0 × 10−8−2.3 × 10−6), and the correlation coefficient of the GBT effect estimates obtained from imputed and from fully sequenced data was 0.999 (Fig. 4d, Supplementary Data 3).
Scatter plots of −log10-transformed p-values (a) and effect sizes (b) for single variant (ExWAS) and −log10-transformed p-values (c) and effect sizes (d) for the gene-based test (GBT) analyses comparing association statistics from partially imputed (x-axis) and fully sequenced (y-axis) data. Single variant results are color-coded by minor allele frequency (MAF), and GBT results are color-coded by p-value (blue—p-value below the significance threshold of 6.7 × 10−7, red—above threshold). r denotes the Pearson correlation coefficient. For panel a, two-sided p-values were obtained from linear mixed effect models (REGENIE) of effect allele dosage on phenotypes. For panel c, two-sided p-values were obtained from linear regression models of mask variant risk allele dosage on phenotypes.
Next, we performed a systematic comparison of our results to those from the largest phenome-wide WES study that also analyzed kidney-related biomarkers in the UK Biobank14. We revealed an overlap of 55 genes using the statistical significance definition of our study (Methods). Our approach identified 50 additional genes, while 39 of the genes identified by Backman and colleagues were not present in our main results (Supplementary Results, Supplementary Fig. 8). More detailed investigations of genes specific to one or the other study revealed that the majority of them was also nominally significant in the respective other studies.
Kidney-related genes are highly expressed in specific kidney cell types
The expression of genes identified through gene-based tests was assessed at single-cell resolution using publicly available single-cell RNA-seq data from human mature kidney cells25. Earlier studies26,27 have reported that human monogenic and complex kidney disease genes are expressed primarily in a single kidney cell type. Comparing expression levels across kidney cell types confirmed this conclusion also for the genes identified in our project (Fig. 5). The majority of genes related to eGFRcrea, eGFRcys, and serum urate, were highly expressed in the proximal tubule, followed by epithelial progenitor cells for eGFR-related genes. Cell type-specific expression of genes associated with UACR reflected the known pathophysiology of the respective gene: COL4A3 and COL4A4 were found to be expressed specifically in podocytes, consistent with their role in the glomerular filter, and CUBN in the proximal tubule, consistent with its role in the tubular reabsorption of filtered albumin.
Heatmaps showing cell type-specific expression of associated genes in eGFRcrea, eGFRcys, urea, UACR, and urate. The expression z-score values are based on single-cell RNA-seq data from Stewart et al.25 Fifteen non-immune kidney cell types are grouped into nephron, endothelium, and stroma, with 9, 4, and 2 cell types each. Genes in each heatmap are ordered by the maximum expression z-score along the 15 cell types.
Kidney-related genes are associated with additional clinical biomarkers and diseases
We next examined whether the genes we identified in association with the five kidney traits were associated with clinical biomarkers and diseases using publicly available association results from the UK Biobank15 (Methods). This can identify associations with non-kidney traits and diseases that may go unnoticed when only studying individual patients in clinical genetic studies. Across the phenome, there were numerous instances where the assumed loss-of-function of the evaluated genes resulted in increased disease risk or changes in continuous markers of disease (Fig. 6, Supplementary Data 5). Often, these associations reflected known clinical signs and symptoms observed among patients with the resulting monogenic diseases. For example, carriers of rare damaging variants in both COL4A3 and COL4A4 showed higher odds of hematuria, urinary symptoms and kidney problems, with ORs as high as 6 for COL4A4 and hematuria (Fig. 6a). COL4A4 variant carriers also showed higher odds of hypertensive disease, consistent with nephritic syndrome commonly observed in Alport’s syndrome patients (MIM #203780), and lower hemoglobin concentrations and hematocrit percentage, consistent with hematuria (Fig. 6b). Similarly, carriers of rare variants in SLC34A3 showed lower phosphate levels and higher odds of urolithiasis (Fig. 6a), consistent with phenotypes of patients affected by hypophosphatemic rickets with hypercalciuria due to recessive SLC34A3 mutations (MIM #241530).
Phenome-wide association study of genes identified in our single variant ExWAS or gene-based study with other phenotypes in the UK Biobank. For every gene, the odds ratio or beta of the UK Biobank phenotype with the smallest p-value of a gene-based association test is displayed. Genes with multiple significant phenotype associations are marked with an asterisk (*). Full results, including numbers of biologically independent samples for all shown UK phenotypes, are available in Supplementary Data 5. Only UK Biobank associations with p-value < 5 × 10−8 (as reported by the respective studies), and more than 5 cases (for binary traits) or more than 5 individuals (for quantitative traits) were considered. a Odd ratios are shown as center points with error bars representing 95% confidence intervals for binary traits. b Betas are shown as center points with error bars representing 95% confidence intervals for quantitative traits, resulting from a linear regression model correcting for age, sex, and age × sex.
Enrichment analyses highlight biologically plausible pathways
We performed Gene Ontology (GO) enrichment analyses to identify biological processes, molecular functions, and cellular components that were significantly enriched for genes identified by our WES analysis and compared the results to those obtained when using genes identified by published GWAS4,18,28 of the corresponding trait as input. To ensure sufficient power, the analyses were carried out for eGFR and for serum urate, where WES identified >5 genes. Pathways that were significantly enriched in both WES-based as well as GWAS-based analyses contained many examples that are highly plausible (Supplementary Data 6). For instance, pathways related to eGFR contained kidney/renal system/urogenital development as well as those related to transport processes across the apical and basolateral plasma membrane (Supplementary Fig. 9A), whereas implicated pathways for serum urate contained “urate metabolic process” as well as those related to the brush border membrane (Supplementary Fig. 9B), where the most important urate transport proteins that greatly influence serum urate levels are expressed.
Experimental validation of a previously unreported eGFR-associated CLDN10 frameshift allele
While previously unreported, putatively pathogenic variants such as the frameshift variant in PKD2 can be validated through orthogonal evidence from hospital diagnostic codes, experimental validation remains the gold-standard to establish variant pathogenicity. As proof of principle, we, therefore, performed experimental studies of a previously unreported eGFR-associated frameshift allele in CLDN10, encoding the tight junction protein claudin-10. This allele was detected in 32 heterozygous carriers in the imputed WES dataset and significantly associated with its lead trait eGFRcys (carriers had 0.99 standard deviations lower transformed eGFRcys values compared to non-carriers, p = 4.2 × 10−12) as well as with other kidney-related traits. The corresponding results from the directly sequenced 167 K UK Biobank participants available at the outset of this project were 13 carriers, 0.96 standard deviations, and a non-significant p = 8.3 × 10−5.
Isoform claudin-10a is expressed in the kidney proximal tubule and the uterus, claudin-10b in the thick ascending limb of Henle’s loop, and various exocrine glands29,30,31. Mutations in CLDN10 can cause the autosomal recessively inherited HELIX syndrome32, which features hypohidrosis, electrolyte imbalance, lacrimal gland dysfunction, ichthyosis, and xerostomia. Abnormal eGFR was found for some mutations33,34,35,36, but not for others35,36,37,38. In our study, the monoallelic deletion (p.Asp223GlufsTer34, 13:95577994:GAT:G, Supplementary Fig. 10) was associated with lower eGFRcys, higher urea, and higher odds of CKD. The resulting frameshift (fs) causes loss of the C-terminal pdz-binding motif needed for binding of the tight junction protein claudin-10 to the scaffolding protein ZO-139. A previously described single nucleotide deletion (13:95577980 del) in two Saudi Arabian families (claudin-10 fs(SA))37 also caused pdz-binding motif loss and resulted in HELIX syndrome in homozygous carriers. Heterozygous family members were unaffected.
Overexpression of fluorescent protein-claudin-10b fusion proteins in HEK 293 cells showed that, similar to claudin-10b wildtype (wt), claudin-10b fs (but not claudin-10b fs(SA)) was able to enrich in contacts between two transfected cells, indicating the formation of tight junction-like structures and suggesting wt-like trans-interaction of claudin-10b fs (Fig. 7; Supplementary Fig. 11). However, claudin-10b fs enrichments had an impaired appearance (predominantly smooth in wt, irregular in fs), that was not corrected by the co-transfection of claudin-10b wt and was mirrored by reduced FRET efficiency of claudin-10b fs within these contacts as a measure of claudin cis interaction (Fig. 7). Whereas claudin-10b fs(SA) neither interacted with claudin-10 wt nor affected its localization, claudin-10b fs interfered with claudin-10 wt localization, likely explaining a mild dominant effect that is distinct from the molecular phenotype of the HELIX syndrome causing mutation. These findings underscore the value of experimental studies for the characterization of alleles that do not result in typical monogenic disease presentations, to yield a more complete picture of the allelic spectrum. Imputation-powered studies of the coding genome, such as our study, can identify and prioritize such variants for the required further experimental investigations.
Upper panels: co-transfection of YFP (red) and CFP(cyan)-tagged Cldn10b wt, fs, or fs(SA), respectively. The majority of wt–wt contacts were long and had a smooth appearance, whereas the majority of fs–fs contacts were short, and had an interrupted (dashed or ‘ragged’) appearance, likely due to claudin-10b within vesicles close to the plasma membrane rather than truly contact-enriched claudin-10b. Cldn10b wt co-localized with claudin-10b fs in cell–cell contacts (merge); however, these contacts had an appearance similar to the contacts observed in cells expressing only Cldn10b fs. As previously described (Alzahrani et al., 2021), Cldn10b fs(SA) did not insert into the plasma membrane but was retained in intracellular compartments (endoplasmic reticulum). The co-expressed Cldn10b wt was unaffected by the presence of Cldn10b fs(SA). Cell–cell contacts had a wt-like appearance. Bars: 5 µm. Lower panel: FRET efficiency as an indicator for cis-interaction was highest when only Cldn10b wt was present (a, gray triangles, n = 46 from m = 5 independent transfections). When only Cldn10b fs (blue diamonds, n = 41, m = 5) was present, or when Cldn10b fs was combined with Cldn10b wt (b; squares; YFP-wt–CFP-fs, red symbols, n = 34, YFP-fs–CFP-wt, yellow symbols m = 4; n = 43, m = 4), FRET was highly significantly lower (a vs. b, 3.96E−12). When Cldn10b fs(SA) was combined with Cldn10b wt (c; green circles, n = 38, m = 4), FRET efficiency was highly significantly lower than FRET efficiencies observed under all other conditions (a vs. c: p = 2.16E−08; b vs. c: p < 1E−16). ANOVA and Tukey Posthoc test. Different transfections per condition are indicated by different shades of the symbols. Red lines indicate mean FRET efficiency ± SEM.
Discussion
We performed ExWAS and gene-level analyses of five measures of kidney function and disease in up to 408,513 white British participants of the UK Biobank study. To increase statistical power, genotypes for 241,620 individuals with available chip genotypes but no sequencing data at the time of our study were imputed for 7,575,566 variants using the available WES data. We sought to characterize our findings across different kidney phenotypes, cell types, and tissues; and to explore the relationship of identified genes across the phenome. We identified associations at 158 unique single variants and at 57 genes with gene-level tests, all at or driven by rare variants. We validated these imputation-based associations using recently released exome sequences from the full set of UK Biobank participants.
Our study complements previous phenome-wide ExWAS screens in the UK Biobank13,40 by being the first dedicated effort to assess associations of kidney function and disease in this dataset. Whereas previous broad screens included kidney-relevant biomarkers such as creatinine and cystatin C as two out of thousands of phenotypes, we used these biomarkers to estimate the most common clinical measure of kidney function, the GFR, compared and contrasted associations across different kidney function markers, and validated results by examining ICD-10 and biomarker-based binary phenotypes and kidney diseases. We were thereby able to distinguish genes that are likely involved in biomarker metabolism from those truly related to reduced kidney function or kidney damage. In addition, we showed that most rare variant associations were independent of common variant associations in nearby GWAS loci. Moreover, we extended a previously introduced WES imputation approach16 to show that a three-times larger reference panel improves the imputation quality further and facilitates the discovery of many additional significant associations. This has implications for future studies, that may use the now available WES data from the full UK Biobank to impute additional study samples.
In our study, the detection of numerous genes and variants known to cause monogenic kidney diseases when mutated underscore the validity of the results and emphasize that imputation-powered studies of the exome are a feasible and attractive option when whole exome sequencing can only be financed in a subset of the study sample. This holds true even for imputed variants with a MAC < 10, as highlighted by the rare PKD2 variant p.Phe472*fs that we detected in association with eGFR. Although this variant is absent from public databases, all carriers had ICD codes related to cystic kidney disease. Thus, the variant can be considered a novel disease-causing mutation that was uncovered by an imputation-powered association study of the coding genome.
As proof of principle, we experimentally established that a previously unreported eGFR-associated frameshift variant in CLDN10 is a functional allele. The monoallelic deletion causes loss of the C-terminal pdz-binding motif needed for binding of the tight junction protein claudin-10 to the scaffolding protein ZO-1. The claudin-10b frameshift protein interfered with the localization of the wild type, which is likely explaining the mild dominant-negative effect.
In terms of genetic architecture of the studied kidney function markers, there was substantial overlap between genes identified in association with eGFRcrea, eGFRcys, and urea, as would be expected for these complementary filtration markers. Conversely, none of the genes associated with UACR and only a few of the genes associated with serum urate were also associated with the filtration markers. This observation supports the separate study of the genetic architecture of GFR-related traits4, UACR9, and serum urate8,18, as is current practice for common variant studies.
The findings of this study have to be seen in the light of some limitations. To maximize imputation quality by increasing the sample size of the reference panel, we focused on samples of white British ancestry only, which limits the generalizability of our results. The presented associations have been chosen after rigorous statistical analysis; however, we did not replicate them in an independent external data set. Finally, imputation quality depends highly on how often an allele is observed in the reference panel, which makes it impossible to impute variants private to samples not in the reference panel, as observed for PKD1, and could lead to poor imputation results for ultra-rare variants such as singletons or doubletons.
In summary, we identified 105 unique genes and characterized 174 rare variant-associations and 83 gene-associations for five measures of kidney function and disease in a WES-based imputed data set of the UK Biobank and made these results publicly available using PheWeb. These findings revealed genetic determinants of kidney function and will help to direct future functional and clinical studies.
Methods
Sample selection
Analogous to other studies16,17, 408,511 participants that were part of the white British ancestry subset (mean age 48.9 ± 8, 54% female) according to the genotype quality control of the UK Biobank were included in this study. Informed consent was obtained by the UK Biobank for all participants.
Phenotype definition
We considered five kidney-related phenotypes: eGFRcrea (based on creatinine, UKB field 30,700, instance 0), eGFRcys (based on cystatin C, UKB field 30720, instance 0), UACR (UKB fields 30,500 and 30,510), serum urate (UKB field 30,880, instance 0), and urea (UKB field 30,670, instance 0). eGFRcrea and eGFRcys were calculated using the CKD-EPI equations41 and winsorized to 15 and 200 ml/min/1.73 m2. To calculate UACR, values for urinary albumin below the detection limit were set to the detection limit value. All phenotypes were inverse-normal transformed.
We fitted linear regression models to the phenotypes, adjusting for sex, age, and the first 40 genetic principal components, as provided by the UK Biobank. For secondary analyses, the same models were additionally adjusted for 639 SNPs for eGFR28, 63 SNPs for UACR9, and 184 SNPs for urate18 to account for the potential effect of common variants.
CKD and gout were defined using ICD10 codes from hospital inpatient records (N18.*, M10.*, UKB field 41270). Microalbuminuria was defined as UACR > 30 mg/g. ExWAS were carried out for these clinically relevant outcomes and used to annotate the findings for continuous kidney markers with respect to the direction and significance of their association with disease. To further characterize the risk allele carriers of selected trait-associated variants, kidney disease was additionally defined by ICD codes for acute kidney injury (N17.9), CKD (N18.3, N18.4, N18.5, N18.9), polycystic kidney disease (Q61.2, Q61.3), and another kidney (N28.1) or ureter (N39.0) disease. Information on allopurinol treatment was obtained from a verbal interview on medication usage.
Genotype imputation
First, genotyped variants (hap_v2) of all 408,511 individuals of White British ancestry (WBA) were lifted to GRCh38 to match the genotype build of the WES calls. To generate the reference panel for the within-cohort imputation of the WES variants into the remainder of the cohort, autosomal WES variants with MAC > 1 of all 166,891 samples of WBA were merged with genotyped variants with MAC > 1 on the same set of individuals. For the target scaffold, directly genotyped variants with a MAF > 0.0001 in the remainder of the WBA cohort (n = 241,620) were selected. Both panels were split into matching chunks of roughly 30,000 variants with 1 Mb overlap, generating a total of 324 chunks. Each chunk was phased with Eagle2 v2.4.1 (reference panel: --Kpbwt=50000 --pbwtIters=5; target scaffold: --Kpbwt=100000 --pbwtIters=5 --pbwtOnly)42. Imputation was performed with minimac4 on each chunk43. Imputed chunks were concatenated phase-aware per chromosome by cutting off 0.5 Mb of each end. In total, 7,596,602 variants that have not been directly genotyped were imputed in the 241,620 genotyped individuals. The imputed calls were merged with the sequenced calls, keeping only sites with maximum missingness of 0.05 and estimated imputation accuracy rsq > 0.3, resulting in 7,479,293 variants in 408,511 individuals.
For validation purposes, the imputation was repeated once, as described above, randomly removing 10,000 individuals from the reference panel. Imputation quality was assessed in these individuals by comparing the correlation of imputed dosages as well as hard calls with the genotypes derived from the WES, excluding directly genotyped sites, which resulted in n = 2,191,400 variants for validation. To assess imputation accuracy also for the very rare variants, the validation set was divided into tranches by MAC and MAF of the variants in the reference panel, with one tranche per MAC up to 9, followed by larger tranches for low-frequency and common variants. To enable a comparison to the imputation quality achieved by Barton et al. on a reference panel of ~50 K16, the exact same MAF range was used. Imputation quality statistics restricting to different classes of variants (SNPs or indels) or different levels of missingness for rare and common variants are available in Supplementary Fig. 12 and Supplementary Data 7.
Functional annotation
Variant annotation was performed using the Ensembl Variant Effect Predictor (VEP)44 version 101 with standard settings, which comprises annotations of the canonical transcripts, gene symbols, and frequencies from the Genome aggregation database (gnomAD v2.1). VEP plugins were used to add the REVEL score (v2020-5)45 and the CADD score (v3.0)46. Loss-of-function variants were rated using the LoFtee VEP plugin (version 2020-8)47 and classified as high-confidence or low-confidence. The in-silico scores LRT (likelihood-ratio test for deleteriousness), M.CAP (Mendelian Clinically Applicable Pathogenicity Score), MetaSVM (meta-score incorporating multiple in-silico scores)48 and FathMM.XF (Prediction score of pathogenic point mutations)49 were added using the dbNSFP (v4.1a)50.
Significance threshold definition
For the single variant analysis, variants in canonical transcripts and HGNC genes with MAC ≥ 5, MAF < 1%, imputation info score ≥0.5, estimated imputation quality rsq ≥ 0.3, VEP impact ≥LOW and VEP consequence not “synonymous” were considered (n = 1,844,188). Quantile-quantile (QQ) and Manhattan plots with different filter settings were assessed. Because of the strong between-phenotype correlation (Pearson correlation coefficient between eGFRcrea and eGFRcys = 0.61; Supplementary Fig. 13), Bonferroni multiple-testing correction was applied by accounting for 4 rather than 5 phenotypes, and the significance threshold was set at p = 0.05/4/1844188 = 6.78 × 10−9. Similarly, for the gene level analysis, where 18,727 genes were tested, the significance threshold was set at 0.05/18727/4 = 6.67 × 10−7.
Single variant analysis
A single variant, whole-exome linear mixed model association analyses were performed with the REGENIE software package51 v2.0.2 in two steps:
REGENIE Step 1: Whole-genome regression model using the recommended parameter setting. The UK Biobank DNA microarray genotypes (hap_v2) were lifted to build GRCh38 and filtered using the recommended settings (genotype call rate > 0.1; Hardy–Weinberg equilibrium p-value < 1e−15; MAC > 100; MAF > 0.01; sample call rate > 0.90).
REGENIE Step 2: Association analysis was performed on imputed dosage levels (BGEN v1.2 8-bit data format) for 408,511 individuals and 1,844,188 variants. LD was estimated afterward between all pairs of significantly associated variants using PLINK v1.90b252. We restricted the association analysis to variants with MAC ≥ 5, as recommended by the REGENIE developers51.
To calculate association statistics independent of common variants, we extracted 423, 63, and 114 independent, common SNPs previously reported to be associated with eGFR4,28, UACR9, and urate18, respectively, from the imputed UK Biobank data, set. We calculated residuals by regressing eGFR, UACR, and urate on all SNPs for a given trait in one regression analysis, and used these residuals as phenotypes in another ExWAS. Adjusted and unadjusted association results were compared with respect to their effect size estimates. Reported R2 measures originated from a linear regression model.
The PheWeb software (https://github.com/statgen/pheweb) with default settings was used to create a local PheWeb instance that displays the results of the single variant analysis and is available under https://ckdgen-ukbb.gm.eurac.edu/.
Gene-level analysis
Two masks (ptv_dmg, dmg_cadd) that defined variants to be aggregated per gene were created based on the UKBB OQFE exome map using the VEP annotations described above. For ptv_dmg, variants were required to pass the following criteria: LoFtee high-confidence loss-of-function, or (missense consequence and MetaSVM score > 0), or FathMM_XF_coding score > 0.5. Using these settings, the ptv_dmg filter included 15,710 genes with a median of 30 variants per gene (interquartile range, IQR 13–68). For dmg_cadd, variants were included with (the consequence “missense” (or worse), a CADD score > 20), a REVEL score > 0.5, or a M_CAP rating “deleterious”. This resulted in 18,727 genes with a median of 114 variants per gene (IQR 54–203).
The gene-level analysis was performed with the SeqMeta R package (seqMeta: An R package for meta-analyzing region-based tests of rare DNA variants; A Voorman, J Brody, H Chen, T Lumley, B Davis – v1.6.7, 2013). As genotype input, imputed hard call genotypes filtered for MAF < 0.01 and estimated imputation quality rsq ≥ 0.3 were used.
To determine how the variants aggregated by the masks for the respective gene contribute to the association signal of the gene test, variants were ordered by their single variant ExWAS p-value from lowest to highest (n variants). Then, two metrics were defined: (1) add-one-in: for i from 1 to n, the burden test was computed using only the i variants with the lowest single variant p-value; (2) leave-one-out: for i from 0 to n − 1, the burden test was computed, removing the i variants with the lowest single variant p-values. Let p1, …, pi,…pn be the p-values of the burden tests for the “add-one-in” tests. We call a gene-trait association a “multi-variant signal” if SD{−log10(p1),.., -log10(pn)} ≥ 0.5, that is, the p-values of the burden tests with different variant sets show some variance. To facilitate interpretation, we assign these associations into three categories. Category 1 associations comprise multi-variant signals, where at least two variants are needed to reach significance (p1 ≥ 6.7 × 10−7, see above). Multi-variant associations where one variant is sufficient to reach significance (p1 < 6.7 × 10−7) are assigned to category 2. Associations that are not multi-variant signals were assigned to category 3.
Validation of imputation-based signals using data from whole exome sequencing of all corresponding UK Biobank participants
ExWAS were repeated for the 174 significant (p < 6.8 × 10−9) associations as well as the analyses for significant (p < 6.7 × 10−7) associations derived from GBT of aggregated variants, using sequence information for the same set of 408,511 individuals for which imputed sequences were available. Genotype calls were extracted from the final UKBB population-level OQFE exome variants (field: 23,158, version: Jul 2022) using PLINK; association analyses were performed with the same phenotype files and the same software packages as described above.
Cell type-specific expression of the associated genes in the kidney
To examine the expression of the associated genes in human kidney cell types, a single-cell RNA-seq dataset was downloaded from https://www.kidneycellatlas.org/25. The dataset included expression profiling of 40,268 human mature kidney cells in 27 cell clusters. The dataset was processed using the R package Seurat v353. The averaging expression values of cells in the same cluster were calculated and then transformed to z-scores so that an expression matrix of genes and cell clusters was obtained. We then used the Bioconductor package ComplexHeatmap v2.9.3 to generate heatmaps of the associated genes for each phenotype based on the expression matrix54. Immune cells were not included in the heatmaps.
GO-term enrichment analyses
We performed GO enrichment analyses using Bioconductor packages clusterProfiler v3.18.155. The input genes were from our WES analyses as well as those from published GWAS4,18,28. The EnrichGO function of clusterProfiler was used. GO terms were considered significantly enriched if the FDR-adjusted p-value < 0.05.
Experimental studies on a previously unreported CLDN10 frameshift mutation
Generation of claudin-10 mutant construct
The CLDN10b frameshift (fs) mutant was generated by 2-step PCR, using a previously published human CLDN10b wildtype (wt) construct29 and human kidney cDNA as templates. The following custom-made primers (Eurofins Genomics, Ebersberg, Germany) were employed: FOR1a 5′-aaaggatccAatggctagcacggc-3′; REV1a 5′-ctcttttagacataagcatttttcaaactgttttgaaggg-3′; FOR2b 5′-cccttcaaaacagtttgaaaaatgcttatgtctaaaagag-3′; REV1b 5′-ttgaggaatattctcagattgcccccatg-3′; REV2 5′-TTTgatatcttatgggagggccttgatgggatc-3′. The final PCR product was cloned into pcDNA 3.1-based vectors containing the sequence for enhanced yellow (EYFP) or cyan (ECFP) fluorescent protein, using BamHI and EcoRV restriction sites, to obtain N-terminally tagged claudin fusion proteins.
Culture and transfection of human embryonic kidney (HEK) 293 cells
HEK293 cells (CRL-1573, A.T.C.C., Manassas, VA, USA) were grown in 25 cm2 culture flasks in MEM-Earle’s media (Sigma-Aldrich, Munich, Germany) supplemented with 10% (v/v) fetal bovine serum, 100 U/mL penicillin, and 100 µg/ml streptomycin (Sigma-Aldrich), at 37 °C in a humidified 5% CO2 atmosphere. Cell line identity was confirmed by PCR single locus technology (Promega, PowerPlex 21 PCR Kit; Eurofins Medigenomix Forensik GmbH, Ebersberg, Germany) in 2018.
For transient transfection, HEK293 cells were seeded on poly-L-lysine (Sigma–Aldrich) coated coverslips in 6-well plates, at a density of 600,000 cells per well. 24 h later, cells were transfected by adding 500 µl cell culture medium (without supplements) per well, containing 10 µl polyethylenimine solution (Sigma-Aldrich, stock concentration 1 µg/µl), 2 µg EYFP- and 2 µg ECFP-plasmid DNA. Approximately 48 h after transfection, HEK293 cells were used for confocal laser scanning microscopy.
The following plasmid combinations were used: EYFP-Cldn10b wt–ECFP-Cldn10b wt, EYFP-Cldn10b fs–ECFP-Cldn10b fs, EYFP-Cldn10b wt–ECFP-Cldn10b fs, EYFP-Cldn10b fs–ECFP- Cldn10b wt. For comparison, the frameshift mutant previously found in Saudi Arabian HELIX syndrome patients37 was also included: EYFP-Cldn10b fs(SA)–ECFP-Cldn10b wt.
Live cell imaging: Förster resonance energy transfer (FRET) and enrichment assays
Live cell imaging was employed to quantify cis (FRET assay, Supplementary Fig. 14) and trans (enrichment assay, Supplementary Fig. 15) interaction of claudins56,57. Cover slips with transfected HEK293 cells were transferred into a HEPES-buffered, HCO3−-free bath solution (in mM: 134.6 NaCl, 2.4 Na2HPO4, 0.6 NaH2PO4, 5.4 KCl, 1.2 CaCl2, 1 MgSO4, 10 HEPES, and 10 d(+)-glucose, pH 7.4) and placed in a heated microscope chamber (37 °C) of a Zeiss LSM 780 confocal microscope. ECFP and EYFP fluorescence was excited at 458 nm and 514 nm, and emission was detected at 470–500 nm and 530–600 nm, respectively.
For the enrichment assay, fluorescence intensity was determined at cell–cell contacts of two claudin-expressing cells (Ic) and at the contacts of these two cells with cells not expressing claudins (Im1, Im2). The enrichment factor, EnF, was calculated as Ic/(Im1 + Im2), as depicted in Supplementary Fig. 15, and considered positive if the value was >1.
For the FRET assay, YFP was bleached 3 times 15 pulses of the 514 nm laser line at 100% intensity, and the relative change in CFP fluorescence intensity before (Ib) and after (Ia) acceptor bleach was evaluated (FRET efficiency = (Ia − Ib)/Ia × 100%), as depicted in Supplementary Fig. 1457. Ratios of YFP fluorescence intensity before bleach and CFP fluorescence intensity after bleach (YFP/CFP ratio) were calculated to assess the comparability of obtained FRET efficiencies.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
We used publicly available individual-level genotype and phenotype data from the UK Biobank (https://biobank.ndph.ox.ac.uk/showcase/). Access to these data needs to be requested from the UK Biobank. Our results are shared with the community using a comprehensive online resource: https://ckdgen-ukbb.gm.eurac.edu/
This work was conducted within approved UK Biobank application numbers 20272 and 64806.
Code availability
Custom scripts and code are available online at https://github.com/genepi-freiburg/seqmeta (https://doi.org/10.5281/zenodo.7564424). Code for other central tools is available online (REGENIE: https://rgcgithub.github.io/regenie, SeqMeta: https://rdrr.io/cran/seqMeta/, PLINK: https://www.cog-genomics.org/plink/).
References
Eckardt, K.-U. et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet 382, 158–169 (2013).
Köttgen, A. et al. New loci associated with kidney function and chronic kidney disease. Nat. Genet. 42, 376–384 (2010).
Pattaro, C. et al. Genetic associations at 53 loci highlight cell types and biological pathways relevant for kidney function. Nat. Commun. 7, 10023 (2016).
Wuttke, M. et al. A catalog of genetic loci associated with kidney function from analyses of a million individuals. Nat. Genet. 51, 957–972 (2019).
Okada, Y. et al. Meta-analysis identifies multiple loci associated with kidney function-related traits in east Asian populations. Nat. Genet. 44, 904–909 (2012).
Morris, A. P. et al. Trans-ethnic kidney function association study reveals putative causal genes and effects on kidney-specific disease aetiologies. Nat. Commun. 10, 29 (2019).
Böger, C. A. et al. CUBN is a gene locus for albuminuria. J. Am. Soc. Nephrol. 22, 555–570 (2011).
Haas, M. E. et al. Genetic association of albuminuria with cardiometabolic disease and blood pressure. Am. J. Hum. Genet. 103, 461–473 (2018).
Teumer, A. et al. Genome-wide association meta-analyses and fine-mapping elucidate pathways influencing albuminuria. Nat. Commun. 10, 4130 (2019).
Tin, A. et al. Large-scale whole-exome sequencing association studies identify rare functional variants influencing serum urate levels. Nat. Commun. 9, 4228 (2018).
Lin, B. M. et al. Whole genome sequence analyses of eGFR in 23,732 people representing multiple ancestries in the NHLBI trans-omics for precision medicine (TOPMed) consortium. EBioMedicine 63, 103157 (2021).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018).
Van Hout, C. V. et al. Exome sequencing and characterization of 49,960 individuals in the UK Biobank. Nature 586, 749–756 (2020).
Backman, J. D. et al. Exome sequencing and analysis of 454,787 UK Biobank participants. Nature 599, 628–634 (2021).
Wang, Q. et al. Rare variant contribution to human disease in 281,104 UK Biobank exomes. Nature 597, 527–532 (2021).
Barton, A. R., Sherman, M. A., Mukamel, R. E. & Loh, P.-R. Whole-exome imputation within UK Biobank powers rare coding variant association and fine-mapping analyses. Nat. Genet. 53, 1260–1269 (2021).
Nag, A. et al. Assessing the contribution of rare-to-common protein-coding variants to circulating metabolic biomarker levels via 412,394 UK Biobank exome sequences. https://doi.org/10.1101/2021.12.24.21268381 (2021).
Tin, A. et al. Target genes, variants, tissues and transcriptional pathways influencing human serum urate levels. Nat. Genet. 51, 1459–1474 (2019).
Rasouly, H. M. et al. The burden of candidate pathogenic variants for kidney and genitourinary disorders emerging from exome sequencing. Ann. Intern. Med. 170, 11–21 (2019).
Hasumi, H. et al. Folliculin-interacting proteins Fnip1 and Fnip2 play critical roles in kidney tumor suppression in cooperation with Flcn. Proc. Natl Acad. Sci. USA 112, E1624–E1631 (2015).
Centini, R. et al. Loss of Fnip1 alters kidney developmental transcriptional program and synergizes with TSC1 loss to promote mTORC1 activation and renal cyst formation. PloS ONE 13, e0197973 (2018).
Domingo-Gallego, A. et al. Clinical and genetic characterization of a cohort of proteinuric patients with biallelic CUBN variants. Nephrol. Dial. Transplant. https://doi.org/10.1093/ndt/gfab285 (2021).
Domingo-Gallego, A. et al. Clinical utility of genetic testing in early-onset kidney disease: seven genes are the main players. Nephrol. Dial. Transplant. 37, 687–696 (2022).
Ewing, R. M. et al. Large-scale mapping of human protein-protein interactions by mass spectrometry. Mol. Syst. Biol. 3, 89 (2007).
Stewart, B. J. et al. Spatiotemporal immune zonation of the human kidney. Science 365, 1461–1466 (2019).
Park, J. et al. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 360, 758–763 (2018).
Wu, H. et al. Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell 23, 869–881.e8 (2018).
Stanzick, K. J. et al. Discovery and prioritization of variants and genes for kidney function in >1.2 million individuals. Nat. Commun. 12, 4350 (2021).
Günzel, D. et al. Claudin-10 exists in six alternatively spliced isoforms that exhibit distinct localization and function. J. Cell Sci. 122, 1507–1517 (2009).
Milatz, S. et al. Mosaic expression of claudins in thick ascending limbs of Henle results in spatial separation of paracellular Na+ and Mg2+ transport. Proc. Natl Acad. Sci. USA 114, E219–E227 (2017).
Breiderhoff, T. et al. Claudin-10a deficiency shifts proximal tubular cl- permeability to cation selectivity via claudin-2 redistribution. J. Am. Soc. Nephrol. 33, 699–717 (2022).
Milatz, S. A novel claudinopathy based on claudin-10 mutations. Int. J. Mol. Sci. 20, E5396 (2019).
Klar, J. et al. Altered paracellular cation permeability due to a rare CLDN10B variant causes anhidrosis and kidney damage. PLoS Genet. 13, e1006897 (2017).
Meyers, N. et al. Hypokalemia associated with a claudin 10 mutation: a case report. Am. J. Kidney Dis. 73, 425–428 (2019).
Hadj-Rabia, S. et al. Multiplex epithelium dysfunction due to CLDN10 mutation: the HELIX syndrome. Genet. Med. 20, 190–201 (2018).
Bongers, E. M. H. F. et al. A novel hypokalemic-alkalotic salt-losing tubulopathy in patients with CLDN10 mutations. J. Am. Soc. Nephrol. 28, 3118–3128 (2017).
Alzahrani, A. S. et al. A novel claudin-10 mutation with a unique mechanism in two unrelated families with HELIX syndrome. Kidney Int. 100, 415–429 (2021).
Sewerin, S. et al. Defective claudin-10 causes a novel variation of HELIX syndrome through compromised tight junction strand assembly. Genes Dis. 9, 1301–1314 (2022).
Günzel, D. & Yu, A. S. L. Claudins and the modulation of tight junction permeability. Physiol. Rev. 93, 525–569 (2013).
Szustakowski, J. D. et al. Advancing human genetics research and drug discovery through exome sequencing of the UK Biobank. Nat. Genet. 53, 942–948 (2021).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612 (2009).
Loh, P.-R. et al. Reference-based phasing using the Haplotype Reference Consortium panel. Nat. Genet. 48, 1443–1448 (2016).
Das, S. et al. Next-generation genotype imputation service and methods. Nat. Genet. 48, 1284–1287 (2016).
McLaren, W. et al. The Ensembl Variant Effect Predictor. Genome Biol. 17, 122 (2016).
Ioannidis, N. M. et al. REVEL: an ensemble method for predicting the pathogenicity of rare missense variants. Am. J. Hum. Genet. 99, 877–885 (2016).
Rentzsch, P., Witten, D., Cooper, G. M., Shendure, J. & Kircher, M. CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 47, D886–D894 (2019).
Karczewski, K. J. et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581, 434–443 (2020).
Dong, C. et al. Comparison and integration of deleteriousness prediction methods for nonsynonymous SNVs in whole exome sequencing studies. Hum. Mol. Genet. 24, 2125–2137 (2015).
Rogers, M. F. et al. FATHMM-XF: accurate prediction of pathogenic point mutations via extended features. Bioinformatics 34, 511–513 (2018).
Liu, X., Li, C., Mou, C., Dong, Y. & Tu, Y. dbNSFP v4: a comprehensive database of transcript-specific functional predictions and annotations for human nonsynonymous and splice-site SNVs. Genome Med. 12, 103 (2020).
Mbatchou, J. et al. Computationally efficient whole-genome regression for quantitative and binary traits. Nat. Genet. 53, 1097–1103 (2021).
Chang, C. C. et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience 4, 7 (2015).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902.e21 (2019).
Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849 (2016).
Yu, G., Wang, L.-G., Han, Y. & He, Q.-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics J. Integr. Biol. 16, 284–287 (2012).
Milatz, S. et al. Tight junction strand formation by claudin-10 isoforms and claudin-10a/-10b chimeras. Ann. N. Y. Acad. Sci. 1405, 102–115 (2017).
Milatz, S. et al. Probing the cis-arrangement of prototype tight junction proteins claudin-1 and claudin-3. Biochem. J. 468, 449–458 (2015).
Acknowledgements
The work of M.W. and A.K. was supported by German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) Project ID 431984000, SFB 1453. A.T. was funded by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) Project ID 455978266. C.F. was supported by the National Institutes of Health grant R01 HG009976. M.L. was funded by an International Mobility grant of the Autonomous Province of Bolzano under Project number D54I18000150003. D.G. was funded by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) Project ID 318374368 and GRK 2318 Project ID 318905415. This research has been conducted using the UK Biobank Resource under Application Numbers 20272 and 64806.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
A.K. and C.F. designed and supervised the study. M.W. performed the ExWAS, and QC analysis, and compiled results. E.K. performed and validated the imputation. M.A.K., H.K., A.T., and Y.L. performed additional analysis. M.W., E.K., M.A.K., H.K., A.T., Y.L., B.G., S.K.F., and D.G. created the figures. M.W. and A.K. created the tables. S.K.F. and D.G. performed the biological experiments. A.K., C.F, M.W., M.L., E.K., A.T., H.K., Y.L., D.G., and C.P. contributed to the writing and proof reading of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Dongjing Liu and Stefan Stender for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wuttke, M., König, E., Katsara, MA. et al. Imputation-powered whole-exome analysis identifies genes associated with kidney function and disease in the UK Biobank. Nat Commun 14, 1287 (2023). https://doi.org/10.1038/s41467-023-36864-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-36864-8
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.