Abstract
A reaction intermediate is a key molecular entity that has been used in explaining how starting materials converts into the final products in the reaction, and it is usually unstable, highly reactive, and short-lived. Extensive efforts have been devoted in identifying and characterizing such species via advanced physico-chemical analytical techniques. As an appealing alternative, trapping experiments are powerful tools in this field. This trapping strategy opens an opportunity to discover multicomponent reactions. In this work, we report various highly diastereoselective and enantioselective four-component reactions (containing alcohols, diazoesters, enamines/indoles and aldehydes) which involve the coupling of in situ generated intermediates (iminium and enol). The reaction conditions presented herein to produce over 100 examples of four-component reaction products proceed under mild reaction conditions and show high functional group tolerance to a broad range of substrates. Based on experimental and computational analyses, a plausible mechanism of this multicomponent reaction is proposed.
Similar content being viewed by others
Introduction
Reaction intermediates are molecular entities that form from starting materials and readily convert to final products. The identification of such intermediates is necessary towards understanding the mechanism of a reaction1,2. However, these intermediates are highly reactive, short-lived, and their concentration is often very low3. Therefore, studies on this entity have remained a tremendous challenge in regarding to its structure, reactivity, and synthetic applications3,4,5. Over the years, remarkable progress has been made in identifying and characterizing the structure of these intermediate species via advanced physico-chemical analytical techniques (e.g. time-resolved spectroscopy, mass spectrometry)6. Recently, the interception of the reaction intermediate with bench-stable chemicals represents an appealing alternative, not only providing the evidence for the existence of specific intermediates, but also offering an efficient approach to observe its chemical reactivity4,7,8. Additionally, this method opens an opportunity to discovering new chemical reactions, which could have a great impact on in the pharmaceutical, material and fine chemical industry9.
Multicomponent reactions (MCRs) that involve multiple starting materials typically react in a stepwise manner to rapidly yield complex products in a greener and more economical manner due to having greater than three highly diversifiable starting materials10,11,12. Unlike traditional reactions, multicomponent reactions increase the accessible chemical space exponentially with each additional reaction component13,14. The discovery of MCRs, especially with a ‘higher order’ variant, are particularly challenging and have emerged as the frontiers in contemporary organic synthesis15,16. Furthermore, stereochemical identification can be challenging while the formation of multiple chemical bonds takes place while various types of highly reactive intermediates are rapidly produced in the process10. For example, the Ugi four-component reaction utilizes an aldehyde, amine, carboxylic acid, and isocyanide to afford α-aminoacyl amide derivatives is the most well-known multicomponent reaction and was first reported by Ugi and coworkers in 195911. An enantioselective version of this reaction has only recently been published by Tan and coworkers in 201817, although its four-component product owns only one chiral center. Considering increasing demand for the development of highly attainable and selective MCRs, the robust and general strategy that can introduce new transformations with a ‘higher order’ variant is highly needed15,16.
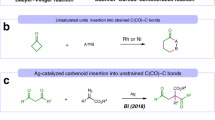
As shown in Fig. 1a, previously developed three-component reactions involved using a bench-stable chemical to trap the reaction intermediate generated from traditional reactions18. Through the sequential assembly of reaction intermediates and trapping reagents, rare examples of MCRs with four variants (four-component reactions) have been discovered in history17,19,20. Given that various bench-stable components could successfully trap the transient reaction intermediate, we further questioned whether it would be possible to trap such intermediate with another intermediate generated by another independent process. The resultant process would be used to discover MCRs with four variants if distinct substrates generate both reaction intermediates15,21,22. However, this strategy suffered numerous challenges in a spatial-temporal version, requiring each intermediate should be generated simultaneously at similar rates. The reaction of these two intermediates occurred in a highly efficient, specific, and selective manner3,8,23. Otherwise, incompatibility of various reactants, uncontrollable sequence of chemical bond formation, and irreversible side reactions would hamper the development of such types of transformations24. Our previous findings suggested that the catalyst system heavily influences the generation of reaction intermediates and their compatibility with trapping reagents (Fig. 1b, c)25,26,27,28,29,30. With an proper choice of catalytic systems31,32,33,34,35,36,37,38,39,40,41,42,43,44,45, simultaneous generation of two types of reaction intermediates with compatible chemical reactivity via distinct catalytic cycles could be possible3,23, thus providing an opportunity to be of use in discovering different MCRs.
a Lego-like synergistic organic synthesis. b General process of trapping type MCRs developed by our group. c The representative examples of 3/4CRs involving the use of CPA/diazo esters. d This 4CR via intermediate cross interception. NOTE: Nu is short for nucleophile, Co-cat. Is short for co-catalyst, CPA is short for chiral phosphoric acid, [MLn] is short for metal catalyst, [Am] is short for amine catalyst, [H+] is short for Brønsted acid catalyst.
It has been extensively explored that diazoester could react with alcohol under the catalysis of transition metal to afford oxonium ylide intermediate (or its enol form), a highly reactive nucleophilic species, which various electrophiles could trap according to previous findings18. In addition, the generation of α,β-unsaturated iminium intermediate using indole and aldehyde under the catalysis of both Brønsted acid and amine via Knoevenagel-type reaction was well-documented46. Coupling the two intermediates above would finally deliver the desired 4CRs (Fig. 1d). To our best knowledge, this intermediate cross interception above has not been disclosed. Although this designed strategy was straightforward, numerous potential side reactions would irreversibly consume newly generated reactive intermediates to produce an array of undesired side products47,48,49. For example, the metal carbene species has been reported to react with various reagents50,51, including aldehydes, imines, indoles, and amines, in the chemical system, generating those undesired ylides or zwitterionic intermediates18. Other potential side reactions of oxonium ylide with aldehydes or imines are also possible in this design30. Furthermore, the stereocontrol issue remains a formidable challenge, especially involving two vicinal quaternary/tertiary stereocenters52.
In this work, we report various highly diastereoselective and enantioselective four-component reactions (containing alcohols, diazoesters, enamines/indoles and aldehydes) which involve the coupling of in situ generated intermediates (iminium and enol).
Results and discussion
Substrate scope
To validate our conceptual hypothesis, we firstly explored the proposed four-component reaction with 4-bromobenzyl alcohol 1a, tert-butyl phenyldiazoesters 2a, 2-methylindole 3A, and 3-phenylpropiolaldehyde 4a as model substrates. Thorough optimizations involved the evaluation of reaction parameters and ultimately led to the following optimal reaction conditions: 5 mol% Pd(CH3CN)2Cl2, 10 mol% SPINOL-derived chiral phosphoric acid 6a, and 50 mol% 2,5-bis(trifluoromethyl)aniline 5a at −20 °C in DCE (Supplementary Tables 1 and 3).
Under the optimized conditions, the substrate scopes of this asymmetric four-component reaction (A4CR) were then investigated (Fig. 2, Supplementary Fig. 1, Supplementary Data). Initially, we examined the primary alcohols 1 and found that benzyl alcohols with diverse functional groups were proven to be suitable substrates (7–10). We were pleased to observe that heteroaromatic alcohols, such as those with furanyl and benzothienyl groups, could readily be employed efficiently (11–12). Moreover, cinnamyl alcohol, allyl alcohol, and 3-phenyl-2-propyn-1-ol were highly applicable to the present reaction and gave desired products in synthetically useful yields without compromising the diastereo- and enantioselectivity (13–15). Concerning the α-diazoesters, a variety of ortho-, meta-, and para- substituted α-aryl-α-diazoesters with various functional groups underwent efficient and highly A4CRs (16–21), and the absolute configuration of 21 was confirmed by X-ray crystallographic analysis (Supplementary Fig. 5). 2-Ethyl indole also resulted in high ee (93%) of the desired product. Notably, 2,5 or 2,6-disubstituted indoles containing bromo, alkyl, phenyl, Bpin, amino, and sulfanyl groups were converted to their corresponding products in satisfying yields with good to excellent enantioselectivity (22–29). Concerning the aldehyde, various substituted (hetero)aromatic ynals were well tolerated in condition A, leading to corresponding products in good yield and diastereo- and enantioselectivity (30–37). Additionally, we investigated the substrate limitation of this A4CR. For example, secondary alcohol, tertiary alcohol, unprotected indoles, indole with a phenyl group at 2-position, and simple aryl aldehyde could not convert to their related products under the catalytic condition A (Supplementary Fig. 1b).
Standard conditions: 1/2/3/4/5a/[Pd]/6a = 0.6/0.3/0.2/0.6/0.1/0.01/0.02 mmol. 2, 3 in 4.0 mL dry DCE was added into a solution of 1, 4, 5a (50 mol%), [Pd] (5 mol%), 6a (10 mol%), and 4 Å MS (50 mg) in 4.0 mL dry DCE via a syringe pump for 60 min, and the resulting mixture was stirred for another 12 h at −20 °C.
We next explored the extension of the present A4CR to other compounds containing enamine motifs. 4-(1-Phenylvinyl)morpholine was identified as a proper substrate under condition B (Supplementary Tables 2 and 4). Figure 3 summarizes the detailed results for this A4CR. A broad spectrum of alcohols, α-diazoesters, enamines, and aldehydes was then investigated (38–86) (Supplementary Fig. 2, Supplementary Data). Like the A4CRs above, the primary alcohols and α-diazoesters with various functional groups and heterocycles were well-tolerated, delivering the corresponding four-component products with satisfied reactivity and selectivity (38–65). The absolute stereochemistry of 41 was concluded by X-ray crystallographic analysis of its derivative (Supplementary Fig. 6). Of particularly noted, we were delighted to find that α-alkyl-α-diazoesters also readily participate in this reaction (66 & 67), demonstrating the diversity of substituents. Enamines with different substituents were also amenable to this protocol, affording the desired products (68–70) in good yields and with excellent diastereo- and enantioselectivities. We next evaluated the scope of aldehydes. Diverse propiolaldehydes showed excellent reactivity regardless of whether the substituents were electron-donating or electron-withdrawing groups (71–77). To further broaden the utility of this protocol, we further explored α,β-unsaturated aldehydes. Interestingly, α,β-unsaturated aldehydes bearing ester and ketone groups were perfectly tolerated under the condition B, furnishing the desired products (78–82) in good yields and with excellent diastereo- and enantioselectivities. Moreover, aromatic aldehydes could also function efficiently in this four-component coupling protocol. For example, para-nitro aldehyde and its 3-methyl analog readily reacted with various alcohols in the current protocol (83–86) (Fig. 3, Supplementary Fig. 2a). Additionally, we investigated the substrate limitation of this A4CR. For example, secondary alcohol, tertiary alcohol, diazoacetate, alkyl enamine and simple aryl aldehyde could not convert to their related products under the catalytic condition B (Supplementary Fig. 2b).
Standard condition: 1/2/3/4/5b/[PdCl(allyl)]2/6b = 0.18/0.18/0.18/0.15/0.075/0.0075/0.015 mmol, 2 and 3a in 1.0 mL dry DCM were added into a solution of 1, 4, [PdCl(allyl)]2, 6b, aniline 5b and 100 mg 4 Å MS in 1.5 mL dry DCM via a syringe pump under a nitrogen atmosphere for 3 h, and the resulting mixture was stirred for another 1 h at −10 °C.
Formal higher-order MCRs design and synthetic applications
The high functional group tolerance encouraged us to further investigate the practical utility of this protocol. Notably, several classic MCR products are also compatible with this enantioselective 4CR, including Passerini-3CR11, Petasis-3CR53, Hantzsch-4CR54, Catellani-4CR55, Ugi-Tetrazole-4CR14, and enantioselective Ugi-4CR11,17, affording the desired products (87–92) with 6 or 7 formal variants in satisfying yields and excellent enantioselectivities in two steps (Fig. 4a, Supplementary Fig. 3, Supplementary Methods). We also performed several transformations of the obtained four-component products, shown in Fig. 4b. For example, when 38 was reduced by H2 in the presence of Pd/C, the product 39 containing three chiral carbon centers was obtained in satisfying yield with excellent diastereoselectivity, and its absolute configuration was predicted by Electronic circular dichroism (Supplementary Fig. 7). 42 could readily be converted into benzoxepine 94 in 72% yield via a palladium-mediated reductive Mizoroki–Heck cyclization, and we were delighted to observe the excellent diastereoselectivity and geometric selectivity (Z:E > 20:1) of the desired product. Notably, treatment of 83 with 20 mol% Yb(OTf)3 could effectively remove the tert-butyl group to form the carboxylic acid 95 in 75% yield without losing the diastereo- and enantioselectivity. In addition, treatment of 47 with 1.0 equiv. trifluoroacetic acid (TFA) readily afforded the formal product of the four-component reaction with H2O (96) in 60% yield. When 20.0 equiv. TFA was used, and we obtained the α-hydroxyl acid 97 in 65% yield without losing the diastereo- and enantioselectivity (Supplementary Methods, Supplementary Fig. 4).
Mechanistic investigations
DFT calculations were performed to explore the mechanism of this A4CR and the origin of stereoselectivity (Supplementary Discussion, Supplementary Figs. 8–13). Combining with the mechanistic studies (Supplementary Discussion), a catalytic cycle was proposed in Fig. 5a. In the amine-CPA catalyzed cycle, intermediate INT-2 formed via a CPA catalyzed nucleophilic addition of aniline 5b on aldehyde 4a and elimination of a water molecule. Subsequently, INT-2 was attacked by enamine 3a, resulting in the formation of INT-4. After a subsequent CPA anion-assisted proton transfer, INT-5 was generated, which delivered the key intermediate INT-6 and aniline 5b via transition state TS-5. Calculations showed that the overall energy barrier was 19.4 kcal/mol for the formation of intermediate INT-6. In the transition-metal-catalyzed cycle, the dissociation of palladium dimer precatalyst by imine gave INT-7, which took an exchange with diazoester 2a to give INT-8. Then palladium-catalyzed decomposition of diazoester 2a led to carbene intermediate INT-9 and the nitrogen release. Oxonium ylide INT-10 was formed from carbene INT-9 by the attack of benzyl alcohol and then transformed into a more stable Pd-associated enol INT-11 through a quick intramolecular [1,3]-proton shift. In an exergonic process, an exchange between INT-11 and imine gave free enol INT-12 and regenerated palladium catalyst. For the formation of free enol INT-12, the overall barrier was 21.0 kcal/mol. Finally, cross interception of the two active intermediates, INT-6 and INT-12, took place in the chiral pocket of CPA 6b anion, providing the four-component product with simultaneously regenerated the CPA 6b. The main challenge of cross interception of the in situ formed two active intermediates is that they need a comparable formation rate to avoid consumption before cross interception occurs. DFT calculations demonstrated that the generation of the two active intermediates, INT-6 and free enol INT-12, had matched energy barriers (19.4 kcal/mol versus 21.0 kcal/mol), enabling the four-component reaction to proceed smoothly.
a Proposed catalytic cycle. b Optimized transition state geometries of symmetric Michael addition and origin of stereoselectivity. c Control experiments. In a and b, the Gibbs free energies were computed with CPCM(DCM)-M06-2X/6-311+G(d,p)[SDD for Pd]//CPCM(DCM)-B3LYP/6-31G(d)[LANL2DZ for Pd]. Most hydrogen atoms are omitted for clarity. All distances are in angstroms.
Asymmetric Michael addition afforded products with successive α,β-stereocenters, and the stereoisomeric TSs are presented in Fig. 5b. Among them, TS-SR had lower free energy than that of TS-RS and TS-SS by 2.4 and 3.8 kcal/mol, respectively. This is in accordance with the 92% ee and 94:6 dr obtained experimentally. In comparing TS-SR with the others (TS-RS, TS-RR, and TS-SS), favorable C-H…π and C-H…O interactions between CPA and the large tert-butyl group in enol led to the preference for the formation of the predominant product. While less C-H…π interactions were present in the other transition states. This result indicated that non-covalent interaction plays a critical role in controlling stereoselectivity56. Additional DFT calculations were conducted to clarify the importance of nonbonding interactions between CPA and substrate (Fig. 5b, bottom). Replacing tert-butyl group by a much smaller methyl group in enol, the C-H…π and C-H…O interactions disappeared in the asymmetric Michael addition transition states. As a result, the relative free Gibbs energy difference between TS-SR-Me and TS-RS-Me (∆∆G‡ = 0.4 kcal/mol) became smaller than that between TS-SR and TS-RS (∆∆G‡ = 2.4 kcal/mol), indicating a poor stereoselectivity without a tert-butyl group. Moreover, using 6c, a CPA with a smaller chiral pocket, and diazoester 2a with a large tert-butyl group, TS-SR-Ph and TS-RS-Ph became flexible where these non-covalent interactions were also not present. The significant decrease in relative free Gibbs energy (∆∆G‡ = 0.2 kcal/mol) demonstrated that the 9-anthryl group was essential in determining the stereoselectivity. Control experiments were carried out and shown in Fig. 5c, and poor selectivity was obtained using either CPA 6b and diazoester 2b (21% ee, 83:17 dr) or CPA 6c and diazoester 2a (6% ee, 84:16 dr). It is clear that both the tert-butyl group in diazoester (2a) and the 9-anthryl group in CPA (6b) are critical to enabling the asymmetric Michael addition with high selectivities through non-covalent interactions.
In conclusion, the four-component reactions of alcohols, diazoesters, enamines/indoles, and aldehydes are developed, affording the corresponding products in satisfying yields with good to excellent chemo-, diastereo- and enantioselectivity. These four-component reactions proceed under mild reaction conditions and show high functional group tolerance. In addition, some classic MCRs products are well-compatible with the present four-component processes, affording several unprecedented examples of formal higher-order MCRs. Mechanism studies demonstrated that the comparable energy barriers of iminium and enol intermediates formation through independent processes ensure the success of this enantioselective four-component reaction. Both CPA and diazoester are critical to our reaction and responsible for acquiring excellent stereoselectivity. Furthermore, these multicomponent methods open more opportunities for developing the MCRs by assembling two reaction intermediates generated by independent catalytic processes.
Methods
General procedure for enantioselective four-component reactions of alcohol, diazoester, indole and aldehyde derivatives
To a flame-dried 20-mL Schlenk flask charged with a magnetic stirring bar, alcohol 1 (0.6 mmol), aldehyde 4 (0.6 mmol), 2,5-bis(trifluoromethyl)aniline 5a (50 mol%), Pd (CH3CN)2Cl2 (10 mol%), (S)-6a (10 mol%) and 4 Å MS (100 mg) in DCE (4.0 mL), was added a mixture of diazoacetate 2 (0.3 mmol) and indole 3A-3I (0.2 mmol) in DCE (4.0 mL) for 1.0 h via a syringe pump at −20 °C. The mixture was stirred for another 12 h at −20 °C. After the completion of the reaction, the reaction mixture was filtrated and the filtrate was evaporated in vacuo to give the crude product. And then the crude product was purified by flash chromatography on silica gel (eluent: PE: EA = 20: 1) to afford the pure products.
General procedure for enantioselective four-component reactions of alcohol, diazoester, enamine and aldehyde derivatives
To a flame-dried 10-mL Schlenk flask charged with a magnetic stirring bar, 3,4-dimethoxyaniline 5b (0.075 mmol), 5.0 mol% [PdCl(allyl)]2, 10.0 mol% (R)-6b, alcohol 1(0.18 mmol), aldehyde 4 (0.15 mmol) and 100 mg 4 Å MS in 2 mL DCM were sequentially added at −10 °C. Diazoacetate 2 (0.18 mmol) and enamine 3a–3c (0.18 mmol) dissolved in DCM (1.5 mL) were added by syringe pump over 3 h. The mixture was stirred for another 1.0 h at −10 °C. After the completion of the reaction, the reaction mixture was filtrated and the filtrate was evaporated in vacuo to give the crude product. And then the crude product was purified by flash chromatography on silica gel (eluent: PE/EA = 50:1 ~ 30:1, v/v) to give the pure product (If the reaction was carried out on more than 0.15 mmol scale, the decreased ee value of corresponding product might be detected).
Data availability
CCDC (2156063&1948222) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Experimental procedures, characterization of all new compounds, computational details, Supplementary Tables and Supplementary Figures are available in the Supplementary Information. The authors declare that the data supporting the manuscript are included in the manuscript and supplementary materials.
References
Trost, B. M. & Tracy, J. S. Catalytically generated vanadium enolates formed via interruption of the Meyer-Schuster rearrangement as useful reactive intermediates. Acc. Chem. Res. 53, 1568–1579 (2020).
Seath, C. P., Trowbridge, A. D., Muir, T. W. & MacMillan, D. W. C. Reactive intermediates for interactome mapping. Chem. Soc. Rev. 50, 2911–2926 (2021).
Allen, A. E. M. & Macmillan, D. W. Synergistic catalysis: a powerful synthetic strategy for new reaction development. Chem. Sci. 2012, 633–658 (2012).
Kalek, M. & Himo, F. Combining Meyer-Schuster rearrangement with aldol and Mannich reactions: theoretical study of the intermediate interception strategy. J. Am. Chem. Soc. 134, 19159–19169 (2012).
Wendlandt, A. E., Vangal, P. & Jacobsen, E. N. Quaternary stereocentres via an enantioconvergent catalytic SN1 reaction. Nature 556, 447–451 (2018).
Roithova, J. Characterization of reaction intermediates by ion spectroscopy. Chem. Soc. Rev. 41, 547–559 (2012).
Yamano, M. M. et al. Intercepting fleeting cyclic allenes with asymmetric nickel catalysis. Nature 586, 242–247 (2020).
Trost, B. M., Luan, X. & Miller, Y. Contemporaneous dual catalysis: chemoselective cross-coupling of catalytic vanadium-allenoate and pi-allylpalladium intermediates. J. Am. Chem. Soc. 133, 12824–12833 (2011).
Romiti, F. et al. Different strategies for designing dual-catalytic enantioselective processes: from fully cooperative to non-cooperative systems. J. Am. Chem. Soc. 141, 17952–17961 (2019).
Ruijter, E., Scheffelaar, R. & Orru, R. V. Multicomponent reaction design in the quest for molecular complexity and diversity. Angew. Chem. Int. Ed. 50, 6234–6246 (2011).
Wang, Q., Wang, D. X., Wang, M. X. & Zhu, J. Still unconquered: enantioselective Passerini and Ugi multicomponent reactions. Acc. Chem. Res. 51, 1290–1300 (2018).
Trost, B. M. The atom economy-a search for synthetic efficiency. Science 254, 1471–1477 (1991).
Domling, A., Wang, W. & Wang, K. Chemistry and biology of multicomponent reactions. Chem. Rev. 112, 3083–3135 (2012).
Neochoritis, C. G., Zhao, T. & Domling, A. Tetrazoles via multicomponent reactions. Chem. Rev. 119, 1970–2042 (2019).
Brauch, S., van Berkel, S. S. & Westermann, B. Higher-order multicomponent reactions: beyond four reactants. Chem. Soc. Rev. 42, 4948–4962 (2013).
Zarganes-Tzitzikas, T., Chandgude, A. L. & Domling, A. Multicomponent reactions, union of MCRs and beyond. Chem. Rec. 15, 981–996 (2015).
Zhang, J. et al. Asymmetric phosphoric acid-catalyzed four-component Ugi reaction. Science 361, eaas8707 (2018).
Zhang, D. & Hu, W. Asymmetric multicomponent reactions based on trapping of active intermediates. Chem. Rec. 17, 739–753 (2017).
Xiong, Q., Dong, S., Chen, Y., Liu, X. & Feng, X. Asymmetric synthesis of tetrazole and dihydroisoquinoline derivatives by isocyanide-based multicomponent reactions. Nat. Commun. 10, 2116 (2019).
Zhang, S. et al. Delayed catalyst function enables direct enantioselective conversion of nitriles to NH2-amines. Science 364, 45–51 (2019).
Wender, P. A. et al. Structural complexity through multicomponent cycloaddition cascades enabled by dual-purpose, reactivity regenerating 1,2,3-triene equivalents. Nat. Chem. 6, 448–452 (2014).
Bakulina, O., Inyutina, A., Dar’in, D. & Krasavin, M. Multicomponent reactions involving diazo reagents: a 5-year update. Molecules 26, 6563 (2021).
Kim, B., Kim, Y. & Lee, S. Y. Stereodivergent carbon-carbon bond formation between iminium and enolate intermediates by synergistic organocatalysis. J. Am. Chem. Soc. 143, 73–79 (2021).
Xu, J. H., Zheng, S. C., Zhang, J. W., Liu, X. Y. & Tan, B. Construction of tropane derivatives by the organocatalytic asymmetric dearomatization of isoquinolines. Angew. Chem. Int. Ed. 55, 11834–11839 (2016).
Kang, Z. et al. Ternary catalysis enabled three-component asymmetric allylic alkylation as a concise track to chiral alpha,alpha-disubstituted ketones. J. Am. Chem. Soc. 143, 20818–20827 (2021).
Hu, W. et al. Cooperative catalysis with chiral Brønsted acid-Rh2(OAc)4: highly enantioselective three-component reactions of diazo compounds with alcohols and imines. J. Am. Chem. Soc. 130, 7782–7783 (2008).
Qiu, H. et al. Highly enantioselective trapping of zwitterionic intermediates by imines. Nat. Chem. 4, 733–738 (2012).
Zhang, D., Zhou, J., Xia, F., Kang, Z. & Hu, W. Bond cleavage, fragment modification and reassembly in enantioselective three-component reactions. Nat. Commun. 6, 5801 (2015).
Xu, X., Zhou, J., Yang, L. & Hu, W. Selectivity control in enantioselective four-component reactions of aryl diazoacetates with alcohols, aldehydes and amines: an efficient approach to synthesizing chiral beta-amino-alpha-hydroxyesters. Chem. Commun. 6564–6566 (2008).
Guo, X. & Hu, W. Novel multicomponent reactions via trapping of protic onium ylides with electrophiles. Acc. Chem. Res. 46, 2427–2440 (2013).
Sancheti, S. P., Urvashi, Shah, M. P. & Patil, N. T. Ternary catalysis a stepping stone towards multicatalysis. ACS Catal. 10, 3462–3489 (2020).
Brak, K. & Jacobsen, E. N. Asymmetric ion-pairing catalysis. Angew. Chem. Int. Ed. 52, 534–561 (2013).
Milo, A., Neel, A. J., Toste, F. D. & Sigman, M. S. A data-intensive approach to mechanistic elucidation applied to chiral anion catalysis. Science 347, 737–743 (2015).
Mahlau, M. & List, B. Asymmetric counteranion-directed catalysis: concept, definition, and applications. Angew. Chem. Int. Ed. 52, 518–533 (2013).
Wang, P. S., Lin, H. C., Zhai, Y. J., Han, Z. Y. & Gong, L. Z. Chiral counteranion strategy for asymmetric oxidative C(sp(3))-H/C(sp(3))-H coupling: enantioselective alpha-allylation of aldehydes with terminal alkenes. Angew. Chem. Int. Ed. 53, 12218–12221 (2014).
Capacci, A. G., Malinowski, J. T., McAlpine, N. J., Kuhne, J. & MacMillan, D. W. C. Direct, enantioselective alpha-alkylation of aldehydes using simple olefins. Nat. Chem. 9, 1073–1077 (2017).
Phipps, R. J., Hamilton, G. L. & Toste, F. D. The progression of chiral anions from concepts to applications in asymmetric catalysis. Nat. Chem. 4, 603–614 (2012).
Li, M. L., Yu, J. H., Li, Y. H., Zhu, S. F. & Zhou, Q. L. Highly enantioselective carbene insertion into N–H bonds of aliphatic amines. Science 366, 990–994 (2019).
Fogg, D. E. & dos Santos, E. N. Tandem catalysis: a taxonomy and illustrative review. Coord. Chem. Rev. 248, 2365–2379 (2004).
Wasilke, J. C., Obrey, S. J., Baker, R. T. & Bazan, G. C. Concurrent tandem catalysis. Chem. Rev. 105, 1001–1020 (2005).
Zhou, J. Recent advances in multicatalyst promoted asymmetric tandem reactions. Chem. Asian J. 5, 422–434 (2010).
Patil, N. T., Shinde, V. S. & Gajula, B. A one-pot catalysis: the strategic classification with some recent examples. Org. Biomol. Chem. 10, 211–224 (2012).
Pellissier, H. & Pellissier, H. Recent developments in enantioselective multicatalysed tandem reactions. Tetrahedron 69, 7171–7210 (2013).
Inamdar, S. M., Shinde, V. S. & Patil, N. T. Enantioselective cooperative catalysis. Org. Biomol. Chem. 13, 8116–8162 (2015).
Martinez, S., Veth, L., Lainer, B. & Dydio, P. Challenges and opportunities in multicatalysis. Acs. Catal. 11, 3891–3915 (2021).
Lancianesi, S., Palmieri, A. & Petrini, M. Synthetic approaches to 3-(2-nitroalkyl) indoles and their use to access tryptamines and related bioactive compounds. Chem. Rev. 114, 7108–7149 (2014).
Padwa, A., Dean, D. C., Osterhout, M. H., Precedo, L. & Semones, M. A. Synthesis of functionalized azomethine ylides via the Rh (II)-catalyzed cyclization of. alpha-diazo carbonyls onto imino. pi-bonds. J. Org. Chem. 59, 5347–5357 (1994).
Terada, M. & Toda, Y. Relay catalysis using a rhodium complex/chiral Bronsted acid binary system: enantioselective reduction of a carbonyl ylide as the reactive intermediate. Angew. Chem. Int. Ed. 51, 2093–2097 (2012).
Yan, M., Zhao, W. J., Huang, D. & Ji, S. J. Unusual reaction of aryldiazoacetates with enamines: highly effective synthesis of γ-ketoesters. Tetrahedron Lett. 45, 6365–6367 (2004).
Doyle, M. P. Electrophilic metal carbenes as reaction intermediates in catalytic reactions. Acc. Chem. Res. 19, 348–356 (1986).
Davies, H. M. L. & Liao, K. Dirhodium tetracarboxylates as catalysts for selective intermolecular C-H functionalization. Nat. Rev. Chem. 3, 347–360 (2019).
Krautwald, S., Sarlah, D., Schafroth, M. A. & Carreira, E. M. Enantio- and diastereodivergent dual catalysis: a-allylation of branched aldehydes. Science 340, 1065–1068 (2013).
Wu, P., Givskov, M. & Nielsen, T. E. Reactivity and synthetic applications of multicomponent Petasis reactions. Chem. Rev. 119, 11245–11290 (2019).
Evans, C. G. & Gestwicki, J. E. Enantioselective organocatalytic Hantzsch synthesis of polyhydroquinolines. Org. Lett. 11, 2957–2959 (2009).
Chen, S. et al. The discovery of a palladium(II)-initiated borono-Catellani reaction. Angew. Chem. Int. Ed. 57, 7161–7165 (2018).
Li, B., Xu, H., Dang, Y. & Houk, K. N. Dispersion and steric effects on enantio-/diastereoselectivities in synergistic dual transition-metal catalysis. J. Am. Chem. Soc. 144, 1971–1985 (2022).
Acknowledgements
We thank Michael. P. Doyle for comments and suggestions on the preparation of the manuscript. We thank Bin Tan for generously providing chiral SPINOL phosphoric acid S6l. We also thank Chi Xiao, Gengxin Liu, Shanshui Meng, Zhenhui Kang, Zongwen Mo for giving help and suggestions in completing the project. W.H. and H.Q. acknowledge the financial support from Guangdong Innovative and Entrepreneurial Research Team Program (No. 2016ZT06Y337). W.H. and H.Q. acknowledge the National Natural Science Foundation of China (NO. 92056201). H.Q. acknowledges support from NSFC grant 21801255. Y.L. acknowledges support from the Fundamental Research Funds for the Central Universities (020514380253, 020514380277), the Natural Science Foundation of Jiangsu Province (BK20211555), and the Jiangsu Innovation & Entrepreneurship Talents Plan. We thank the High Performance Computing Center (HPCC) of Nanjing University for doing the numerical calculations in this paper on its blade cluster system.
Author information
Authors and Affiliations
Contributions
S.Y., H.Q. and W.H conceived and designed the project; S.Y. and R.H. developed the catalytic asymmetric four-component reactions, and conducted most of the experiments; M.Z., X.J. performed parts of substrate scope experiments and synthetic applications; W.C. and W.Z. conducted the DFT calculations and provided mechanism analysis; J.C. conducted the ECD calculations; Y.L. directed the DFT calculations and mechanism; D.Z. helped the direction of the project; S.Y., W.C., R.H., H.Q., Y.L., and W.H. prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Kevin Schofield, and the other, anonymous, reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, S., Chang, W., Hua, R. et al. An enantioselective four-component reaction via assembling two reaction intermediates. Nat Commun 13, 7088 (2022). https://doi.org/10.1038/s41467-022-34913-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-34913-2
This article is cited by
-
Bifunctionality of dirhodium tetracarboxylates in metallaphotocatalysis
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.