Abstract
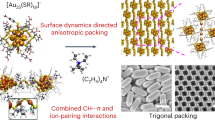
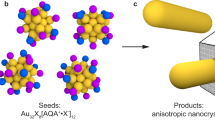
The growth of nanoparticles along one or two directions leads to anisotropic nanoparticles, but the nucleation (i.e., the formation of small seeds of specific shape) has long been elusive. Here, we show the total structure of a seed-sized Au56 nanoprism, in which the side Au{100} facets are surrounded by bridging thiolates, whereas the top/bottom {111} facets are capped by phosphine ligands at the corners and Br− at the center. The bromide has been proved to be the key to effectively stabilize the Au{111} to fulfill a complete face-centered-cubic core. In femtosecond electron dynamics analysis, the non-evolution of transient absorption spectra of Au56 is similar to that of larger-sized gold nanoclusters (n > 100), which is ascribed to the completeness of the prismatic Au56 core and an effective electron relaxation pathway created by the stronger Au-Au bonds inside. This work provides some insights for the understanding of plasmonic nanoprism formation.
Similar content being viewed by others
Introduction
In nanoscience research, anisotropic nanoparticles (NPs) are particularly attractive owing to their extraordinary optical, electronic and catalytic properties1,2,3,4,5. In 2001, Mirkin and coworkers first reported a bulk solution phase synthesis of Ag nanoprisms and identified quadrupole plasmon resonances6. This breakthrough stimulated wide interest in this unique type of anisotropic NPs; for example, subsequent work explored the use of plasmon excitation to control the chemical process of nanoprism growth7 and later the plasmon-induced photocatalysis8,9,10, as well as many other scenarios. The structure of nanoprisms (triangular or hexagonal) possesses atomically flat {111} top and bottom facets, whereas the sides are typically {100} or {110} facets11. The preferential growth of nanoprisms was explained by the collective effects of the seed structure and plasmon effect12,13,14, the crystal face selective blocking mechanism15, the different surface energies of crystal facets and the adsorption of halide ions16,17,18, among the proposed mechanisms for different syntheses. After a long-time debate, it has become clear that halide anions play a significant role in metal nanoprism growth18,19. A controlled amount of iodide anions (in addition to Br–) leads to the production of Au nanoprisms (tens to hundreds of nm in edge length)18,19,20. Halide anions are also required for the formation of Au and Ag nanorods (tens to hundreds nm in length)2,18,19,20. However, the bonding structure of halides on metal facets is still elusive.
In preparing anisotropic NPs, the early-stage seeds are often critical but remain difficult to study such ultrasmall seeds (typically up to a few nm in size)2,5,6. In recent research, atomically precise metal nanoclusters (NCs) of 1–3 nm in diameter (e.g., stabilized by thiolates (SR) or other ligands) have emerged as a new class of nanomaterials, which can serve as models to relate the detailed structures to the assembly and various properties21,22,23,24,25,26,27,28, but no relationship between the anisotropic NPs and NCs has been discussed in the literature.
The advantage of pursuing anisotropic NCs with atomic precision lies in the possible determination of total structures (i.e., not only the metal core, but also the surface bonding and arrangement of ligands)29, hence, providing atomic-level insights into the “seeds”. Due to the thermodynamics, the vast majority of structurally determined NCs are spherical30,31, while anisotropic NCs are rare32,33,34,35,36. A rod-shaped Au8n+4(SR)4n+8 series of NCs is worthy of a comment, as these NCs can be defined as elongated quantum boxes enclosed exclusively by {100} facets (six total), and their growth is by successively adding Au8 layers along the [001] direction37. Atomically precise gold NCs co-protected by thiolate, phosphine and halides (X = Cl/Br) are more likely of the rod shape38,39, and there is no success yet in obtaining the prismatic shape, which is thus highly desirable. The information about the ligand distribution and surface bonding geometry on Au(111) facets of nanoprisms is critically needed but still unknown. This motivated us to obtain atomically precise NCs of prismatic shape in hope of solving their atomic structure, as such information will offer a glimpse of the long sought-after seeds of nanoprisms and also hopefully bridge up the two research domains1,2,3,4,5,6,7,25,40,41.
Herein, we report the attainment of a prism-shaped [Au56(SPh-tBu)24(P(Ph-4-X)3)6Br2]2+ nanocluster of face-centered-cubic (fcc) structure (abbrev. Au56 below, and X = CF3, Cl, F, or H). This NC possesses ternary ligands that are relevant to plasmonic nanoprism synthesis6,40 and also exhibits relatively extended Au(111) facets. By taking advantages of the atomically precise NCs of molecular purity, we successfully crystallized Au56 NCs into a macroscopic, coherent superlattice (i.e., a single crystal), and X-ray crystallography analysis determined its total structure. The Au56 structure offers a glimpse into the distribution of ternary ligands on the prismatic structure as well as the specific surface bonding. The optical and electronic properties of Au56 differ from those of comparable gold NCs42 and plasmonic nanoprisms. Overall, the attainment of a prismatic Au56 seed sheds light on the formation and surface protection mechanisms of regular sized nanoprisms with plasmon resonances. Future attempts of seeded growth might also offer a possibility for the synthesis of atomically precise, plasmonic nanoprisms.
Results
Synthesis and characterization
In a typical synthesis of Au56, 0.2 mmol HAuCl4·3H2O, 0.28 mmol tetraoctylammonium bromide (TOAB), 0.20 mmol phosphine ligand (any of P(Ph-4-CF3)3, P(Ph-4-Cl)3, P(Ph-4-F)3, or PPh3), and 200 μL tert-butyl-benzenethiol were mixed in solvents of CH3CH2OH and CH2Cl2 (v:v = 1:9). Then, 1.5 mmol (CH3)3CNH2·BH3, (was added to reduce the gold precursor to NCs, and the reaction was allowed to continue for 12 h. The product was thoroughly washed by n-hexane and further purified by crystallization. The crystallization was conducted by vapor diffusion of n-hexane into a CH2Cl2 solution of Au56 NCs (see Method for details).
The optical absorption spectra of the NCs are slightly different before and after crystallization (Supplementary Fig. 1), indicating the elimination of impurities after crystallization. With redissolved Au56 crystals in solution, a distinct step-wise optical absorption spectrum is obtained (Fig. 1a), with peaks at 390, 430, 505, 610, and 750 nm for the case of phosphine = P(Ph-4-CF3)3. When P(Ph-4-Cl)3, P(Ph-4-F)3 or PPh3 is applied, the spectra of all Au56 NCs are the same (Fig. 1b) as the case of P(Ph-4-CF3)3. The spectra of Au56 NCs indicate a nonmetallic state42, i.e., the peaks are single-electron transitions (as opposed to the collective electron excitation) and are thus fundamentally different from the in-plane/out-of-plane dipole and quadruple plasmon resonances observed in Au or Ag nanoprisms in metallic state (edge length 40–100 nm, thickness 5–50 nm)43,44. The optical absorption onset of Au56 is ~1.2 eV (Supplementary Fig. 2). In contrast, differential pulse voltammetry (DPV) gives a HOMO-LUMO gap of 0.93 V (i.e., Eg = 0.93 eV); note: the electrochemical gap is 1.21 V (Supplementary Fig. 3, the first oxidation/reduction peak at +0.92 and −0.29 V, respectively) and subtracting the charging energy gives the 0.93 V gap. Thus, Au56 NCs have a forbidden HOMO-LUMO transition. On a note, the rod-shaped [Au25(SR)5(PPh3)10Cl2]2+ also had a forbidden HOMO-LUMO transition45. Electrospray ionization mass spectrometry analysis (ESI-MS) of the [Au56(SPh-tBu)24(P(Ph-4-CF3)3)6Br2]2+ indicates the molecular purity (Fig. 1c), so are other Au56 NCs with different phosphine ligands (Fig. 1d, e, f).
Experimental UV-vis optical spectra of a [Au56(SPh-tBu)24(P(Ph-4-CF3)3)6Br2]2+ NC and b [Au56(SPh-tBu)24(P(Ph-4-X)3)6Br2]2+ (X = F, Cl, or H) NCs. c–f The ESI-MS spectra of the corresponding NCs. The gray profiles in all insets are the simulated isotope patterns which match well with the experimental data of ESI-MS.
X-ray crystallography
We further succeeded in crystallization of Au56 NCs. We illustrate with the Au56 protected by P(Ph-4-CF3)3, thiolate and Br− ligands. The single crystal data shows that each Au56 is associated with two SbF6– counterions (Fig. 2a, b, Supplementary Table 1), thus, a 2+ charge of the NC. The 56 Au atoms are arranged into a fcc structure of prismatic shape, hence, an ultrasmall nanoprism (diameter 15 Å × height 9 Å, Fig. 2b). Note that the aspect ratio of nanoprisms is usually above 3 while that of Au56 is only ~1.7, indicating that the anisotropic growth of the NC is still at an initial ‘seed’ stage. On the top/bottom of the prismatic Au56, 10 Au atoms form a triangular top facet, so is the bottom facet. The side of Au56 is comprised of six {100} and six smallest {111} facets (Fig. 2c).
The prismatic Au56 structure contains four atomic layers (Fig. 2d, A/B/C/A), i.e., a top Au10 triangle, two Au18 truncated triangles, and a bottom Au10 triangle. The truncated triangular layer shows a degree of truncation, T = b/a = 0.34, consistent with that measured in truncated Ag nanoprisms with edge length ~68 nm46, suggesting that the truncation in nanoprisms can be traced to ultrasmall seed-sized NCs. As the A/B bilayer and C/A bilayer are arranged in a staggered manner (θ = 60° to each other, Fig. 2d), Au56 exhibits a hexagonal-prism shape. If the two halves were arranged in an eclipsed manner, a triangular nanoprism would be obtained with a twin plane (Supplementary Fig. 4)47,48.
While the Au56 with P(Ph-4-CF3)3 phosphine ligand had some residual electron densities in the core, which can be attributed to the inadequate absorption correction of gold, which strongly absorbs X-rays. Another two Au56 NCs with different phosphine ligands had better crystal quality and were also solved, and all have the same core structure (see the supporting cif files and Supplementary Tables 2 and 3). The counterions to the [Au56(SPh-tBu)24(P(Ph-4-X)3)6Br2]2+ (X = F or Cl) are two Cl–. It is clear that the ligands and the associated counterions are critical in the orientation control on the assembly of the NCs with the same cluster structure (Supplementary Fig. 5), as the possibly formed hydrogen bonds, the volume of counterions, and their steric and electrostatic effect often cause differences29. Specifically, Au56 with P(Ph-4-CF3)3 forms a triclinic unit cell, and Au56 with P(Ph-4-F)3 gives rise to a monoclinic one, whereas the unit cell of Au56 with P(Ph-4-Cl)3 is trigonal.
According to the Au-Au bond length distribution inside the core, the Au56 structure can alternatively be divided into four parts: a central rhombohedral Au8 with average bond length of 2.808 Å (Fig. 3a, marked in purple, 2.833(12) Å and 2.784(12) Å, respectively); six Au4 tetrahedrons (Fig. 3a, three on the top and another three at the bottom, marked in red) in which the Au-Au bonds are very short (avg. 2.760 Å: 2.763(1), 2.767(10), 2.780(10), 2.764(10), 2.792(10), 2.697(11) Å), and such Au4 units were previously observed in other fcc Au NCs49; a Au12 ring (Fig. 3a, marked in pink, 2.684(11) Å and 2.827(10) Å, respectively) to connect the central Au8 with six Au4 units surrounding at the waist (Fig. 3b), and the distance between the Au12 ring and Au8 is 2.873(1) and 2.890(10) Å, respectively, whereas that between Au12 and the tip of Au4 is 2.722(10) and 2.801(10) Å, respectively; by contrast, the perimetric 12 Au atoms (Fig. 3c, marked in orange) are loosely bonded to the central part of the core. Thus, there is an effective pathway within the Au56 NC by having stronger Au-Au bonds (i.e. shorter bond lengths), which presumably endows the NC some interesting dynamics in ultrafast electron relaxation (vide infra).
In previous work on Au or Ag nanoprisms, the surface-protecting ligands and bonding structure were not known. Here, with the Au56 structure solved, one can gain a glimpse into such issues. We found that thiolates on Au(100) facets show a bridging mode of bonding, rather than the terminal mode as commonly thought, and the organic parts of the thiolates tilt towards the same direction (Fig. 4a). More importantly, the protection of Au(111) facets by phosphine and bromide is interesting (Fig. 4b). One of the three Ph-4-CF3 branches of one phosphine ligand (three phosphine ligands total) points to the central Br− on Au(111), whereas the other two Ph-4-CF3 branches stretch outwards (Fig. 4a). The bromide resides on top of the Au atom within Au(111) (Fig. 4a, highlighted by a blue circle) and is critical in stabilizing the Au(111) facets in the Au56 NC. It is interesting to see that although I– is critical in directing the formation of gold nanoprisms18,50, for Au56, Br– is necessary to selectively stabilize the Au(111) facet51. We further suppose that, due to the higher surface energy of Au{100} facets, stronger Au-SR bonds are preferred; whereas on the Au{111} facets of lower surface energy, relatively weaker Au-Br bonds are accommodated.
Electron dynamics
Due to the completeness of the fcc Au56 core structure, we expect distinct electron dynamics in this NC and thus performed femtosecond transient absorption (fs-TA) measurements to probe the excited-state dynamics of Au56. According to the TA data map probed between −1 and 7000 ps, after excitation at 400 nm, one can observe positive excited-state absorption (ESA) bands at 365, 540 and 700 nm, together with negative ground-state bleaching (GSB) at 400, 500 and 600 nm (Fig. 5a). Between 0 and 3 ps, a rapid decay for all ESA bands is clear, and the TA spectra of Au56 remain almost unchanged between 3 ps and 7000 ps (Fig. 5b, c). Data fitting shows that a fast decay (0.8 ps) followed by a very slow decay (40 ns measured by ns pump-probe, Supplementary Fig. 6) can well fit the TA dynamics at all wavelengths. It is interesting to see that there is almost no spectral evolution in the TA data map; the TA spectral profile at 0.3 ps is almost identical to that at 1000 ps. After normalization, the TA spectra only exhibit a slight blue-shift between 0.3 and 1000 ps (Supplementary Fig. 7). The 40 ns lifetime is comparable to that of small-sized Au NCs (<50 gold atoms)42, while the non-evolution of TA spectra is reminiscent of large-sized Au NCs (>100 gold atoms)42.
Previous work on similar sized Au52(SR)32 reported that the profile of ESA bands from higher excited-states is drastically different from that of S1 state52, which is in contrast to the observation in Au56 here. The lack of evolution of the TA spectra of Au56 suggests that the ESA of both Sn and S1 states should be a featureless band covering the entire detection range (350–750 nm). Such an observation should arise from the unique packing structure of the Au56 NC, i.e., a complete fcc core structure which is larger than that of Au-SR NCs of similar size with motifs, as well as the specific electron motion pathway within Au56 in which a Au12 ring effectively connects the six Au4 units and the central Au8 with strong Au–Au bonds. By contrast, in Au52(SR)32, the Au–Au bond lengths within each Au4-coiled helix is shorter than that between the two helices49. Besides, the larger fcc core of Au56 should eventually lead to more condensed higher excited-states, hence, featureless ESA in the visible range if the negative ground-state bleaching bands were not there.
Discussion
The bromide on each Au(111) facet should come from TOAB in the reactants, which is similar to cetyltrimethylammonium bromide (CTAB) acting as a cationic surfactant widely used to stabilize colloidal metal NPs1,2. A chemical reduction of gold precursor in the presence of Br– is important for synthesizing the Au56 prismatic NC with stabilized Au(111) facets, along with the choice of ligands (thiol and phosphine). When we replaced Br− (from TOAB) with Cl− (TBAC: tetrabutylammonium chloride) in the experiment, no Au56 NC was formed (Supplementary Fig. 8).
In previous work on the synthesis of regular-sized nanoprisms, phosphine (e.g., bis(p-sulfonatophenyl) phenylphosphine dihydrate dipotassium salt) and citrate6, as well as thiolate40, were used for Ag nanoprisms, whereas CTAB was used to produce Au nanoprisms with the help of iodide44,53. The addition of a small amount of I– was previously found to be essential to suppress the growth along the Au[111] direction18,19,20, resulting in nanoprisms of high aspect ratios. We rationalize that for NCs with ~1 nm size, Br– is effective enough to restrain the growth along the Au[111] direction, and the surface bonding of Br– on Au(111) facets is revealed in the structure of Au56. Due to the restrained Au[111] growth, the size expansion of Au(111) facet should be favored, however, it is confined by phosphine ligands at the three corners, otherwise the Au{100} facets decorated by thiolates would extend to even larger sizes32.
We also noticed that although the atomic packing inside the Au56 NC can be considered as fcc, the typical Au-Au bond length of 2.88 Å for bulk Au is not throughout the structure; instead, there are a strongly bonded part and a loosely bonded part. The Au-Au bond lengths within the central rhombohedral Au8 and the six tetrahedral Au4 units, as well as the Au12 ring to connect them together are shorter than the 2.88 Å value in bulk Au. On the other hand, the distances between the Au4 units are rather long (3.155 (11) Å), and the perimetric 12 Au atoms are loosely connected to the inner part with avg. 3.047 Å. The effective pathway created by stronger Au-Au bonds inside the Au56 NC should be responsible for the special feature of electron relaxation dynamics being similar to what is observed in large-sized Au NCs with more than 100 gold atoms.
In summary, a prism-shaped Au56 NC is successfully obtained. The Au(111) facet is protected by three phosphine ligands at the corners and one Br− at the center, indicating the growth mechanism for anisotropic NCs. The presence of Br– is important for stabilizing the Au(111) facets and achieving a complete prism-shaped Au56 NC. Such insights are expected to be helpful for understanding the system of conventional NPs. Femtosecond transient absorption measurements of Au56 indicates a picture of more congested excited-states than that of similar-sized Au NCs in the literature, which becomes similar to that of those large-sized Au NCs (>100 gold atoms). This can be explained by the strongly bonded pathway inside the Au56 with a complete prism in shape. Overall, the atomic structure of seed-sized nanoprism sheds light on the initial stage of nanoprisms and provides useful information for rationalization of the formation and growth mechanism. Future work may explore the use of such precise seeds for possible achievements of atomically precise, larger nanoprisms and thus bridge up the two domains4,5,6,7,40.
Methods
Reagents
Tetrachloroauric(III) acid (HAuCl4·3H2O, ≥ 99.99% metals basis, Aladdin), tetraoctylammonium bromide (TOAB, 98%, Aladdin), tetrabutylammonium chloride (TBAC, 98%, Aladdin), borane-tert-butylamine complex ((CH3)3CNH2·BH3, ≥ 95.0%, Aladdin), tris(4-fluorophenyl)phosphine (P(Ph-4-F)3, 98%, Aladdin), tris(4-chlorophenyl)phosphine (P(Ph-4-Cl)3, 98%, Aladdin), tris(4-trifluoromethylphenyl)phosphine (P(Ph-4-CF3)3, 97%, Aladdin), triphenylphosphine, (PPh3, ≥ 95%, Aladdin), 4-tert-butylbenzenethiolate (SPh-tBu, ≥ 98.5%, Aladdin), ethanol (HPLC, Aladdin), dichloromethane (HPLC, Aladdin), n-hexane (HPLC, Aladdin). All reagents and solvents were commercially available and used as received without further purification.
Synthesis of Au56 NCs
To prepare the Au56(SPh-tBu)24(P(Ph-4-X)3)6Br2 NC, 0.2 mmol HAuCl4·3H2O, 0.28 mmol TOAB were first dissolved in a mixed solvent of CH3CH2OH and CH2Cl2 (v:v = 1:9) for 15 min, and then, 0.20 mmol phosphine (i.e., P(Ph-4-F)3, P(Ph-4-Cl)3, P(Ph-4-CF3)3, or PPh3) was added. After 30 min, 200 μL HSPh-tBu was added, and the solution was stirred for another 30 min. When the solution became almost clear, 1.5 mmol reducing agent ((CH3)3CNH2·BH3) was added, and the reaction was continued for 12 h. The product was thoroughly washed by n-hexane, and extracted by CH2Cl2. 20 mg NaSbF6 was added to the CH2Cl2 solution of Au56 NCs and mixed for 30 min. The Au56 NCs were further purified during the crystallization which was conducted by vapor diffusing n-hexane into the CH2Cl2 solution of the NCs for a week.
Characterization
Crystals were re-dissolved in CH2Cl2 and then used as the samples for all the measurements. The UV-vis absorption spectra were collected with an Agilent HP8453 diode array spectrometer. Electrospray ionization (ESI) mass spectra were acquired using a Waters UPLC H-class/Xevo G2-XS QTof mass spectrometer. The sample was dissolved in CH3OH/CH2Cl2 (v:v = 1:1) solution with a concentration of ~0.1 mg ml−1. The sample was infused at 20 μL min−1 directly. The source temperature was kept at 80 °C with the spray voltage kept at 3.0 kV.
X-ray crystallographic determination
A suitable crystal was mounted onto a MiTeGen capillary with fluorolube and performed on a STOE STADIVARI diffractometer equipped with CuKα X-ray source (λ = 1.54186 Å). The crystal was kept at 120 K during the data collection. Using Olex254, the structure was solved with the olex2.solve55 structure solution program using Charge Flipping and refined with the olex2.refine56 refinement package using Gauss-Newton minimization. All the Au, P, Br, S, Cl and Sb atoms were found directly. Remaining non-hydrogen atoms were generated via subsequent difference Fourier syntheses. All the non-hydrogen atoms were refined anisotropically. All the hydrogen atoms were set in geometrically calculated positions and refined isotropically using a riding model. The diffuse electron densities resulted from the residual solvent molecules were removed from the data set using the SQUEEZE routine of PLATON and the refined data was further generated.
Crystal data
For [Au56(SPh-tBu)24(P(Ph-4-CF3)3)6Br2](SbF6)2 (M = 18425.37 g mol−1): triclinic, space group P-1, a = 21.9736(8) Å, b = 24.0131(14) Å, c = 25.8161(12) Å, α = 108.841(4)°, β = 100.243(3)°, γ = 106.585(4)°, V = 11795.6(10) Å3, Z = 1, T = 120(2) K, μ(GaKα) = 34.661 mm−1, ρcalc = 2.594 g cm−3, 69387 reflections measured (7.698° ≤ 2θ ≤ 119.996°), 33856 unique (Rint = 0.0630, Rsigma = 0.0719), which were used in all calculations. The final R1 was 0.0877 (I > 2σ(I)) and wR2 was 0.2646 (all data). The residual electron densities left inside the cluster core can be attributed to the inadequate absorption correction of gold which strongly absorbs X-rays.
For [Au56(SPh-tBu)24(P(Ph-4-F)3)6Br2]Cl2 (M = 17124.63 g mol−1): monoclinic, space group C2/c, a = 45.473 Å, b = 28.445 Å, c = 42.222 Å, α = γ = 90°, β = 121.57°, V = 46531.3 Å3, Z = 4, T = 120(2) K, μ(CuKα) = 34.089 mm−1, ρcalc = 2.444 g cm−3, 216121 reflections measured (7.518° ≤ 2θ ≤ 125°), 37013 unique (Rint = 0.0715, Rsigma = 0.0348), which were used in all calculations. The final R1 was 0.0918 (I > 2σ(I)) and wR2 was 0.2735 (all data).
For [Au56(SPh-tBu)24(P(Ph-4-Cl)3)6Br2]Cl2 (M = 17346.24 g mol−1): trigonal, space group R-3, a = b = 27.1253(17) Å, c = 50.856(6) Å, α = β = 90°, γ = 120°, V = 32406(5) Å3, Z = 3, T = 120(2) K, μ(CuKα) = 37.558 mm−1, ρcalc = 2.666 g cm−3, 30861 reflections measured (8.35° ≤ 2θ ≤ 124.982°), 11310 unique (Rint = 0.0518, Rsigma = 0.0623), which were used in all calculations. The final R1 was 0.0594 (I > 2σ(I)) and wR2 was 0.1796 (all data).
Nanosecond spectral measurement
The nanosecond transient absorption measurements were performed using a nanosecond flash photolysis setup Edinburgh LP920 spectrometer (Edinburgh Instruments Ltd.), combined with a Nd:YAG laser at 355 nm (Surelite II, Continuum Inc.).
Ultrafast transient absorption
The femtosecond transient absorption spectra were measured at ∼150 fs time-resolution using a commercial femtosecond broadband pump−probe setup (Harpia-TA, Light Conversion)57 with an amplified femtosecond Ti:sapphire laser (Coherent Astrella, 7 mJ, 40 fs, 800 nm, 1 kHz). The pumping wavelengths were generated by an optical parametric amplifier (TOPAS-C, Coherent), and the probe pulses were obtained with a CaF2 plate (2 mm thick). The solution samples were in 1 mm optical path quartz cuvettes. Data analysis was performed with R-package TIMP software with the graphical interface Glotaran (Target Analysis model) and CarpetView (Light Conversion).
Data availability
The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number CCDC 2015959, 2021449, 2021439. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
References
Murphy, C. J. et al. Anisotropic metal nanoparticles: synthesis, assembly, and optical applications. J. Phys. Chem. B 109, 13857–13870 (2005).
Lohse, S. E., Burrows, N. D., Scarabelli, L., Liz-Marzán, L. M. & Murphy, C. J. Anisotropic noble metal nanocrystal gowth: the role of halides. Chem. Mater. 26, 34–43 (2014).
Sherry, L. J., Jin, R., Mirkin, C. A., Schatz, G. C. & Van Duyne, R. P. Localized surface plasmon resonance spectroscopy of single silver triangular nanoprisms. Nano Lett. 6, 2060–2065 (2006).
Zhai, Y. et al. Polyvinylpyrrolidone-induced anisotropic growth of gold nanoprisms in plasmon-driven synthesis. Nat. Mater. 15, 889–895 (2016).
Stangherlin, S., Cathcart, N., Sato, F. & Kitaev, V. Gold nanoprisms: synthetic approaches for mastering plasmonic properties and implications for biomedical applications. ACS Appl. Nano Mater. 3, 8304–8318 (2020).
Jin, R. et al. Photoinduced conversion of silver nanospheres to nanoprisms. Science 294, 1901–1903 (2001).
Jin, R. et al. Controlling anisotropic nanoparticle growth through plasmon excitation. Nature 425, 487–490 (2003).
Christopher, P., Xin, H. & Linic, S. Visible-light-enhanced catalytic oxidation reactions on plasmonic silver nanostructures. Nat. Chem. 3, 467–472 (2011).
Wang, P., Huang, B., Dai, Y. & Whangbo, M.-H. Plasmonic photocatalysts: harvesting visible light with noble metal nanoparticles. Phys. Chem. Chem. Phys. 14, 9813–9825 (2012).
Clavero, C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photonics 8, 95–103 (2014).
Millstone, J. E., Hurst, S. J., Métraux, G. S., Cutler, J. I. & Mirkin, C. A. Colloidal gold and silver triangular nanoprisms. Small 5, 646–664 (2009).
Millstone, J. E., Métraux, G. S. & Mirkin, C. A. Controlling the edge length of gold nanoprisms via a seed-mediated approach. Adv. Funct. Mater. 16, 1209–1214 (2006).
Xue, C., Millstone, J. E., Li, S. & Mirkin, C. A. Plasmon-driven synthesis of triangular core–shell nanoprisms from gold seeds. Angew. Chem. Int. Ed. 46, 8436–8439 (2007).
Shahjamali, M. M. et al. Gold coating of silver nanoprisms. Adv. Funct. Mater. 22, 849–854 (2012).
Xia, Y., Xiong, Y., Lim, B. & Skrabalak, S. E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics? Angew. Chem. Int. Ed. 48, 60–103 (2009).
Langille, M. R., Personick, M. L., Zhang, J. & Mirkin, C. A. Defining rules for the shape evolution of gold nanoparticles. J. Am. Chem. Soc. 134, 14542–14554 (2012).
Elechiguerra, J. L., Reyes-Gasga, J. & Yacaman, M. J. The role of twinning in shape evolution of anisotropic noble metal nanostructures. J. Mater. Chem. 16, 3906–3919 (2006).
DuChene, J. S. et al. Halide anions as shape-directing agents for obtaining high-quality anisotropic gold nanostructures. Chem. Mater. 25, 1392–1399 (2013).
Rai, A., Singh, A., Ahmad, A. & Sastry, M. Role of halide ions and temperature on the morphology of biologically synthesized gold nanotriangles. Langmuir 22, 736–741 (2006).
Ha, T. H., Koo, H.-J. & Chung, B. H. Shape-controlled syntheses of gold nanoprisms and nanorods influenced by specific adsorption of halide ions. J. Phys. Chem. C. 111, 1123–1130 (2007).
Li, Y. et al. Double-helical assembly of heterodimeric nanoclusters into supercrystals. Nature 594, 380–384 (2021).
Agrachev, M., Ruzzi, M., Venzo, A. & Maran, F. Nuclear and electron magnetic resonance spectroscopies of atomically precise gold nanoclusters. Acc. Chem. Res. 52, 44–52 (2019).
Kwak, K. & Lee, D. Electrochemistry of atomically precise metal nanoclusters. Acc. Chem. Res. 52, 12–22 (2019).
Li, Y., Higaki, T., Du, X. & Jin, R. Chirality and surface bonding correlation in atomically precise metal nanoclusters. Adv. Mater. 32, 1905488 (2020).
Jin, R., Li, G., Sharma, S., Li, Y. & Du, X. Toward active-site tailoring in heterogeneous catalysis by atomically precise metal nanoclusters with crystallographic structures. Chem. Rev. 121, 567–648 (2020).
Li, Y. et al. Heterometal-doped M23 (M = Au/Ag/Cd) nanoclusters with large dipole moments. ACS Nano 14, 6599–6606 (2020).
Chakraborty, P., Nag, A., Chakraborty, A. & Pradeep, T. Approaching materials with atomic precision using supramolecular cluster assemblies. Acc. Chem. Res. 52, 2–11 (2019).
Zhou, M. et al. Three-orders-of-magnitude variation of carrier lifetimes with crystal phase of gold nanoclusters. Science 364, 279–282 (2019).
Li, Y. & Jin, R. Seeing ligands on nanoclusters and in their assemblies by X-ray crystallography: atomically precise nanochemistry and beyond. J. Am. Chem. Soc. 142, 13627–13644 (2020).
Jin, R., Zeng, C., Zhou, M. & Chen, Y. Atomically precise colloidal metal nanoclusters and nanoparticles: fundamentals and opportunities. Chem. Rev. 116, 10346–10413 (2016).
Yan, J., Teo, B. K. & Zheng, N. Surface chemistry of atomically precise coinage-metal nanoclusters: from structural control to surface reactivity and catalysis. Acc. Chem. Res. 51, 3084–3093 (2018).
Zeng, C., Liu, C., Chen, Y., Rosi, N. L. & Jin, R. Atomic structure of self-assembled monolayer of thiolates on a tetragonal Au92 nanocrystal. J. Am. Chem. Soc. 138, 8710–8713 (2016).
Chen, Y. et al. Crystal structure of barrel-shaped chiral Au130(p-MBT)50 nanocluster. J. Am. Chem. Soc. 137, 10076–10079 (2015).
Li, Y. et al. Heteroatom tracing reveals the 30-Atom Au-Ag bimetallic nanocluster as a dimeric structure. J. Phys. Chem. Lett. 11, 7307–7312 (2020).
Alhilaly, M. J. et al. [Ag67(SPhMe2)32(PPh3)8]3+: synthesis, total structure, and optical properties of a large box-shaped silver nanocluster. J. Am. Chem. Soc. 138, 14727–14732 (2016).
Diecke, M., Schrenk, C. & Schnepf, A. Synthesis and characterization of the highly unstable metalloid cluster Ag64(P-nBu3)16Cl6. Angew. Chem. Int. Ed. 59, 14418–14422 (2020).
Zeng, C. et al. Gold quantum boxes: on the periodicities and the quantum confinement in the Au28, Au36, Au44, and Au52 magic series. J. Am. Chem. Soc. 138, 3950–3953 (2016).
Shichibu, Y. et al. Biicosahedral gold clusters [Au25(PPh3)10(SCnH2n+1)5Cl2]2+ (n = 2-18): a stepping stone to cluster-assembled materials. J. Phys. Chem. C. 111, 7845–7847 (2007).
Jin, R. et al. Tri-icosahedral gold nanocluster [Au37(PPh3)10(SC2H4Ph)10X2]+: linear assembly of icosahedral building blocks. ACS Nano 9, 8530–8536 (2015).
Cathcart, N. & Kitaev, V. Monodisperse hexagonal silver nanoprisms: synthesis via thiolate-protected cluster precursors and chiral, ligand-imprinted self-assembly. ACS Nano 5, 7411–7425 (2011).
Li, Y. et al. Atom-by-atom evolution of the same ligand-protected Au21, Au22, Au22Cd1, and Au24 nanocluster series. J. Am. Chem. Soc. 142, 20426–20433 (2020).
Zhou, M. et al. Three-stage evolution from nonscalable to scalable optical properties of thiolate-protected gold nanoclusters. J. Am. Chem. Soc. 141, 19754–19764 (2019).
Xue, C. & Mirkin, C. A. pH-switchable silver nanoprism growth pathways. Angew. Chem. Int. Ed. 46, 2036–2038 (2007).
Millstone, J. E. et al. Observation of a quadrupole plasmon mode for a colloidal solution of gold nanoprisms. J. Am. Chem. Soc. 127, 5312–5313 (2005).
Park, S. & Lee, D. Synthesis and electrochemical and spectroscopic characterization of biicosahedral Au25 clusters. Langmuir 28, 7049–7054 (2012).
Chen, S. & Carroll, D. L. Synthesis and characterization of truncated triangular silver nanoplates. Nano Lett. 2, 1003–1007 (2002).
Aherne, D., Ledwith, D. M., Gara, M. & Kelly, J. M. Optical properties and growth aspects of silver nanoprisms produced by a highly reproducible and rapid synthesis at room temperature. Adv. Funct. Mater. 18, 2005–2016 (2008).
Wang, Y., Peng, H.-C., Liu, J., Huang, C. Z. & Xia, Y. Use of reduction rate as a quantitative knob for controlling the twin structure and shape of palladium nanocrystals. Nano Lett. 15, 1445–1450 (2015).
Zeng, C. et al. Gold tetrahedra coil up: Kelulé-like and double helical superstructures. Sci. Adv. 1, e1500425 (2015).
Millstone, J. E., Wei, W., Jones, M. R., Yoo, H. & Mirkin, C. A. Iodide ions control seed-mediated growth of anisotropic gold nanoparticles. Nano Lett. 8, 2526–2529 (2008).
Wan, X.-K., Wang, J.-Q. & Wang, Q.-M. Ligand-protected Au55 with a novel structure and remarkable CO2 electroreduction performance. Angew. Chem. Int. Ed. 133, 20916–20921 (2021).
Zhou, M. et al. Evolution of excited-state dynamics in periodic Au28, Au36, Au44, and Au52 nanoclusters. J. Phys. Chem. Lett. 8, 4023–4030 (2017).
Grzelczak, M., Pérez-Juste, J., Mulvaney, P. & Liz-Marzán, L. M. Shape control in gold nanoparticle synthesis. Chem. Soc. Rev. 37, 1783–1791 (2008).
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. & Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42, 339–341 (2009).
Bourhis, L. J., Dolomanov, O. V., Gildea, R. J., Howard, J. A. & Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment – Olex2 dissected. Acta Crystallogr A71, 59–75 (2015).
Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. C. C71, 3–8 (2015).
Huo, D. et al. Intramolecular energy transfer in a series of star-shaped molecules with a central porphyrin core and four oligocarbazole arms. J. Phys. Chem. C. 124, 27356–27365 (2020).
Acknowledgements
Y.S. acknowledges financial support by the NSFC (Grant 21801001 and 22171007) and Grant for Scientific Research of BSKY (No: XJ2020026). M.Z. acknowledges financial support by the NSFC (Grants 21871001 and 21631001), the Ministry of Education, and the Education Department of Anhui Province.
Author information
Authors and Affiliations
Contributions
Y.S. conceived and carried out experiments. Y.S. and Y.L. analyzed the data. Y.L. wrote the paper under the supervision of M. Zhu and R.J.. H.L., T.X., C.Z. and K.F. assisted in the synthesis. M. Zhou, D.H., Y.W. and J.J. carried out the TA measurement and analyses. W.X. carried out some calculations. All authors contributed to the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Indranath Chakraborty, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Song, Y., Li, Y., Zhou, M. et al. Atomic structure of a seed-sized gold nanoprism. Nat Commun 13, 1235 (2022). https://doi.org/10.1038/s41467-022-28829-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-28829-0
This article is cited by
-
Atomically precise nanoclusters predominantly seed gold nanoparticle syntheses
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.