Abstract
Streams and rivers emit substantial amounts of nitrous oxide (N2O) and are therefore an essential component of global nitrogen (N) cycle. Permafrost soils store a large reservoir of dormant N that, upon thawing, can enter fluvial networks and partly degrade to N2O, yet the role of waterborne release of N2O in permafrost regions is unclear. Here we report N2O concentrations and fluxes during different seasons between 2016 and 2018 in four watersheds on the East Qinghai-Tibet Plateau. Thawing permafrost soils are known to emit N2O at a high rate, but permafrost rivers draining the East Qinghai-Tibet Plateau behave as unexpectedly minor sources of atmospheric N2O. Such low N2O fluxes are associated with low riverine dissolved inorganic N (DIN) after terrestrial plant uptake, unfavorable conditions for N2O generation via denitrification, and low N2O yield due to a small ratio of nitrite reductase: nitrous oxide reductase in these rivers. We estimate fluvial N2O emissions of 0.432 − 0.463 Gg N2O-N yr−1 from permafrost landscapes on the entire Qinghai-Tibet Plateau, which is marginal (~0.15%) given their areal contribution to global streams and rivers (0.7%). However, we suggest that these permafrost-affected rivers can shift from minor sources to strong emitters in the warmer future, likely giving rise to the permafrost non-carbon feedback that intensifies warming.
Similar content being viewed by others
Introduction
Nitrous oxide (N2O) is a major stratospheric ozone destroyer and the third most important long-lived greenhouse gas (GHG)1. Sources of this powerful GHG are poorly constrained in general, and for global streams and rivers in particular2. Studies of lotic N2O dynamics have focused almost exclusively on human-impacted lowland systems across diverse climate zones, where anthropogenic nitrogen (N) enrichment has been linked to elevated N2O fluxes3. Unfortunately, data are extremely sparse for more pristine regions, including the rapidly changing permafrost-rich cryospheres at high altitudes and latitudes. As a result, the current estimate of global fluvial emissions of 291.3 ± 58.6 Gg N2O-N yr−1 has substantial uncertainty4.
Massive amounts of organic carbon (OC, ~1014 Pg C) are stored in the top 3 m of Northern Hemisphere permafrost soils5. As these deposits thaw, ice-locked carbon is liberated and can be transported to adjacent running waters, where it becomes available for processing and loss to the atmosphere as carbon dioxide (CO2) and methane (CH4)6,7,8,9. Consequently, fluvial emissions of these gases in some cryosphere regions are now occurring at elevated and increasing rates10,11. However, the magnitude of N2O emissions from streams and rivers in regions where permafrost thaw is afoot is unknown despite the fact that Northern Hemisphere permafrost soils contain 67 Pg N to a depth of 3 m (excluding N pools in the active layer), and are evident or even substantial sources of N2O12. Studies of Alaskan streams have emphasized sustained delivery of N from thawing permafrost soils resulting in elevated inorganic N13,14 and often-supersaturated N2O concentrations in these receiving waters13. However, in the absence of direct measurements of N2O emissions from permafrost-affected streams and rivers, it is not clear if these results are representative and translate to overall elevated N2O emissions.
To address this knowledge gap, we provide a cross-regional and seasonal direct measurement of fluvial N2O concentrations and fluxes, generated in four headwater catchments that vary in altitude from 1650 to 4600 m and cover an area of ca. 73.6 × 104 km2 on the East Qinghai-Tibet Plateau (EQTP; Fig. 1; Supplementary Fig. 1, Table 1 and 2), and identify likely mechanisms shaping N2O dynamics in these rivers. Areal CH4 emissions from streams and rivers in this region are strongly affected by permafrost thaw, and are among the highest reported rates globally11, suggesting that a similar effect may be underway for N2O in these rivers as well. This region is the largest cryosphere outside the Arctic and Antarctic15, with vast Pleistocene-aged permafrost16 that contains an estimated 1.8 Pg N in the upper 3 m of soil17. As the ‘Water Tower of Asia’, meltwater from the Qinghai-Tibet Plateau (QTP) feeds ten great Asian rivers and ubiquitous ponds, lakes, and wetlands18. Such alpine streams and rivers are tightly connected to permafrost soils11 and have high turbulence-induced gas exchange19, and these features potentially promote their capacity to process terrestrial N and sustain high N2O emissions. Further, many parts of the QTP have experienced significant human population growth over the last six decades accompanied by similar rapid increases in livestock20, again adding to the potential for enhanced riverine N2O emissions. However, as we show below, these alpine permafrost-affected streams and rivers of the EQTP are unexpectedly minor atmospheric N2O sources. Yet there are signs that developing climate warming and enhanced anthropogenic influences may lead to a substantial increase in fluvial N2O emissions from high altitudes and latitudes in the coming decades. This study offers a guide to include permafrost streams and rivers in current and future N2O inventories, given their potentially important implications for N2O budget.
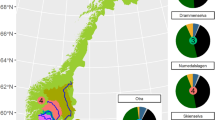
Sampling sites within the four catchments. The blue shading represents permafrost extent on the EQTP (Data for the permafrost extent courtesy of ref. 57). The inset highlights the study area on a map of China.
Results and discussion
Variability of N2O concentrations and fluxes
All sampled streams and rivers were supersaturated on all dates (117.9–242.5%, n = 342 samples from 114 site visits) in N2O with respect to the atmosphere. Dissolved N2O concentrations fluctuated between 10.2 and 18.9 nmol L−1 with an average of 12.4 ± 1.7 nmol L−1, which is one-third of the global average3 (37.5 nmol L−1; Supplementary Table 3). Significantly higher N2O concentrations were observed in spring (P < 0.001), followed by fall and summer (Supplementary Fig. 2a). Despite differences in catchment attributes including permafrost fraction and population densities (Supplementary Table 2), N2O concentrations were not significantly different among the four river systems (Supplementary Fig. 2a).
Diffusive N2O fluxes from EQTP rivers were predominantly positive (to the atmosphere), ranging from −14.0 to 40.6 µmol m−2 d−1 with an average of 9.4 ± 6.2 µmol m−2 d−1 (n = 436 samples from 114 site visits). This mean flux is an order of magnitude lower than the global average3 (94.3 µmol m−2 d−1; Supplementary Table 3). Diffusive N2O fluxes were similar in summer and fall, and significantly higher than those in spring (P < 0.05; Supplementary Fig. 2b). The asynchronous seasonal patterns between concentrations and fluxes are likely caused by water temperature and precipitation (Supplementary Discussion 1). As with concentration, no significant differences were found for N2O diffusion among the four rivers (Supplementary Fig. 2b).
N2O ebullition has seldom been documented in lotic ecosystems, although it can coincide with CH4 bubble release21. Because of the presence of large organic reserves in surrounding permafrost, shallow water depths, and their exposure to low barometric pressure, streams and rivers on the EQTP are notable hotspots of CH4 ebullition11. Thus, we had hypothesized that N2O would also be entrained during the widespread processes of CH4 bubble release. The mean N2O ebullition rate from EQTP rivers was 0.74 ± 2.47 µmol m−2 d−1, and accounted for 4.1 ± 11.9% of total N2O fluxes (diffusion + ebullition) across all sites. Despite this potential for high ebullition rates, accounting for this flux had a little overall effect, as the average total N2O flux (10.2 ± 7.1 µmol m−2 d−1) was still nine-fold lower than the global mean diffusive N2O.
Terrestrial processes modulating N2O dynamics
The availability of inorganic N is often the primary determinant of rates of N2O production and emission in both permafrost-affected soils12 and fluvial networks2. In the QTP, dissolved N released from both thawing permafrost soils and animal manure (Supplementary Discussion 2) is biologically available for plant uptake22,23,24 or instead may be exported to river channels. Because plant growth is N-limited in this region24, we predicted that an increase in vegetation cover would result in greater plant uptake of terrestrial N, and consequently lower inputs to, and concentrations of N in streams and rivers, up to a point. Indeed, riverine dissolved inorganic N (DIN) concentrations decreased with increasing vegetation cover for all sites (Fig. 2a). However, mechanisms causing the observed decline in riverine DIN cannot be elucidated from vegetation coverage alone, as productivity and greenness per unit plant cover decline at higher elevations25. Thus, we also examined normalized difference vegetation index (NDVI, a measure of productivity and greenness) to indicate plant N uptake. Sites with high NDVI had low riverine DIN in non-continuous (namely discontinuous, sporadic, and isolated) permafrost zones (Fig. 2b), in line with the hypothesis of greater plant influence on riverine N availability when and where vegetation productivity and greenness were higher. In contrast, in areas with continuous permafrost, sites with high NDVI had high riverine DIN (Fig. 2b), suggesting that terrestrial N was sufficient to support plant growth and associated productivity/greening regardless of season, and indeed likely exceeded the N demand of plant community26. If so, then the surplus DIN can be exported to river corridors. Terrestrial N2O can also be transported along with DIN to surrounding watercourses, and higher N2O concentrations in some sites draining permafrost areas could be supported by transport of terrestrial N2O and/or greater in situ N2O production supported by more DIN inputs. Even so, EQTP streams and rivers collectively received reduced terrestrial N after plant uptake. DIN concentrations (0.54 ± 0.30 mgN L−1) in EQTP waterways were at the lower end of the range reported for global streams and rivers3 (0.002–21.2 mgN L−1) and constrained within a relatively narrow range. The low N2O concentrations and fluxes are consistent with the low N availability in these rivers.
a Correlation between vegetation coverage (see Methods) and riverine DIN for all sites across all permafrost categories. The red line represents the fit of a linear regression through the observed data. b Riverine DIN for each specific normalized difference vegetation index (NDVI) interval (≤0.3; 0.3–0.6; ≥0.6) in continuous and non-continuous permafrost zones across different seasons (one-way ANOVA with Tukey’s post-hoc test: *P < 0.05; **P < 0.01; ***P < 0.001). Boxes represent the 25th and 75th percentiles, and error bars show the 95th percentiles. Black circles and horizontal lines indicate the arithmetic means and medians, respectively. Gray circles are outliers.
Biogeochemical processes regulating N2O dynamics
Riverine N2O concentrations could not be effectively predicted by simple linear regressions with environmental variables [R2 ≤ 0.1 in most cases, including dissolved oxygen (DO, P > 0.05, R2 = 0.004) and NH4+ concentrations (P < 0.001, R2 = 0.1); Supplementary Table 4]. However, we found a strong positive relationship between NO3− and N2O concentrations when DO saturation (%O2) was undersaturated (<100%) in the water column (Fig. 3a). This result was validated by a regression tree analysis that identified %O2 as the primary control on N2O concentration, and that higher N2O concentrations occurred when %O2 < 100% and NO3− ≥ 0.58 mgN L−1 (Fig. 3b). The tree had a greater explanatory power (R2 = 0.56) than the simple linear regression of NO3− and N2O concentrations (R2 = 0.23; Supplementary Table 4). N2O concentrations were uniformly low and weakly related to NO3− when %O2 ≥ 100% (Fig. 3a), suggesting that N2O present at these sites was derived from rare surface sediment patches in the channel that maintain hypoxic-anoxic conditions despite abundant O2 in the water column or from external sources including inputs of dissolved N2O from permafrost soils, intermediate runoff (Supplementary Fig. 3), and upwelling groundwater27 to maintain such low to modest but supersaturated N2O concentrations. Regardless of the specific source, this result means that the widespread occurrence of well-oxygenated overlying waters of EQTP rivers (139 of 227 samples) limits the extent of hypoxic-anoxic regimes needed for N2O generation via denitrification.
a N2O concentrations as functions of NO3− concentrations for samples with supersaturated O2 (blue symbols) and undersaturated O2 (red symbols). b Regression tree describing predictors of N2O concentrations in EQTP rivers. Parameters entering the model were %O2 and NO3−. Values at the ends of each terminal node indicate the N2O concentrations (nmol L−1 ± 1 SD) and number of observations (n). Cross-validated relative error was 1.70 ± 0.02 and R2 was 0.56. c \({{{{{{\rm{F}}}}}}}_{{{{{{{\rm{N}}}}}}}_{2}{{{{{\rm{O}}}}}}}\) in relation to stream order across EQTP rivers. All error bars represent ± 1 SE.
The emission factor EF5-r (EF5-r = N2O-N/NO3−-N) is a surrogate for the conversion of riverine NO3- to dissolved N2O28. Mean EF5-r for EQTP rivers (0.17%) was lower than both the global average (0.22%; Supplementary Table 3) and Intergovernmental Panel on Climate Change (IPCC) default value (0.26%) and is indicative of a small portion of riverine NO3- being converted to dissolved N2O in EQTP rivers. According to our regression tree analysis, the discrepancies between EF5-r for EQTP rivers and IPCC estimate corroborate past studies that a simple linear NO3− model does not adequately predict actual N2O29,30, because N2O concentrations will not necessarily increase with NO3- loads in oxic environments. We therefore recommend that the IPCC methodology should be revised to consider nonlinear relationships or interactions among multiple environmental variables.
Microbial processes underlying N2O dynamics
N2O yield [ΔN2O/(ΔN2O + ΔN2) × 100%] is a useful metric of relative N2O generation27, and in EQTP rivers, the N2O yield (0.003–0.87%, average 0.23%) was 11 times lower than has been reported for lotic settings (0.01–53.8%, average 2.47%; Supplementary Table 5). This small percentage indicates that N processing in EQTP streams and rivers predominantly generates dinitrogen (N2) instead of N2O. Laboratory determination of benthic N2O production rates confirmed the consistent conversion of N2O to N2, as these rates were negative (N2O was consumed) for two-thirds of the sites (Supplementary Table 6). Low N2O yields have been associated with the availability of ample OC to support the complete reduction of NO3− to N22, and in addition to supplying N, thawing permafrost is also a source of biolabile OC to EQTP streams and rivers11 (Fig. 4a).
a Correlation between dissolved organic carbon (DOC) concentrations and N2O yields. Grey points denote samples affected by reservoirs, and were excluded from the correlation analysis. b Correlation between dissolved oxygen saturation (%O2) and ratios of (nirS + nirK)/nosZ. The red lines represent the fit of a linear regression through the observed data and the vertical dashed line denotes the boundary between super- and sub-saturated dissolved oxygen.
Examination of relative gene abundances involved in N2O production and consumption provides further insights regarding the reason for the low N2O yield in EQTP rivers. The key enzymes for N2O production are two types of nitrite reductase (nirS and nirK)31, and N2O consumption is mediated by clade I and II nitrous oxide reductase (nosZ) which catalyzes N2O reduction to N232. A high ratio of (nirS + nirK)/nosZ indicates an amplified capacity for N2O production relative to its loss, leading to high N2O concentrations in the water column. This ratio for EQTP riverbed sediments (average 1.96) was far below values reported from other lotic settings worldwide that vary from 2.16 to 3.24 × 106 (average 19.8; Supplementary Table 7), providing compelling evidence for a molecular basis for the low N2O yield in EQTP rivers. Unexpectedly, we also found a negative correlation between the (nirS + nirK)/nosZ ratio and %O2 (Fig. 4b; Supplementary Discussion 3), illustrating that the microbial community increasingly favors the reduction of N2O to N2 as DO saturation increased33.
Physicochemical processes governing N2O dynamics
To understand potential controls on N2O fluxes (\({{{{{{\rm{F}}}}}}}_{{{{{{{\rm{N}}}}}}}_{2}{{{{{\rm{O}}}}}}}\)), we used stepwise regression to assess the relationships between \({{{{{{\rm{F}}}}}}}_{{{{{{{\rm{N}}}}}}}_{2}{{{{{\rm{O}}}}}}}\) and multiple environmental variables known to influence \({{{{{{\rm{F}}}}}}}_{{{{{{{\rm{N}}}}}}}_{2}{{{{{\rm{O}}}}}}}\). The analysis showed that %O2, pH, water temperature, total phosphorus, and NO3− all had significant but weak relationships with \({{{{{{\rm{F}}}}}}}_{{{{{{{\rm{N}}}}}}}_{2}{{{{{\rm{O}}}}}}}\) (P < 0.001, R2 = 0.14; Supplementary Table 8).
Local hydrogeomorphology is, at least qualitatively, a reliable predictor of \({{{{{{\rm{F}}}}}}}_{{{{{{{\rm{N}}}}}}}_{2}{{{{{\rm{O}}}}}}}\) downstream trends2. Along the longitudinal continuum, \({{{{{{\rm{F}}}}}}}_{{{{{{{\rm{N}}}}}}}_{2}{{{{{\rm{O}}}}}}}\) was highest in 3rd-order (headwater) streams, declined in 4th- and 5th-order (medium-sized) rivers, and was slightly elevated in 6th- and 7th-order (large) rivers (Fig. 3c). The decline in flux from 3rd- to 5th-order streams may reflect the reduced perimeter-to-surface-area ratio (ratio of wetted perimeter to cross-sectional area) and hyporheic exchange rates (exchange rates of dissolved substances between surface water and groundwater beneath and alongside the river channel) with increasing stream order34,35,36, while the increase in 6th- and 7th-order sites might be due to increasing riverine DIN concentrations2 (Supplementary Fig. 4). Furthermore, increased suspended sediment loads can enhance N2O generation in larger turbid channels, as suspended particles provide micro-niches that support N transformations37, and thus facilitate N2O production in the water column2,36. Suspended sediment concentration increased with stream order for these rivers11, lending support to the hypothesis that this mechanism contributes to higher fluxes observed in 6th- and 7th-order channels.
Regional and global implications
Based on our flux measurements, we estimated that EQTP 3rd- to 7th-order streams and rivers emitted 0.206 Gg N2O-N yr−1 (5–95th percentiles: 0.129–0.291 Gg N2O-N yr−1, 2603 km2 of river channel area). Our upscaling did not include 1st- and 2nd-order streams, which can contribute disproportionately high areal fluxes to overall fluvial N2O emissions36,38. Low-order streams are always well connected to continuous permafrost and hence should receive high N inputs while having reduced N2O solubility owing to high altitudes, and these conditions are expected to lead to high N2O fluxes. Based on this logic, we estimated a total emission of 0.275 Gg N2O-N yr−1 from 1st- to 7th-order streams and rivers (5–95th percentiles: 0.162–0.400 Gg N2O-N yr−1, 3049 km2) by extrapolating the relationship in Fig. 3c to include 1st- and 2nd-order streams.
Despite large uncertainties due to a lack of observational data from 1st- and 2nd-order streams (Supplementary Discussion 4), the upscaling exercise enables us to place our estimates in a broader context of both regional N2O budgets and fluvial emissions at the global scale. The percentage of N2O in total GHG (CO2 + CH4 + N2O) emissions expressed as CO2 equivalents corresponded to 1.0% for EQTP 3rd- to 7th-order drainages, then dwindled to 0.4% for EQTP 1st- to 7th-order streams and rivers, falling within the range of pristine rivers (0.2–1.2%)39,40. These values contrast those from human-impacted fluvial networks, where N2O percentages are generally much higher (2.8–13.9%, average 6.8%; Supplementary Table 9) due to often elevated inputs of fertilizer- or sewage-derived N that boost N2O emissions. Expressed as per unit stream/river and basin area, EQTP 1st- to 7th-order streams and rivers released a total of 0.08 t N2O-N km−2 yr−1 and 0.32 kg N2O-N km−2 yr−1, respectively, to the atmosphere, which are one order of magnitude lower than those from lotic systems worldwide [0.65 (range: 0.08–2.55) t N2O-N km−2 yr−1 and 2.44 (range: 0.55–5.78) kg N2O-N km−2 yr−1, respectively; Supplementary Table 9]. Applying the emission rate per unit stream/river and basin area to the entire QTP 1st- to 7th-order drainage networks, we obtained a riverine N2O emission of 0.432–0.463 Gg N2O-N yr−1, which is minor (~0.15%) given their areal contribution (0.7%) to global streams and rivers41. In addition, these N2O estimates are probably overestimated. The emission of N2O accumulated underneath the ice during winter was estimated to be 15% of the annual emission39. This ice-melt outgassing of winter N2O was included in the above annual N2O emissions; however, this flux may be very limited in permafrost-affected systems due to minimum N inputs from frozen soils in winter42. These alpine permafrost waterways emit large amounts of CH411, but fortunately they are currently small contributors of N2O delivery to the atmosphere, demonstrating CH4 and N2O dynamics are uncoupled within these systems.
Although QTP fluxes were small, existing global estimates do not effectively capture this natural fluvial source of N2O, nor are the major drivers of N2O dynamics well known for these systems. Our study is a step forward in quantifying fluvial N2O evasion from a cryospheric biome, and highlights the unique dynamic nature of N2O concentrations and fluxes in high-altitude environments. Our finding that oxygen saturation was the first and primary correlate of N2O concentrations may also have broader implications for aquatic N2O dynamics in other high-altitudinal streams.
Future fluvial N2O emissions under warming climate
Temperatures are rising faster in high altitudes and latitudes than in other regions43. As warming continues, permafrost thaw is expected to increase, liberating substantial amounts of dissolved N12. As this process progresses into deeper soil layers below the rhizosphere, diminished plant uptake24 should favor greater export of N to streamflow via deep flow paths14,44,45. Meanwhile, warmer water temperatures reduce gas solubility and enhance hypoxia and denitrification at the expense of anammox46, directing more N towards denitrification, and concomitant N2O production and evasion to the atmosphere (Fig. 5). Moreover, the duration of the ice-free season across the cryosphere is rapidly increasing and will continue to increase47, suggesting a potential proxy for riverine N2O release. Furthermore, human perturbations may bring an extra N burden to the cryosphere and exacerbate these impacts. Taken together, these processes might render streams and rivers draining permafrost catchments across the globe to become hotspots of N2O to the atmosphere in the future, leading to positive non-carbon climate feedback of currently unanticipated magnitude because of an increase in fluvial N2O production following the development of climate change and escalation of anthropogenic influence.
Future warming and associated permafrost thaw, together with enhanced human perturbations will increase terrestrial N input into surrounding river networks. Warming also raises water temperatures (highlighted in orange), promoting N2O production via denitrification with suppressing the reduction of N2O to N2, eventually resulting in elevated N2O emissions.
The high degree of spatiotemporal variability in riverine N2O observed here is likely to exist in other unexplored cryospheres. Further progress in understanding how aquatic ecosystems in these climate-sensitive regions will respond to ongoing global warming would benefit greatly from more N2O measurements from glacial and permafrost-affected lotic and lentic systems at high spatial and temporal resolution. Alongside N2O measurements, capturing the fate of thawed N in cryospheric aquatic systems are indispensable to stitching together pathways and processes into a holistic framework. It is time to improve our understanding of cryospheric aquatic N2O emissions in shaping the global N2O budget, and how this contribution might be altered in progressively warming high altitudes and latitudes.
Methods
Site description
The ~73.6 × 104 km2 study area of the East Qinghai-Tibet Plateau (EQTP) is located between the boundary of the Loess Plateau (Gansu Province, 36° N) and the southern frigid limit (Yunnan Province, 28° N). This region is characterized by a high-relief landscape ranging from 1650 to 7000 m above sea level. The elevational gradient, along with abundant but variably distributed permafrost creates geomorphological, hydrological, vegetative, and climatological heterogeneities in the alpine landscape. The EQTP includes four great Asian rivers—the Yangtze, Yellow, Lancang-Mekong and Nu-Salween Rivers. We sampled a broad range of streams and rivers in these basins that ranged from Strahler order 3 to 7. All sampling sites were visited during the daytime in spring (May–June), summer (July–August), and fall (September–October) between 2016 and 2018. The Yellow River was sampled seven times, the Yangtze River four times, and the Lancang and Nu Rivers were both sampled on three dates. We grouped the sampling sites into four categories that represent different permafrost zones: continuous, discontinuous, sporadic, and isolated (Fig. 1). We then merged discontinuous, sporadic, and isolated permafrost zones together under the non-continuous permafrost group in response to different NDVI trends.
N2O concentrations and fluxes
Triplicate samples for dissolved N2O concentrations were collected by completely filling 120 mL glass serum bottles at wrist depth below the water surface at each site. After preserving samples with 0.5 mL saturated ZnCl2 solution, the serum bottles were sealed with butyl stoppers, crimped with aluminum caps, and stored at ambient temperature in the dark. Local ambient air samples were also taken and used to back-calculate N2O concentration in water in equilibrium with the atmosphere. N2O concentrations were determined via the headspace equilibration method48 on a gas chromatography equipped with an electron capture detection for N2O (Agilent 7890B GC-µECD). The partial pressure of CO2 (pCO2) in surface water was determined following our earlier work11. Sampling and analysis of dissolved N2 concentrations are described in Supplementary Methods 1.
\({{{{{{\rm{F}}}}}}}_{{{{{{{\rm{N}}}}}}}_{2}{{{{{\rm{O}}}}}}}\) was measured simultaneously with dissolved N2O concentration collection. Four floating chambers were held in place at each transect, covering depth gradients from the river bank to the mid-channel to capture the spatial heterogeneity. Measurements lasted for 1 h at each site, and 50 mL gas extracted from inside the chambers at 0, 5, 10, 20, 40, and 60 min intervals were injected into air-tight gas sampling bags for analysis in the laboratory by GC-µECD. These chambers were of the same size and shape and streamlined with a flexible plastic foil collar to minimize the effects of chamber-induced turbulence when measuring fluxes49 and were covered with aluminum foil to reflect the sunlight and minimize internal heating.
Surface water and sediment samples were collected simultaneously with gas samples for physicochemical and microbial analyses at each site, respectively. Air temperature, air pressure, and wind speed were measured in situ with a portable anemometer (Testo 480). DO, pH, ORP, conductivity, and water temperature were measured in situ with portable field probes (Hach HQ40d). Annual air temperature and precipitation were obtained from the National Meteorological Information Center (http://data.cma.cn/).
Flux computation
Total \({{{{{{\rm{F}}}}}}}_{{{{{{{\rm{N}}}}}}}_{2}{{{{{\rm{O}}}}}}}\) were calculated according to the equation below:
where, nt and n0 are the number of moles of N2O in the chamber at time t and time zero (mol), respectively; A is the surface area of water covered by the chamber (m2) and t is the measurement duration time (min). Diffusive and ebullitive N2O fluxes were separated using the Campeau et al. approach50. Briefly, we assumed that Ft for CO2 (FCO2) is exclusively diffusive (that is, CO2 ebullition is negligible). FCO2 was computed from the linear regression of pCO2 against time to eliminate possible bias due to gas accumulation in the chamber headspace that can affect the flux rates. We then used FCO2 to calculate kCO2 by rearranging the equation for Fick’s law of gas diffusion51:
where KH is the temperature-adjusted Henry’s constant, and ΔpCO2 is the CO2 partial pressure difference between surface water and the atmosphere. Finally, the theoretical diffusive k for N2O was calculated based upon kCO2 as follows52:
where kN2O and kCO2 are gas transfer velocity of N2O and CO2, respectively; Sc is the Schmidt number. n = 1/2 is used for rippled or turbulent surface water conditions, or n = 2/3 is used for a smooth water surface53. We then calculated the theoretical diffusive \({{{{{{\rm{F}}}}}}}_{{{{{{{\rm{N}}}}}}}_{2}{{{{{\rm{O}}}}}}}\) according to
where k is gas transfer velocity (m·d−1), Cwater is water gas concentration (mol m−3), and Ceq is gas concentration in water in equilibrium with the local atmosphere corrected for temperature-induced changes in solubility according to the Henry’s law (mol m−3). Thus, the difference between the total and diffusive N2O fluxes is attributable to ebullition.
Physicochemical analyses
Duplicate filtered water samples were analyzed for NH4+-N, NO2−-N, and NO3−-N, as well as dissolved organic carbon (DOC) following detailed methods described in ref. 11. Total phosphorus (TP) was measured on a UV − Vis spectrophotometer (Agilent 1200) using the standard molybdenum blue method after persulfate digestion. Determination of sedimentary N2O production rates is described in Supplementary Methods 2.
Microbial analyses
A total of 26 sediment samples collected across the four rivers between 2016 and 2018 were prepared in triplicate. Genomic DNA was extracted from approximately 0.5 g fresh homogenized sediment using the FastDNA® SPIN Kit for Soil (MP Biomedicals), following the manufacturer’s instructions. Abundances of dissimilatory nitrite reductase (nirS and nirK)31 and nitrous oxide reductase (nosZI and nosZII) genes32 in these river sediments were estimated by real-time quantitative PCR. Detailed information is available in Supplementary Methods 3.
GIS analyses
Vegetation types in the study area are alpine meadow and steppe, and vegetation coverage for each type was extracted using datasets from ref. 54. The vegetation coverage for each river reach was determined within a circular area of 5 km in radius centered around each sampling site using the ArcGIS 10.6 buffer analysis tool. Normalized difference vegetation index (NDVI) data were obtained from the China Monthly Vegetation Index Spatial Distribution Data Set (Resource and Environment Science and Data Center, Chinese Academy of Sciences, https://www.resdc.cn/), with a spatial resolution of 1 × 1 km2 for the period 2016–2018 corresponding to sampling dates. The NDVI values for each river reach were calculated as the mean value within a circular area of 5 km in radius centered around each sampling site using the ArcGIS 10.6 zonal statistics tool.
Hydrological analysis was performed on the ArcGIS 10.4 platform. Streams and rivers were extracted and Strahler stream order for all stream lines in the four catchments was calculated based on Digital Elevation Model (DEM) data. We then calculated the total surface area for each stream order by multiplying the total length with the average width of this stream order. The total length was derived from the distance tool in the ArcGIS. We calculated the average river width for each stream order based on in situ width measurements at the sampling location and 50 additional locations of the corresponding stream order from Google Earth Maps in each catchment. Average stream width for the 1st- and 2nd-order streams were obtained from the width ratios found for rivers of high stream orders according to our earlier work11.
Upscaling technique
We upscaled the magnitude of N2O emissions (and uncertainty) from EQTP streams and rivers with a Monte Carlo simulation (MATLAB R2018b) that ran 1000 iterations for each sampled 3rd- to 7th-stream order. Each iteration randomly selected a N2O flux measurement and simultaneously selected a surface area based on a normal distribution surrounding the mean and standard deviation for that stream in order to generate an order-specific N2O flux per unit of time. This value was then summed across the ice-free season (210 d from April to October) to estimate N2O emission for each stream order. For 1st- and 2nd-order streams, a range of N2O fluxes that extrapolated from the relationship in Fig. 3c and the surface area for this order were used to produce total fluxes and constrain the uncertainty. We summed up the final distribution of N2O emissions from stream orders 1–7, including the mean value and 95% confidence intervals. Finally, we divided the ice-free season emission by 85% to obtain the annual emission. This is because early spring efflux of winter-derived N2O fuels ~15% of the annual emissions39.
Statistical analyses
Pearson correlations and multiple linear stepwise regression analysis were conducted under the Statistical Product and Service Solutions 25.0 software (SPSS) at a significance level of P < 0.05. To uncover complex dependencies among predictor variables, we used a regression tree approach to analyze predictors of N2O concentrations with MATLAB R2018b. This nonparametric method does not require linear relationships and allows for interaction effects among predictors. All data form a single group at the top of the tree, and the tree is grown by repeatedly splitting the data into two subgroups, with each split based on the explanatory variable which makes the resulting groups as different as possible. All values of predictive variables are assessed each time a dichotomy is made55. For each of the two subgroups, the same process was repeated until a tree is formed. Such a tree needed to be pruned to avoid overfitting and improve predictive accuracy. We then applied 10-fold cross-validation and pruned the tree based on the ‘1-SE’ rule. This was a parsimonious approach to find the smallest tree whose cross-validated relative error (CVRE) is within one standard error of the minimum. We report the amount of variance explained as R2 to depict the fit of the tree. We also report the CVRE since it is a suitable measure of prediction56. Predictive variables available for entry into the model were: %O2 and NO3-. Given the high CVRE here, the model is more suggestive than predictive.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data used in this study are available at the Environmental Data Initiative (https://portal.edirepository.org/nis/mapbrowse?packageid=edi.695.1), https://doi.org/10.6073/pasta/ba9340800403c450e7d942d450237dc4.
Code availability
The Monte Carlo model and regression tree analysis code used in this study are available at the Environmental Data Initiative (https://portal.edirepository.org/nis/mapbrowse?packageid=edi.695.1), https://doi.org/10.6073/pasta/ba9340800403c450e7d942d450237dc4
References
Ravishankara, A. R., Daniel, J. S. & Portmann, R. W. Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326, 123–125 (2009).
Quick, A. M. et al. Nitrous oxide from streams and rivers: a review of primary biogeochemical pathways and environmental variables. Earth Sci. Rev. 191, 224–262 (2019).
Hu, M., Chen, D. & Dahlgren, R. A. Modeling nitrous oxide emission from rivers: a global assessment. Glob. Change Biol. 22, 3566–3582 (2016).
Yao, Y. et al. Increased global nitrous oxide emissions from streams and rivers in the Anthropocene. Nat. Clim. Change 10, 138–142 (2020).
Mishra, U. et al. Spatial heterogeneity and environmental predictors of permafrost region soil organic carbon stocks. Sci. Adv. 7, eaaz5236 (2021).
Striegl, R. G., Dornblaser, M. M., McDonald, C. P., Rover, J. R. & Stets, E. G. Carbon dioxide and methane emissions from the Yukon River system. Glob. Biogeochem. Cycles https://doi.org/10.1029/2012gb004306 (2012).
Crawford, J. T., Striegl, R. G., Wickland, K. P., Dornblaser, M. M. & Stanley, E. H. Emissions of carbon dioxide and methane from a headwater stream network of interior Alaska. J. Geophys. Res. Biogeosci. 118, 482–494 (2013).
Zolkos, S., Tank, S. E., Striegl, R. G. & Kokelj, S. V. Thermokarst effects on carbon dioxide and methane fluxes in streams on the Peel Plateau (NWT, Canada). J. Geophys. Res. Biogeosci. 124, 1781–1798 (2019).
Dean, J. F. et al. East Siberian Arctic inland waters emit mostly contemporary carbon. Nat. Commun. 11, 1627 (2020).
Serikova, S. et al. High riverine CO2 emissions at the permafrost boundary of Western Siberia. Nat. Geosci. 11, 852–829 (2018).
Zhang, L. et al. Significant methane ebullition from alpine permafrost rivers on the East Qinghai-Tibet Plateau. Nat. Geosci. 13, 349–354 (2020).
Voigt, C. et al. Nitrous oxide emissions from permafrost-affected soils. Nat. Rev. Earth Environ. 1, 420–434 (2020).
Abbott, B. W., Jones, J. B., Godsey, S. E., Larouche, J. R. & Bowden, W. B. Patterns and persistence of hydrologic carbon and nutrient export from collapsing upland permafrost. Biogeosciences 12, 3725–3740 (2015).
Khosh, M. S. et al. Seasonality of dissolved nitrogen from spring melt to fall freezeup in Alaskan Arctic tundra and mountain streams. J. Geophys. Res. Biogeosci. 122, 1718–1737 (2017).
Yang, M., Wang, X., Pang, G., Wan, G. & Liu, Z. The Tibetan Plateau cryosphere: observations and model simulations for current status and recent changes. Earth Sci. Rev. 190, 353–369 (2019).
Jin, H. J., Chang, X. L. & Wang, S. L. Evolution of permafrost on the Qinghai-Xizang (Tibet) Plateau since the end of the late Pleistocene. J. Geophys. Res. Earth Surface https://doi.org/10.1029/2006jf000521 (2007).
Kou, D. et al. Spatially-explicit estimate of soil nitrogen stock and its implication for land model across Tibetan alpine permafrost region. Sci. Total Environ. 650, 1795–1804 (2019).
Immerzeel, W. W. & Bierkens, M. F. P. Asia’s water balance. Nat. Geosci. 5, 841–842 (2012).
Horgby, Å. et al. Unexpected large evasion fluxes of carbon dioxide from turbulent streams draining the world’s mountains. Nat. Commun. 10, 4888 (2019).
Chen, H. et al. The impacts of climate change and human activities on biogeochemical cycles on the Qinghai-Tibetan Plateau. Glob. Change Biol. 19, 2940–2955 (2013).
Baulch, H. M., Dillon, P. J., Maranger, R. & Schiff, S. L. Diffusive and ebullitive transport of methane and nitrous oxide from streams: are bubble-mediated fluxes important? J. Geophys. Res. Biogeosci. https://doi.org/10.1029/2011jg001656 (2011).
Keuper, F. et al. A frozen feast: thawing permafrost increases plant-available nitrogen in subarctic peatlands. Glob. Change Biol. 18, 1998–2007 (2012).
Liu, X. et al. Nitrate is an important nitrogen source for Arctic tundra plants. Proc. Natl Acad. Sci. USA 115, 3398–3403 (2018).
Kou, D. et al. Progressive nitrogen limitation across the Tibetan alpine permafrost region. Nat. Commun. 11, 3331 (2020).
Gao, M. et al. Divergent changes in the elevational gradient of vegetation activities over the last 30 years. Nat. Commun. 10, 2970 (2019).
Salmon, V. G. et al. Adding depth to our understanding of nitrogen dynamics in permafrost soils. J. Geophys. Res. Biogeosci. 123, 2497–2512 (2018).
Beaulieu, J. J. et al. Nitrous oxide emission from denitrification in stream and river networks. Proc. Natl Acad. Sci. USA 108, 214–219 (2011).
Cooper, R. J., Wexler, S. K., Adams, C. A. & Hiscock, K. M. Hydrogeological controls on regional-scale indirect nitrous oxide emission factors for rivers. Environ. Sci. Technol. 51, 10440–10448 (2017).
Rosamond, M. S., Thuss, S. J. & Schiff, S. L. Dependence of riverine nitrous oxide emissions on dissolved oxygen levels. Nat. Geosci. 5, 715–718 (2012).
Venkiteswaran, J. J., Rosamond, M. S. & Schiff, S. L. Nonlinear response of riverine N2O fluxes to oxygen and temperature. Environ. Sci. Technol. 48, 1566–1573 (2014).
Zumft, W. G. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61, 533–616 (1997).
Hallin, S., Philippot, L., Löffler, F. E., Sanford, R. A. & Jones, C. M. Genomics and ecology of novel N2O-reducing microorganisms. Trends Microbiol. 26, 43–55 (2018).
Rees, A. P. et al. Biological nitrous oxide consumption in oxygenated waters of the high latitude Atlantic Ocean. Commun. Earth Environ. 2, 36 (2021).
Alexander, R. B., Smith, R. A. & Schwarz, G. E. Effect of stream channel size on the delivery of nitrogen to the Gulf of Mexico. Nature 403, 758–761 (2000).
Gomez-Velez, J. D. & Harvey, J. W. A hydrogeomorphic river network model predicts where and why hyporheic exchange is important in large basins. Geophys. Res. Lett. 41, 6403–6412 (2014).
Marzadri, A., Dee, M. M., Tonina, D., Bellin, A. & Tank, J. L. Role of surface and subsurface processes in scaling N2O emissions along riverine networks. Proc. Natl Acad. Sci. USA 114, 4330–4335 (2017).
Xia, X., Jia, Z., Liu, T., Zhang, S. & Zhang, L. Coupled nitrification-denitrification caused by suspended sediment (SPS) in rivers: Importance of SPS size and composition. Environ. Sci. Technol. 51, 212–221 (2017).
Turner, P. A. et al. Indirect nitrous oxide emissions from streams within the US Corn Belt scale with stream order. Proc. Natl Acad. Sci. USA 112, 9839–9843 (2015).
Soued, C., del Giorgio, P. A. & Maranger, R. Nitrous oxide sinks and emissions in boreal aquatic networks in Québec. Nat. Geosci. 9, 116–120 (2016).
Borges, A. V. et al. Variations in dissolved greenhouse gases (CO2, CH4, N2O) in the Congo River network overwhelmingly driven by fluvial-wetland connectivity. Biogeosciences 16, 3801–3834 (2019).
Allen, G. H. & Pavelsky, T. M. Global extent of rivers and streams. Science 361, 585–588 (2018).
Cavaliere, E. & Baulch, H. M. Denitrification under lake ice. Biogeochemistry 137, 285–295 (2018).
Pepin, N. et al. Elevation-dependent warming in mountain regions of the world. Nat. Clim. Change 5, 424–430 (2015).
Harms, T. K. & Jones, J. B. Jr. Thaw depth determines reaction and transport of inorganic nitrogen in valley bottom permafrost soils. Glob. Change Biol. 18, 2958–2968 (2012).
Vonk, J. E. et al. Reviews and syntheses: effects of permafrost thaw on Arctic aquatic ecosystems. Biogeosciences 12, 7129–7167 (2015).
Tan, E. et al. Warming stimulates sediment denitrification at the expense of anaerobic ammonium oxidation. Nat. Clim. Change 10, 349–355 (2020).
Yang, X., Pavelsky, T. M. & Allen, G. H. The past and future of global river ice. Nature 577, 69–73 (2020).
Johnson, K. M., Hughes, J. E., Donaghay, P. L. & Sieburth, J. M. Bottle-calibration static head space method for the determination of methane dissolved in seawater. Anal. Chem. 62, 2408–2412 (1990).
Lorke, A. et al. Technical note: drifting versus anchored flux chambers for measuring greenhouse gas emissions from running waters. Biogeosciences 12, 7013–7024 (2015).
Campeau, A., Lapierre, J.-F., Vachon, D. & del Giorgio, P. A. Regional contribution of CO2 and CH4 fluxes from the fluvial network in a lowland boreal landscape of Québec. Glob. Biogeochem. Cycles 28, 57–69 (2014).
Wanninkhof, R. & Knox, M. Chemical enhancement of CO2 exchange in natural waters. Limnol. Oceanogr. 41, 689–697 (1996).
Raymond, P. A. et al. Scaling the gas transfer velocity and hydraulic geometry in streams and small rivers. Limnol. Oceanogr. Fluids Environ. 2, 41–53 (2012).
Alin, S. R. et al. Physical controls on carbon dioxide transfer velocity and flux in low-gradient river systems and implications for regional carbon budgets. J. Geophys. Res. Biogeosci. https://doi.org/10.1029/2010JG001398 (2011).
Wang, Z. et al. Mapping the vegetation distribution of the permafrost zone on the Qinghai-Tibet Plateau. J. Mountain Sci. 13, 1035–1046 (2016).
De’ath, G. & Fabricius, K. E. Classification and regresstion trees: a powerful yet simple technique for ecological data analysis. Ecology 81, 3178–3192 (2000).
De’ath, G. Multivariate regression trees: a new technique for modeling species-environment relationships. Ecology 83, 1105–1117 (2002).
Obu, J., Westermann, S., Kääb, A. & Bartsch, A. Permafrost Extent and Ground Temperature Map, 2000–2016, Northern Hemisphere Permafrost (https://apgc.awi.de/dataset/pex). doi.pangaea.de/10.1594/PANGAEA.888600 (2018).
Acknowledgements
We thank Lin Zhao and Zhiwei Wang at Northwest Institute of Eco-Environment and Resources, CAS who have graciously shared their GIS data to make our vegetation analysis possible. This research was funded by the National Natural Science Foundation of China (grant no. 92047303, 52039001) and the National Key R&D Program of China (grant no. 2017YFA0605001).
Author information
Authors and Affiliations
Contributions
X.X. and L.Z. designed the research. L.Z. and S.Z. collected and analyzed samples. Q.W. and R.L. conducted GIS, Monte Carlo, and regression tree analyses. X.X., L.Z., S.Z., T.J.B., S.L., Z.Y., J.N., and E.H.S. performed data analysis and wrote the manuscript with significant contribution from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Maija Marushchak and the other, anonymous, reviewer for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, L., Zhang, S., Xia, X. et al. Unexpectedly minor nitrous oxide emissions from fluvial networks draining permafrost catchments of the East Qinghai-Tibet Plateau. Nat Commun 13, 950 (2022). https://doi.org/10.1038/s41467-022-28651-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-28651-8
This article is cited by
-
Andean headwater and piedmont streams are hot spots of carbon dioxide and methane emissions in the Amazon basin
Communications Earth & Environment (2023)
-
Carbon and nitrogen cycling on the Qinghai–Tibetan Plateau
Nature Reviews Earth & Environment (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.