Abstract
In the central nervous system (CNS), functional tasks are often allocated to distinct compartments. This is also evident in the Drosophila CNS where synapses and dendrites are clustered in distinct neuropil regions. The neuropil is separated from neuronal cell bodies by ensheathing glia, which as we show using dye injection experiments, contribute to the formation of an internal diffusion barrier. We find that ensheathing glia are polarized with a basolateral plasma membrane rich in phosphatidylinositol-(3,4,5)-triphosphate (PIP3) and the Na+/K+-ATPase Nervana2 (Nrv2) that abuts an extracellular matrix formed at neuropil-cortex interface. The apical plasma membrane is facing the neuropil and is rich in phosphatidylinositol-(4,5)-bisphosphate (PIP2) that is supported by a sub-membranous ßHeavy-Spectrin cytoskeleton. ßHeavy-spectrin mutant larvae affect ensheathing glial cell polarity with delocalized PIP2 and Nrv2 and exhibit an abnormal locomotion which is similarly shown by ensheathing glia ablated larvae. Thus, polarized glia compartmentalizes the brain and is essential for proper nervous system function.
Similar content being viewed by others
Introduction
The complexity of the nervous system is not only defined by intricate neuronal networks but is also reflected by the cellular diversity of the different glial cells found throughout the nervous system. The number of functions attributed to the different glial cells is steadily growing and ranges from guiding and instructing functions during early brain development to synapse formation and plasticity, and general ion and metabolite homeostasis1,2.
One of the key functions of glial cells lies in the establishment of boundaries that help compartmentalizing neuronal computing. A very tight boundary is the blood–brain barrier. In all invertebrates as well as in primitive vertebrates this barrier is established by glial cells, whereas higher vertebrates transferred this function to endothelial cells3,4,5. Smaller signaling compartments are established by astrocytes that tile the synaptic areas in the brain6,7,8. Oligodendrocytes insulate single segments of up to 20 different axons by forming myelin sheets around them9. Moreover, oligodendrocyte progenitor cells act as innate immune cells and participate in scar formation separating intact brain tissue from the injured site10.
Invertebrates have a less complex nervous system and glial cells are clearly outnumbered by neurons. In the ant Harpegnathos saltator 27% of all brain cells are of glial nature, whereas in the fruit fly Drosophila melanogaster just 10% of all neural cells are of glial nature11,12. As in primitive vertebrates, glial cells form the blood–brain barrier and organize the entire metabolite traffic into and out of the nervous system and also integrate external stimuli such as circadian rhythms with general brain functions13,14,15,16. All neuronal cell bodies are situated beneath the blood–brain barrier and are surrounded by cortex glial cells. These cells regulate neuroblast division, nurture neurons, and phagocytose dying neurons17,18,19,20,21,22,23,24.
Fly neurons project their dendrites and axons into the central neuropil. This morphologically distinct structure is separated from all neuronal cell bodies by the neuropil-associated glia, which comprises astrocyte-like glial cells and ensheathing glial cells25,26,27,28,29. Astrocyte-like cells form numerous fine and highly branched processes that infiltrate the neuropil. As in vertebrates they tile the synaptic neuropil into discrete units. They express a number of neurotransmitter transporters to clear synaptic spillover although they do not form frequent intimate contacts with individual synapses and in addition are able to secrete gliotransmitters29,30,31.
The neuropil encasing ensheathing glia comprises two distinct classes that can be classified by morphological as well as molecular criteria11,27,28,32. In the larval nervous system, just four ensheathing glial cells are formed in each hemineuromer. Two of them embrace the neuropil and do not wrap around individual axons. In contrast, the other two larval ensheathing glial cells found in each abdominal hemineuromere exhibit a more complex morphology. In part they encase the neuropil as the other two ensheathing glial cells but in addition they also enwrap axons between the CNS/PNS boundary and the neuropil. They are thus called ensheathing/wrapping glial cells27,28. In the larva, the organization of the neuropil is relatively simple, but the adult CNS shows complex regional compartmentalization33,34. This is reflected by an increase in the number of ensheathing glial cells that are generated during pupal stages and, as in larval stages, fall into two morphological classes32,35.
Several studies have already shed some light on the functional roles of ensheathing glia in the fly nervous system. First, it was demonstrated that ensheathing glial cells remove neuronal debris after injury utilizing the Draper pathway24,36,37. In addition to this immune and surveillance function, ensheathing glia can participate in neuronal signaling. Mutant analysis indicates the sulfite oxidase Shopper needs to be expressed by ensheathing glia to regulate glutamate homeostasis in the neuropil28. Likewise, the Excitatory amino acid transporter 2 (Eaat2) functions in ensheathing glia to modulate sleep in the adult38. In addition, cell type specific knockdown experiments demonstrate that the voltage-gated potassium channel encoded by the gene seizure (sei) is required in ensheathing glia to protect flies from acute heat-induced seizures39.
Given the apparent role of ensheathing glia in forming neuronal compartments and modulating their function, we initiated a comprehensive analysis of this—as we found—highly polarized cell type. The plasma membrane of the ensheathing glia facing the neuropil is rich in the phospholipid PIP2 and is supported by sub-membranous ßHeavy-Spectrin. Thus, it can be assumed that the apical cell domain is oriented towards the neuropil. The basolateral plasma membrane exhibits an accumulation of the Na+/K+ ATPase Nervana 2 (Nrv2) suggesting that sodium ion dependent transport is polarized in these cells. In addition, basally localized Integrins anchor the ensheathing glia to a specific extracellular matrix (ECM) formed at the interface of neuropil and CNS cortex. To study the functional relevance of ensheathing glia, we ablated these cells using a split Gal4 driver that we had established. The absence of ensheathing glia triggers a compensatory growth of astrocyte-like cells which, however, does not completely restore an internal diffusion barrier normally promoted by ensheathing glia. Animals lacking ensheathing glia show abnormal larval locomotion and reduced longevity. Reduction of ßHeavy-Spectrin expression affects ensheathing glia cell morphology and leads to mislocalization of PIP2. Moreover, larvae with ensheathing glia specific ßHeavy-spectrin knockdown or larvae lacking ensheathing glia show similar abnormal locomotor phenotypes as ßHeavy-spectrin mutant larvae. In conclusion, we show that separation of the neuropil by polarized ensheathing glia is required for nervous system function.
Results
Ensheathing glia encase the neuropil throughout development
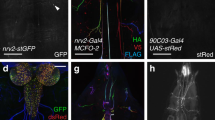
Four ensheathing glial cells are formed in each hemineuromere during mid embryogenesis and are associated with the neuropil27. Two of these cells only encase the neuropil, whereas the two other also wrap axons connecting the peripheral nerves with the neuropil (ensheathing/wrapping glial cells, Fig. 1)27,32,40. To target the larval ensheathing glial cells, we compared the activity of the previously used Gal4 lines (NP6520-Gal4, 56F03-Gal4, nrv2-Gal4, and 83E12-Gal4) and found the 83E12-Gal4 driver as the most specific one for both larval and adult CNS27,28,32,41. Cell counts and 3D reconstructions (see below) suggest that 83E12-Gal4 is active in all larval ensheathing glial cells. However, since no 3D reconstruction has been conducted for the adult TEM volume we cannot exclude that some 83E12-Gal4 negative ensheathing glial cells exist.
Representative images are shown. a–d Dissected larval CNS of increasing age with the genotype [83E12-Gal4, UAS-CD8::GFP], stained for GFP (green), Repo (magenta) and neuronal membranes (anti-HRP, blue), anterior is up. The positions of the orthogonal section shown in a’–d’ is indicated by a dashed line. a, a’ In a stage 16 embryo ensheathing glial cells have not yet covered the neuropil (arrowheads). b, b’ First instar larval CNS. c, c’ Second instar larval CNS. d, d’ CNS of a wandering third instar larva. The arrows point towards dorsal protrusions of the ensheathing glia engulfing dorsal neurons. e MCFO labeling of ensheathing glia in third instar larva stained for the expression of V5 (green), HA (red), and FLAG and OLLAS epitopes (blue). flp expression was induced for one hour during first instar larval stage. Note that ensheathing glia tile the ventral nerve cord. f Two distinct ensheathing/wrapping glia cells cover the nerve root and part of the neuropil (arrowheads). g Ensheathing glia occupy specific territories in the neuropil. h, i Third instar larval nerve cord with the genotype [55B12-Gal4, 83E12-LexA, UAS-CD8::mCherry, LexAop-GFP]. All cortex glia cells are labelled by mCherry expression (magenta). Ensheathing glial cells are labelled by GFP expression (green). The dashed line indicates the position of the orthogonal view shown in (i). j Schematic view on a cross section through a hemineuromere indicating the position of the different glial cells. Astrocyte-like cells and ensheathing glial cells localize close to the neuropil and the axons connecting the neuropil with the periphery. The cortex glia covers lateral and ventral neuronal cell bodies. Scale bars a–i are 50 µm.
During embryonic stages, the ensheathing glia initially cover the dorsal domain of the neuropil (Fig. 1a, a’). In first instar larvae, thin processes of the ensheathing glia begin to encase the neuropil (Fig. 1b, b’). Notably, small gaps between individual ensheathing glial processes are still detectable in the second instar larva (Fig. 1c, c’). In the third larval instar, ensheathing glia appear to form a closed case around the neuropil. The ensheathing glia also send processes around the dorsally located neuronal cell bodies (Fig. 1d’, Supplementary Fig. S1). Multi-color flipout (MCFO) labelling demonstrates that ensheathing glia processes tile the neuropil (Fig. 1e–g, j) to separate it from the CNS cortex. The cortex glia can be visualized using the Gal4 drivers 55B12-Gal4; NP2222-Gal4 and NP0577-Gal428,42. These drivers showed that no cortex glial cells reside at the dorsal surface of the ventral nerve cord (Fig. 1h–j). This notion is further corroborated by split-GFP experiments where GFP is reconstituted at the baso-lateral boundary between cortex and neuropil but not at the dorsal surface of the ventral nerve cord (Supplementary Fig. S1a–f’). Here, several large glutamatergic neurons are found that appear to be encased by processes of the ensheathing glia (Supplementary Fig. S1g–l). In conclusion, the ensheathing glia can wrap axons as they connect with peripheral organs, associate with dorsal neurons, and encase the neuropil (Fig. 1j).
Electron microscopic analysis of larval ensheathing glia
To further study the morphological characteristics of the ensheathing glia, we analyzed serial section transmission electron microscopy (ssTEM) data sets of a first instar larval ventral nerve cord (L1)43. We annotated all neuropil-associated glial cells in the abdominal neuromeres A1-A8/9 using CATMAID44 (Fig. 2). In total, 159 neuropil-associated glial cells were identified that based on their typical morphology could be assigned to one of the three classes of neuropil-associated glial cells27: astrocyte-like glial cells, ensheathing glia and ensheathing/wrapping glia (Fig. 2a, b). The morphological characteristics of the different glial subtypes are already evident in first larval instar and become more pronounced in third instar larval brain (Fig. 2c, d). Astrocyte-like glial cells were identified by a prominent process extending into the neuropil proximal to the astrocyte nucleus29,31. In the abdominal ventral nerve cord of the L1 volume, 96 astrocyte-like glial cells were identified matching the previously known numbers27,29.
a, b Schematic view on a first instar larval central nervous system. All neuropil-associated glial cells were annotated in a serial section TEM volume. Yellow dots indicate the position of the nuclei of astrocyte-like cells, magenta dots indicate the positions of the ensheathing glial cell nuclei, green dots indicate the positions of the ensheathing/wrapping glial nuclei. The arrow points to a segmental position where only one instead of two ensheathing/wrapping glial cell was identified. c Representative section through the fourth abdominal hemineuromer of a L1 larva showing the position of three different ensheathing glial cells around the neuropil. d Representative section through the fourth abdominal neuromere of a third instar larval ventral nerve cord. Note that ensheathing glia completely cover the neuropil. It was not determined whether the red marked glial processes belong to the blue or green ensheathing/wrapping glial cell. Scale bar (c, d): 10 µm, (e–h): 5 µm, (i, j): 0.2 µm. e Ensheathing glia in a first and (f) in a third instar larva. g Ensheathing/wrapping glial cells in a first and (h) in a third instar larva. Note the thin processes that engulf cell bodies of dorsally located neurons (asterisk). i On the ventral face of the neuropil an ensheathing glial cell and a cortex glial cell form a two-layered sheath between cortex and neuropil. j A highly multilayered glial cell layer is found at the dorsal face of the neuropil.
In total 28 ensheathing glial cells were identified in the abdominal neuromeres 1–7 (Fig. 2a). These glial cells extend thin membrane sheaths along the face of the neuropil (Fig. 2e). The nuclei of the central ensheathing glial cell in each neuromere is usually associated with the dorso-ventral channel25. The other ensheathing glia is found at a ventral position at the neuropil cortex interface (see also27).
In total 33 cells were annotated as ensheathing/wrapping glial cells, which encase the neuropil and wrap axons in the nerve root. However, this is not very pronounced in the first instar stage, yet (Fig. 2g). With one exception (Fig. 2b), two cells, both located at the dorsal aspect of the neuropil, are associated with each intersegmental nerve root in neuromeres 1–7 (Fig. 2b). The fused A8/A9 nerve has two nerve roots associated with two ensheathing/wrapping glial cells, each (Fig. 2b).
To determine whether the overall morphology of ensheathing glial cells changes during development, we annotated the ensheathing glia in three abdominal neuromeres (A1-A3) of a third instar larval brain (L3)45. Here, too, two ensheathing and two ensheathing/wrapping glial cells are found in each hemineuromere (Fig. 2f, h). Note, that dorsally to the neuropil, ensheathing glia send protrusions to the blood–brain barrier and thus adopt a more complex polarized morphology (Fig. 2h). In consequence, dorsal neurons are encased by ensheathing glia processes and not by cortex glia (Fig. 1j, Fig. 2h, see below). The ventrolateral ensheathing glial cells do not encase neuronal cell bodies, but form very flat membrane sheets around the neuropil (Fig. 1g–i, Fig. 2e, f).
The light microscopic analysis suggests that ensheathing glial cells encase the neuropil in third instar larvae. This idea is supported by the ssTEM data sets. In L1 ventral nerve cord ensheathing, ensheathing/wrapping and astrocyte-like glial cells form an almost closed structure around the neuropil (Fig. 2c). In contrast, in the L3 larval ventral nerve cord the neuropil encasement is complete (Fig. 2d). In addition, multiple layers of glial processes are found around the neuropil (Fig. 2i, j). At the ventral face of the neuropil, the glial sheath is often made of cell processes of ensheathing glial and cortex glial cells (Fig. 2i).
The complexity of ensheathing glia increases during development
About 112 ensheathing glial cells expressing 83E12-Gal4 can be detected in the third instar larva with only few ensheathing glial cells in the brain lobes (n = 5, with 110, 112, 112, 114, and 116 83E12-Gal4 positive cells, median = 112) (Fig. 3a, m), which corresponds to previously determined numbers27,35,40,46,47 and matches the annotations made in the TEM volume mentioned above. During the subsequent pupal development, the number of the 83E12-Gal4 positive ensheathing glial cell population increases more than 1,100 ensheathing glial cells in the adult (ventral nerve cord and brain without optic lobes, number determined using Imaris) (Fig. 3c, n).
Representative images are shown. a Control third instar larvae with nuclei of ensheathing glia labelled [83E12-Gal4, UAS-Lam::GFP]. Nuclei are stained using DAPI. The boxed area is shown in high magnification in (b). c Adult control brain. The number of ensheathing glial nuclei is quantified in (m, n, n = 5 brains for all genotypes). d–f Expression of an activated FGF-receptor leads to an increase in the number of larval ensheathing glia in larval thoracic neuromeres (arrowheads). The white boxed area is shown in higher magnification (e). g–i Upon expression of string dsRNA in all ensheathing glia the number of larval ensheathing glial cells is slightly reduced. For quantification see (m). The boxed area in the larval CNS (g) is shown in high magnification in (h). i Expression of string dsRNA in all ensheathing glia reduces the number of ensheathing glial cells in the adult CNS. j–l A similar reduction in the number of ensheathing glial cells is observed following expression of fzr. The boxed area in (j) is shown in high magnification in (k). Scale bars larval CNS (a, b, d, e, g, h, j, k) are 50 µm, scale bars for adult CNS (c, f, i, l) are 100 µm. Quantification of the ensheathing glial cell number in five larval brains (m) and in five adult brains (n) using Imaris (unpaired t-test, two-tailed). The standard deviation is indicated. The optic lobes and the tract ensheathing glial cells were excluded in the quantification. For larva: control – stringdsRNA: *p = 0.0192; control – fzr: **p = 0.0013; control - λhtl: ****p =< 0.0001; for adult: control – stringdsRNA: ***p = 0.0002; control – fzr: **p = 0.0018; control - λhtl: **p = 0.0057. (O) Quantification of DAPI intensity in 30 larval abdominal, 30 larval thoracic, and 10 adult thoracic ensheathing glial and neuronal nuclei. Source data are provided as a Source Data file.
It is unclear whether all larval ensheathing glia degenerate or whether some survive to participate in the formation of adult ensheathing glia35,46. To test whether ensheathing glial cells are competent to divide, we expressed activated FGF-receptor which is known to trigger perineurial glial proliferation48,49. Interestingly, a proliferative response upon activated FGF-receptor expression is most prominently observed in thoracic neuromeres (Fig. 3a–f, m, n, Supplementary Fig. S2). We then blocked cell division in the ensheathing glia by RNAi-based silencing of string, which encodes the Drosophila Cdc25 phosphatase homolog required for progression from G2 to M phase50. This expression regime did not severely affect ensheathing glia cell number in the larval CNS (Fig. 3g, h, m, 94 ensheathing glia in string knockdown larvae vs. 112 ensheathing glial cells in control animals, n = 5 with 83, 83, 110, 99, and 94 83E12-Gal4 positive cells, median = 94). In contrast, suppression of string function resulted in 38% less ensheathing glial cells in the adult nervous system (724 [n = 5, 724, 707, 769, 633, and 848 83E12-Gal4 positive cells, median = 724] instead of the expected 1136 ensheathing glial cells (Fig. 3i, n). Similarly, when we expressed the cell cycle regulator Fizzy related (Fzr) which blocks cell division upon overexpression51,52,53 we noted a similar reduction in the number of adult ensheathing glia (Fig. 3j–n). Interestingly, the block of cell proliferation in ensheathing glia mostly affects the adult brain (Fig. 3i, l). To further test whether larval ensheathing glia could divide, we analyzed the ploidy of the ensheathing glia using DAPI staining54. In the larval ventral nerve cord, 30 of 30 tested abdominal glial nuclei are polyploid and thus are likely not to divide (Fig. 3o, Supplementary Fig. S3a–d). In contrast, 10 out of 25 ensheathing glial cells in thoracic neuromeres are diploid and possibly could divide (Fig. 3o, Supplementary Fig. S3a–d). Phospho-histone H3 serves as a specific marker for mitosis. In the larval CNS, we only rarely detected 83E12-Gal4 and phospho-histone H3 positive cells (Supplementary Fig. S3e). Dividing ensheathing glial cells were easily detected in early pupae but not in 42 APF old pupae (Supplementary Fig. S3f–h). In addition, we fed the thymidine analogue 5-ethynyl-2′-deoxyuridine (EdU) which allows to label DNA synthesis during larval development and frequently detected EdU staining in 83E12-Gal4 positive ensheathing glia of larval and young pupal brains (Supplementary Fig. S3i, j). Moreover, when we labelled larval ensheathing glia using a MCFO2 strategy55 in third instar larvae or early pupae, we found labelled ensheathing glia in the adult (Supplementary Fig. S4a-c). In conclusion, at least some larval 83E12-Gal4 expressing ensheathing glial cells are diploid and can proliferate.
Ensheathing glial cells are not required for viability
To test the functional relevance of the ensheathing glia, we performed ablation experiments. Expression of hid and reaper (rpr) using repo-Gal4 or 83E12-Gal4 resulted in early larval lethality. Since 83E12-Gal4 is also expressed in the midgut, we used a split-Gal4 approach to restrict Gal4 expression to only the ensheathing glial cells of the CNS56,57. We generated a construct that allowed the expression of a Gal4 activation domain under the control of the 83E12 enhancer [83E12-Gal4AD]. When crossed to flies that carry an element that directs expression of the Gal4 DNA binding domain in all glial cells [repo-Gal4DBD], Gal4 activity will be reconstituted only in CNS ensheathing glial cells. Indeed, animals carrying both transgenes [83E12-Gal4AD, repo-Gal4DBD] show the same expression domain as the original 83E12-Gal4 driver in the adult as well as in the larval nervous system (Supplementary Fig. S4d–g).
Flies expressing hid and rpr only in the ensheathing glia [UAS-rpr; 83E12-Gal4AD; repo-Gal4DBD, UAS-hid] survive to adulthood. Possibly, in flies with the genotype [UAS-rpr; 83E12-Gal4AD; repo-Gal4DBD, UAS-hid] low levels of Gal4 based hid and or rpr expression might also occur in the related astrocyte-like cells. To further validate the specificity of ensheathing glia ablation, we stained the specimens for Rumpel and Nazgul. Rumpel is a SLC5A transporter strongly expressed by the ensheathing glia and weakly by astrocyte-like glia (Fig. 4). Nazgul is a NADP-retinol dehydrogenase that is specifically expressed by astrocyte-like cells58. In control, third instar larval brains Rumpel is found along the entire neuropil with Nazgul expressing astrocytes positioned in a characteristic pattern on the dorsal surface of the neuropil (Fig. 4a–c). Likewise, when we ablated the ensheathing glia in a background of a UAS-CD8::GFP transgene [UAS-rpr; 83E12-Gal4AD; repo-Gal4DBD, UAS-hid] no ensheathing glia cells can be detected in the CNS. Only few wrapping glial cells in the peripheral nervous system can still be detected (Supplementary Fig. S4h–j).
Representative images are shown. a–f Third instar larval brains stained for Rumpel (green, expressed by ensheathing glia), Nazgul (magenta, expressed by astrocyte-like cells) and HRP (blue, neuronal membrane marker). a Maximum projection of a confocal stack of a control larval CNS. The dashed line indicates the position of the orthogonal view shown in (c). b Dorsal view of a control ventral nerve cord. Note the regular positioning of the astrocyte-like cells (magenta). c Orthogonal section of astrocyte-like cells which are labelled using anti-Nazgul staining (white, arrowheads point to short protrusions). d, e Upon ablation of the ensheathing glia [UAS-rpr; 83E12-Gal4AD; repo-Gal4DBD, UAS-hid], Rumpel expression is greatly reduced. The dashed line indicates the position of the orthogonal view shown in (f). e Dorsal view of a larval ventral nerve cord following ensheathing glia ablation. The position and the morphology of the astrocyte-like cells appears disorganized. f Orthogonal section. Note, that astrocyte-like glial cells develop long cell processes that appear to encase the entire neuropil in the absence of ensheathing glia (arrowheads). g Dorsal view on a thoracic neuromere of an adult control fly. h Thoracic neuromere of an adult fly lacking ensheathing glial cells. The number of astrocyte-like cells increases. i Quantification of the number of astrocyte-like cells in the ventral nerve cord of adult flies (n = 6 brains, **p = 0.0018, unpaired t-test, two-tailed, standard deviation is indicated). j Upon ablation of the ensheathing glia, longevity is reduced (n = 200 mated females, ****p = 4.67365E-83, Log-rank test, two tailed, standard deviation is indicated). Scale bars are: a–f 50 µm, g, h 100 µm. Source data are provided as a Source Data file.
Astrocytes infiltrate the entire neuropil and form only few processes on the outer surface of the neuropil (Fig. 4c). Upon ablation of the ensheathing glia, only a very faint astrocytic Rumpel signal is detected in the head region (Fig. 4d). In the ventral nerve cord, astrocyte-like cells form additional large cell processes around the neuropil (Fig. 4f). In addition, in adult flies lacking ensheathing glia we noted a 20% increase in the number of Nazgul positive astrocyte-like cells (Fig. 4g–i, n = 6 for each genotype, [control: 427, 459, 379, 458, 368 and 433, median = 430; ablation: 507, 520, 469, 478, 516, and 545, median = 512; **p = 0.0022). Adult flies are fertile but lifespan is reduced by about 40% compared to control animals (Fig. 4j, n = 200 mated females, p = 4,67365E-83). In conclusion, these data show that ensheathing glial cells are not essential for viability. Upon ablation of ensheathing glia, astrocyte-like cells form additional processes encasing the neuropil. This compensatory growth of astrocyte-like cells suggests that barrier establishment is a key function of the ensheathing glia.
Ensheathing glial cells contribute to barrier formation around the neuropil
The third instar larval CNS is covered by subperineurial glial cells that do not allow penetration of labelled 10 kDa dextran. In wild type, subperineurial glial cells block paracellular diffusion across the blood–brain barrier by forming septate junctions59. However, in larvae lacking septate junctions, extensive interdigitations between neighboring subperineurial cells can also provide a barrier function60.
Extensive electron microscopic analyses failed to demonstrate septate junction like structures between different ensheathing glial cells. However, glial processes overlap extensively at the neuropil cortex interface (Fig. 2i, j), which might increase the length of the diffusion path at the boundary between CNS cortex and neuropil. To directly test whether ensheathing glial cells indeed provide a barrier function, we performed dextran diffusion assays in control brains and those lacking ensheathing glia. For this, we carefully dissected third instar larval brains including anterior cuticular structures and placed them on a coverslip. Upon injection of fluorescently labelled 10 kDa dextran directly into the neuropil of one brain lobe employing capillary normally used for DNA injections, we determined diffusion of the labelled dextran within the neuropil through the large brain commissure into the contralateral hemisphere (Fig. 5a). Diffusion of dextran was monitored under the confocal microscope and quantified for 10 minutes (Fig. 5b, d, movies S1,2). The ratio of the fluorescence measured at two identical sized ROIs at the neuropil and the cortex contralateral to the injection site was plotted against time (Fig. 5c). In control larvae, a fluorescence is mostly confined to the neuropil area and only little dye reaches the cortex area. When we performed the injection experiments in larvae lacking ensheathing glia, we noted a significantly faster increase in fluorescence signal in the cortex area relative to the neuropil (Fig. 5c). Thus, we conclude that the ensheathing glia, although they lack specialized occluding junctions, contribute to a barrier function that possibly involves the extensive overlap noted for ensheathing glia cell processes (Fig. 2).
a Schematic depiction of the dye injection experiments. Fluorescently labelled 10 kDa dextran was injected into the left hemisphere of a wandering third instar larva. Diffusion of the dye was monitored at two positions. In the neuropil (N) and in the cortex (C) harboring all neuronal cell bodies. b, b’ Stills of a representative movie of a larval brain lobe with the genotype [n = 5; 83E12-Gal4, UAS-CD8::GFP] following dye injection, time is indicated. c Quantification of dye diffusion rate in larvae of the genotype [83E12-Gal4, UAS-CD8::GFP] (blue line) and [UAS-rpr; 83E12-Gal4AD, repo-Gal4DBD, UAS-hid] (red line) (n = 5, standard deviation is shown, *p = 0.0181; unpaired t-test, two-tailed). d, d’ Stills of a representative movie of a larval brain lobe with the genotype [n = 5, UAS-rpr; 83E12-Gal4AD; repo-Gal4DBD, UAS-hid] following dye injection. Scale bars are 50 µm. Source data are provided as a Source Data file.
Ensheathing glial cells are polarized
Tissue barriers are generally formed by polarized cells which are characterized by a differential distribution of plasma membrane lipids. The apical membrane is generally rich in phosphatidylinositol 4,5-bisphosphate (PIP2), whereas the basolateral cell membrane contains more phosphatidylinositol 3,4,5-triphosphate (PIP3)61,62. To detect a possible differential localization of these phospholipids, we employed different lipid sensors based on distinct PH-domains. PH-PLCδ-mCherry is targeted by PIP263 whereas PH-AKT-GFP preferentially binds PIP364. We focused our analysis on the ensheathing glial cells covering the dorsal aspect of the neuropil, since the ventrolateral ensheathing glia form very thin, less than 200 nm thick, sheets around the neuropil that precludes a further analysis by confocal microscopy.
We co-expressed both sensors in the ensheathing glia using 83E12-Gal4 and quantified the distribution of both sensors by determining the number of green and red fluorescent pixels in 83E12-Gal4 positive cells in orthogonal sections comparing the cell domain close to the neuropil with the one close to the blood–brain barrier (Fig. 6a–g). Here, we noted an increased PH-PLCδ-mCherry localization at the direct interface of the neuropil and the ensheathing glia (Fig. 6b–e). For quantification, we measured the mean fluorescence intensity of PH-PLCδ-mCherry and PH-AKT-GFP (30 dorsal neuropil areas of 10 larvae). This demonstrated an almost twofold enrichment of PIP2 at the plasma membrane domain facing the neuropil. The PIP3 detecting PH-AKT-GFP shows a complimentary distribution with an enrichment in basolateral plasma membrane domains (Fig. 6f, g). A similar polarization of the ensheathing glial cells can be detected in adult stages (Fig. 6f; Supplementary Fig. S5, 30 neuropil areas in 10 adult brains).
Representative images are shown. a Projection of a confocal stack of a third instar larval ventral nerve cord coexpressing PH-AKT-GFP and PH-PLCδ-mCherry in ensheathing glial cells (EG) [83E12-Gal4, UAS-PH-AKT-GFP, UAS-PH-PLCδ-mCherry]. The dashed white line indicates the position of the orthogonal section shown in (b). b Orthogonal section showing the dorsal aspect of the neuropil. The boxed area is shown in higher magnification in (c–e). The dashed areas were subsequently used for quantification of GFP and mCherry localization. f Quantification of GFP/mCherry distribution in larval and adult ensheathing glia. The ratio of red (PLCδ-mCherry shown in magenta) and green (PH-AKT-GFP) fluorescent pixels of the areas indicated in (c) is plotted. The mean and standard deviation is shown. g Schematic view of a dorsal ensheathing glial cell. The neuropil facing domain is characterized by a high PIP2 content, whereas PIP3 is concentrated on the baso-lateral domain, where Nrv2 is predominantly localized, too. The blue dot indicates the position of a neuron. h Coexpression of Nrv2::GFP and 83E12-Gal4, UAS-mCherry in the ventral nerve cord of third instar larva. The boxed area is shown in higher magnification in (i, j). Note the preferential localization of Nrv2 at the basolateral cell domain of the ensheathing glia (arrows). Only little Nrv2 is found at the apical domain (open arrow head). Additional expression of Nrv2 is seen in the blood–brain barrier (filled arrowhead). k Quantification of polarized Nrv2::GFP localization. The mean and standard deviation is shown. Scale bars in a, b, h: 50 µm; in b–e, i, j: 25 µm. Source data are provided as a Source Data file.
Polarized epithelial cells are characterized by an enrichment of the Na+/K+-ATPase Nervana 2 (Nrv2) at the basolateral plasma membrane65. To study the localization of Nrv2, we employed a gene trap insertion66. As found for PIP3, Nrv2::GFP localizes predominantly at the basal side of the ensheathing glia (Fig. 6h–k), which corresponds to the localization of Nrv2 in epithelial cells. Therefore, we conclude that dorsal ensheathing glial cells are polarized cells throughout development, facing their apical-like domain towards the neuropil (Fig. 6g). Although all polarity markers are also expressed by the ventrolateral ensheathing glia, we cannot resolve their polarity due to the very flat shape of these membrane sheets which does not allow the separation of apical and basolateral membrane domains by optical methods.
Integrin α and extracellular matrix components are expressed by adult ensheathing glia
In epithelial cells, apical-basal polarity is also reflected by a basally located integrin receptor which bind the basally located extracellular matrix (ECM) proteins. inflated encodes an alpha-subunit of the integrin receptor and inflated mRNA is expressed by adult perineurial and ensheathing glial cells11,67 (Supplementary Fig. S6). Endogenously YFP-tagged Integrin α (ifCPTI-004152 68) localizes at the blood–brain barrier and around the neuropil in larval brains (Fig. 7a, b, e). In the adult brain, IntegrinYFP α localization around the neuropil appears more pronounced compared to abdominal neuromeres (Fig. 7c, d). Integrin receptors bind ECM components, suggesting that these proteins are also expressed within the nervous system. We thus tested expression of all genes annotated to encode proteins involved in extracellular matrix formation (FlyBase) using published single cell sequencing data of adult brains11 (Supplementary Fig. S6). Eight of these genes appear expressed by the ensheathing glia and GFP-protein trap insertion lines were available for trol (Perlecan), viking (Collagen IV), and dally (Glypican).
Representative images are shown. Expression of Integrin α and different extracellular matrix components as detected by using gene trap insertion lines. a–e Endogenously YFP-tagged Integrin α encoded by the inflated gene. f–j Endogenously GFP-tagged heparan sulfate proteoglycan Dally protein encoded by dally::GFP. k–o Endogenously tagged Collagen IV protein encoded by viking::GFP. p–t Endogenously tagged Perlecan encoded by trol::GFP. The different developmental stages are indicated. a, i, m, s The boxed areas are shown in the bottom row. Glial cells are in magenta (anti-Repo staining), neuronal membranes are in blue (anti-HRP staining) and the GFP-tagged proteins are in green (anti-GFP staining). a, b, e During late larval stages, prominent Inflated expression is seen at the blood–brain barrier (arrows) and weak expression is seen around the neuropil at the position of the ensheathing glia (arrowhead). c, d In adults, Integrin α is still found at the blood–brain barrier (arrows) and becomes more prominent around the neuropil (arrowheads). Note that strongest Inflated expression is detected in larval brains. No Inflated expression can be detected in the abdominal neuromeres (asterisk). f, g During the larval stage Dally is expressed at the blood–brain barrier (arrows) and in the CNS cortex (asterisks). A slightly stronger localization of Dally can be detected at the position of basal ensheathing glia processes (arrowhead). h–j In adults, Dally is enriched at the position of the ensheathing glia (arrowheads). k, l In the larval CNS Collagen IV is found at blood–brain barrier (arrows) but not within the nervous system. m–o Collagen IV is detected at the neuropil-cortex interface in adult stages (arrowheads in m, n, boxed area is shown in (o)). p, q Trol is detected at the larval blood–brain barrier (arrows) but not around the neuropil. r–t In adults, prominent Trol localization is seen at the position of the ensheathing glia (arrowheads). The boxed area in (s) is shown at higher magnification in (t). Scale bars are: larval CNS 50 µm, adult CNS 100 µm except for e: 25 µm; j, o, t: 50 µm.
The heparan sulfate proteoglycan Dally is most strongly detected close to the cells of the larval blood–brain barrier (Fig. 7f). Within the larval CNS, Dally is found in the cortex and is enriched around the larval neuropil (Fig. 7f, g). In adults, Dally localization at the blood–brain barrier ceases but is still found in the CNS cortex. A prominent enrichment of Dally is seen around the neuropil (Fig. 7h–j). In contrast to Dally, we could not detect Trol and Viking around the larval neuropil and only detected strong signals at the blood–brain barrier (Fig. 7k, l, p, q). However, in the adult nervous system both Trol and Viking are detected basally of the ensheathing glia facing the cortex glia with an even distribution in the different parts of the CNS (Fig. 7m–o, r–t). In conclusion, the ensheathing glia express ECM proteins which is characteristic for polarized cell types.
Polar distribution of ßHeavy-Spectrin in larval ensheathing glia
Polarization of cells is also evident in the cytoskeleton underlying the plasma membrane. Whereas α-Spectrin is found below the entire plasma membrane, ß-Spectrin decorates the basolateral plasma membrane and ßHeavy-Spectrin (ßH-Spectrin) is located at the apical domain of the cell69. ß-Spectrin is found in all neural cells in the ventral nerve cord70 and no specific localization can be resolved in the ensheathing glia due to its strong overall expression. ßH-Spectrin is encoded by the karst gene. One available MiMIC insertion allows to target two of the seven isoforms (Fig. 8a). In third instar larval brains, GFP-ßH-Spectrin localizes at the blood–brain barrier (Fig. 8c, d, f, g), and close to the neuropil (Fig. 8c, d, f), indicating expression by the ensheathing glia. Here, ßH-Spectrin is enriched close to the apical-like, PIP2 containing membrane of the ensheathing glia facing towards the neuropil (Fig. 8c, d). To further validate the expression of Karst in the ensheathing glia, we silenced karst expression by expressing double stranded RNA. When karst expression is silenced in all glial cells using repo-Gal4 only expression in trachea as well as a weak background signal at the neural lamella around the CNS is detected (Supplementary Fig. S7a, b). Silencing of karst expression in the blood–brain barrier using moody-Gal4 did not affect localization of GFP around the neuropil (Supplementary Fig. S7c, d). When we silenced karst specifically in the ensheathing glia using 83E12-Gal4 protein localization in the blood–brain barrier is unaffected but no ßH-Spectrin protein can be detected around the neuropil (Fig. 8g).
Representative images are shown. a Schematic view of the karst locus. Transcription is from left to right. Seven different karst mRNAs are generated from two promoters (P1, P2) as indicated (kst-RA - RH). The ßH-Spectrin proteins PE and PG differ in a C-terminal exon indicated by brackets. The position of two MiMIC insertions and the EP insertion used for gain of function experiments is indicated, the blues line denotes the position of the peptide used for immunization. b Confocal view of the surface of a third larval instar brain of a kstMiMIC03134::GFP animal that expresses mCherry in all ensheathing glia [83E12-Gal4, UAS-CD8::mCherry]. The dashed line indicates the position of the orthogonal view shown in (c, d). Expression at the blood–brain barrier forming subperineurial glia (arrows) and at the apical domain of the ensheathing glia (arrowheads) can be detected. For quantification see (k). e Similar view as shown in (b) of a control larva stained for Kst expression using a polyclonal antiserum. The dashed line indicates the position of the orthogonal view shown in (f). f Localization of Kst protein is weakly seen in the blood–brain barrier (arrow) and the ensheathing glia (arrowhead). g Orthogonal view of a kstMiMIC03134::GFP animal expressing kstdsRNA in the ensheathing glia (EG) [83E12-Gal4, UAS-kstdsRNA]. Expression of Karst in ensheathing glia cannot be detected anymore (asterisk). Expression in the blood–brain barrier is unaffected (arrow). h, i The Trojan Gal4 element in kstMiMIC03134 directs Gal4 expression in the kst P2 pattern. Lamin::GFP localizes to ensheathing glial nuclei (arrows), some cells of the blood–brain barrier (arrowheads) and some cells in the position of tracheal cells (asterisks). j Quantification of Karst localization in different membrane domains of ensheathing glial cells, quantification as described in Fig. 6. Scale bars are 50 µm except d: 10 µm. Source data are provided as a Source Data file.
A similar ßH-Spectrin localization was detected using antisera directed against a C-terminal peptide present in all predicted ßH-Spectrin isoforms (Fig. 8a, e, f, for specificity of the generated antiserum see: Supplementary Fig. S7e, f). Furthermore, we generated a Trojan-Gal4 insertion using the same MiMIC insertion that was used to generate the KarstGFP protein trap (Fig. 8a). The kstTrojan-Gal4 allele is expected to report only the expression pattern of the proximal promoter which activates expression of two out of the seven karst splice variants (FlyBase) (Fig. 8a). Indeed, when driving a nuclear GFP reporter (UAS-Lam::GFP), expression in the ensheathing and blood–brain barrier glial cells is apparent (Fig. 8h, i). In addition, cells in the position of tracheal cells express the kstTrojan-Gal4 allele (Fig. 8i). During pupal stages, expression of Karst ceases between 72 and 96 hours after puparium formation (APF) (Supplementary Fig. S8). Adult ensheathing glial cells lack detectable expression of the kstMiMIC::GFP insertion and show no reactivity using the anti-Karst antisera.
ßH-Spectrin is required for ensheathing glial polarity
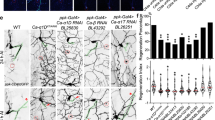
We next tested whether ßH-Spectrin is required for ensheathing glial cell polarity and determined the overall morphology of the ensheathing glia in the karst loss of function karstMiMIC13613 larvae using 83E12-Gal4 driving CD8::GFP. In karst mutants, larval ensheathing glial cells are still present but show an abnormal collapsed morphology. The extension of ensheathing glia cell protrusions around dorsally located neuronal cell bodies is less evident (Fig. 9a, b). When we silenced karst expression using RNAi, we noted a weaker phenotype and dorsal protrusions frequently formed (Fig. 9c). This allowed an analysis of the distribution of PIP2 and PIP3 (Fig. 9c–e). Whereas in ensheathing glial cells of control ventral nerve cords, PIP2 (as detected by PH-PLCδ-mCherry) is found predominantly on the domain facing the neuropil, an almost even distribution is noted upon kst knockdown in larvae (compare Figs. 6f, 9i). Interestingly, the polar distribution of PIP3 does not appear to be affected by kst knockdown (Fig. 9e, i). To further test whether ßH-Spectrin is needed for ensheathing glial polarization, we performed overexpression experiments. To direct expression of wild type Karst protein in ensheathing glia, we utilized the EP-element insertion P{EPgy2}EY01010. Overexpression of karst results in abnormally convoluted and partially collapsed ensheathing glial cell morphology characterized by an even distribution of both PIP2 and PIP3 (Fig. 9f–h, j).
Representative images are shown. a Orthogonal view of the dorsal aspect of a third instar control ventral nerve cord stained for ensheathing glial (EG) morphology [83E12-Gal4, UAS-CD8::GFP]. b Third instar larval brain of a zygotic karst null mutant larvae with the genotype [kstMiMIC13613 / kstMiMIC13613, 83E12-Gal4, UAS-CD8::GFP]. The absence of all ßH-Spectrin protein affects the morphology of the dorsally located ensheathing glial cells. c–e Third instar larval ventral nerve cord with reduced kst expression in ensheathing glia [83E12-Gal4, UAS-kstdsRNA, UAS-PH-AKT-GFP, UAS-PH-PLCδ-Cherry]. d PH-PLCδ-Cherry binds to PIP2 and shows an even distribution between apical and basolateral plasma membrane domains. For quantification see (i). Quantification was performed as described in Fig. 6. e PH-AKT-GFP binds to PIP3 and is distributed in a polarized manner. For quantification see (i). f–h Third instar larval ventral nerve cord with increased kst expression in ensheathing glia [83E12-Gal4, kstP{EPgy2}EY01010, UAS-PH-AKT-GFP, UAS-PH-PLCδ-Cherry]. Note, the variable phenotype noted upon karst overexpression. Ensheathing glia with almost normal morphology (open arrowhead) is next to hyperconvoluted ensheathing glia (asterisk). Quantification of PIP2 and PIP3 localization is shown in (j). i, j Quantification was performed as described in Fig. 6. The mean and the standard deviation is shown. k Quantification of the distribution of Nrv2::GFP using an unpaired t-test, two-tailed. *pcontrol-kstdsRNA = 0.0474; ****pcontrol-kstEP < 0.001. The mean and the standard deviation is shown. l–n In karst mutant larvae, Nrv2 is found in ensheathing glia cell processes (open arrowhead) that face the neuropil. The dashed line indicates the neuropil ensheathing glia boundary. o–q Upon ensheathing glial specific kst knockdown, Nrv2 localizes to the basolateral cell domains, for quantification see (k). Filled arrowhead indicates the blood–brain barrier. r–t Upon overexpression of Karst, polarized localization of Nrv2 is less apparent in ensheathing glial cell processes (normal morphology: open arrowheads, hyperconvoluted morphology: asterisk), for quantification see (k). Scale bars are 50 µm. Source data are provided as a Source Data file.
We next assayed Nrv2 localization in karst null mutants as well as upon 83E12-Gal4 directed kst knockdown. In larvae lacking karst expression ensheathing glial cells show a collapsed morphology and polarized localization of Nrv2 cannot be resolved (Fig. 9l–n). Upon kst knockdown Nrv2 correctly localizes to basolateral protrusions (Fig. 9k, o–q). In kst gain of function larvae, the polarized Nrv2 distribution is lost (Fig. 9k, r–t), supporting the notion that the spectrin cytoskeleton might affect proper positioning of Nrv265,71,72,73.
Polarized ensheathing glia is required for locomotor behavior
The above data show that larval ensheathing glia are polarized, ECM abutting cells that separate the neuropil from the CNS cortex and are required for longevity of the adult fly. To test whether the ensheathing glial cells are required for normal locomotor control during larval stages, we compared locomotion of control animals with those lacking ensheathing glia or with those with reduced ßH-Spectrin or Nrv2 expression using FIM imaging74,75.
Control animals move on long paths interrupted by short reorientation phases that are characterized by increased body bending (Fig. 10a). kst knockdown specifically in ensheathing glial cells [83E12-Gal4AD; repo-Gal4DBD, UAS-kstdsRNA] causes a reduction in the peristalsis efficiency during go-phases (Fig. 10a, b, f, g). Likewise, crawling velocity is reduced significantly (Fig. 10g). This suggests that the specific lack of ßH-Spectrin in ensheathing glia causes a strong locomotor phenotype. The observed locomotor phenotypes might be due to an ectopic neuronal activity of the split-Gal4 driver in addition to its activity in the ensheathing glia. Assuming that the repo promoter is indeed glial cell specific76, neuronal activity is not expected in the split Gal4 approach. Moreover, we have no evidence that karst is expressed by neurons. Furthermore, we analyzed kst mutant larvae to validate the RNAi-induced phenotypes. The split Gal4 driver might have ectopic activity in other glial cells, but given the specific karst knockdown only in ensheathing glia we consider this unlikely (Fig. 8g). The Trojan-Gal4 insertion in the karstMiMIC03134 insertion is expected to affect only isoforms Karst-PE and Karst-PG (Fig. 8a). As control, we used an insertion of the Gal4 element in the opposite, unproductive orientation. Similar as detected for the kst knockdown, we noted a decreased peristalsis efficiency and a reduced crawling velocity (Fig. 10c, i, j). Larvae completely lacking zygotic karst expression [karstMiMIC13613/Df(3 L)ED2083] show a comparable larval locomotion phenotype (Fig. 10d, i, j). This larval locomotion phenotype is similar to the one observed following ablation of the ensheathing glial cells using the genotype [UAS-rpr; 83E12-Gal4AD; repo-Gal4DBD, UAS-hid] (Fig. 10e). Thus, we conclude that polarized ensheathing glia that connect the blood-brain barrier with the dorsal neuropil are required for normal locomotor behavior. In order to perform vectorial transport, the Na+/K+ ATPase must act in a polarized fashion. We thus also compared larval locomotion of animals with reduced nrv2 expression to control animals. Interestingly, larvae with ensheathing glial cells lacking nrv2 expression behave opposite to larvae that lack ßH-spectrin showing an increased peristalsis efficiency as well as an increased crawling velocity (Supplementary Fig. S9). The analysis of the role of polarized Nrv2 distribution for ensheathing glia physiology will thus be an interesting topic for future research.
a Exemplary locomotion tracks of control wandering third instar larvae with the genotype [83E12-Gal4AD, repo-Gal4DBD, UAS-GFPdsRNA]. Larvae move in long run phases. b Upon kst knockdown in ensheathing glia (EG) only [83E12-Gal4AD, repo-Gal4DBD, UAS-kstdsRNA], larvae do not explore the tracking arena. c The insertion of a Trojan Gal4 element into the first coding exon of kst-RE(RG) generates an PE and PG isoform specific mutant (see Fig. 8). Larvae with the genotype [kstTrojanGal4 / Df(3 L)ED208] show a comparable locomotion phenotype as RNAi knockdown larvae. d Locomotion phenotype of zygotic karst null mutant larvae with the genotype [kstMiMIC13613 / Df(3 L)ED208]. e Locomotion phenotype observed following specific ablation of the ensheathing glia [UAS-rpr; 83E12-Gal4AD; repo-Gal4DBD, UAS-hid]. f–h Quantification of peristalsis efficiency, velocity and bending distribution between control larvae, kst knockdown and ensheathing glia ablated animals. Box plots show median (line), boxes represent the first and third percentiles, whiskers show standard deviation, diamonds indicate outliers. i–k Quantification of the same parameters comparing wild type Canton S with kst null larvae and a kstTrojan control strain that carries the Trojan insertion in a non-productive orientation [kstMiMIC03134-Trojan-C in trans to the Df(3 L)ED208] with the isoform specific kstPE, PG mutant [kstMiMIC03134-TrojanGal4 / Df(3 L)ED208. Quantification Wilcoxon rank-sum test, two tailed, n = 50. Peristalsis Efficiency [mm/wave]: GFPdsRNA – kstdsRNA: ****p = 2.65E-32 GFPdsRNA – ablation; ****p = 3.22E-30; velocity [mm/sec]: GFPdsRNA – kstdsRNA: **** p = 3.81E-11; GFPdsRNA – ablation: ****p = 1.54E-05; Peristalsis Efficiency [mm/wave]: Canton S – kstMiMIC / Df: ****p = 3.98E-20; Canton S – Trojan C/ Df: ****p = 4.61E-13; TrojanC – TrojanGal4 / Df: ****p = 1.22E-13; velocity [mm/sec]: Canton S – kstMiMIC / Df: ****p = 1.15E-06; Canton S – TrojanC/ Df: ****p = 1.21E-03; TrojanC – TrojanGal4 / Df: ****p = 5.94E-13. Source data are provided as a Source Data file.
Discussion
Here, we present a comprehensive analysis of the ensheathing glia found in the CNS of Drosophila. We developed a split-Gal4 tool to specifically manipulate the ensheathing glia and show that ensheathing glial cells are highly polarized cells with an apical-like cell domain facing towards the neuropil. Polarized membrane lipids as well as several polar localized proteins were identified. The apical sub-membranous cytoskeleton is organized by ßH-Spectrin encoded by karst. Ensheathing glia cells contribute to the formation of an internal diffusion barrier as determined by dye penetration experiments in ensheathing glia ablated animals. Flies that lack ensheathing glia have increased numbers of astrocytes, they are viable but show reduced longevity. Moreover, we show that ensheathing glial cells lacking Karst have an altered cell polarity and kst mutants as well as cell type specific knockdown larvae show abnormal locomotor behavior.
To study ensheathing glial cell biology, we utilized the 83E12-Gal4 driver which, as described41, appears specific for ensheathing glia. Some of the larval ensheathing glia are diploid and block of cell proliferation reduces their number by 38%, suggesting that proliferation of these cells contributes to the increase of ensheathing glial complexity. These finding support the notion that some larval ensheathing glia can dedifferentiate, proliferate and redifferentiate to form the more complex adult ensheathing glia46. Ablation of ensheathing glia results in an increased number of adult astrocyte-like cells which suggests that the close interdependence of these two cell types identified in embryonic stages27,77 persists to adulthood. Similarly, a plastic interaction also occurs between cortex and ensheathing glia20. Such interactions might also contribute to the functional differences that appear likely to be associated with for example dorsal or ventral ensheathing glial cells. Unfortunately, no specific molecular tools exist that allow to test this hypothesis.
A likely role of ensheathing glial cells is to establish a diffusion barrier around the neuropil, as was demonstrated by our dye injection experiments in ensheathing glia ablated larvae. However, we cannot exclude the possibility that ensheathing glia instruct neighboring cells such as the cortex glia or astrocyte-like glia to form a barrier around the neuropil. The separation of the neuropil from the cortex in the larva might facilitate the formation of signaling compartments specified by astrocyte-like cells. The 10-fold increase in the number of ensheathing glial cells in the adult brain is likely to further organize neuronal functions into discrete domains35,78,79,80.
As also observed in vertebrates, the formation of signaling compartments can be detected on a gross anatomical level as well as on a circuit level. In both cases, the neuropil-associated glia comprising ensheathing glia are at center stage25 and complexity of regional compartmentalization increases in the adult, mirroring the increase in ensheathing glial cell number33,34,81. In vertebrates, a classical example for this fundamental principle can be seen at the rhombomeric organization of the vertebrate hindbrain82. Here, glial cells provide essential functions in boundary formation, too83. Thus, glia may not only structure the nervous system by compartmentalizing synapses but also act on the level of larger functional units.
The establishment and maintenance of cell polarity is a fundamental cell biological feature and is required for the development of different cell types and tissues. For instance, the mammalian nervous system is protected by the blood–brain–barrier established by polar endothelial cells whereas the blood–brain barrier around the invertebrate nervous system is comprised of polarized glial cells4,13,84,85,86. Cell polarity is induced by evolutionarily conserved mechanisms87,88. Apical polarity regulators (APRs) comprise a diverse set of proteins centered around the two apical polarity protein complexes Bazooka/Par3 and Crumbs89. In addition, basolateral regulators including the Scribble complex (Scribble, Discs large and Lethal (2) giant larvae and the kinases PAR-1 and LKB1 participate in the definition of a polar cell phenotype90. Some of these genes (Par1, Scrib, dlg1) appear to be expressed by adult ensheathing glia11. For Scrib but not Par1 or dlg1 this can be confirmed protein trap insertion lines. However, RNAi mediated knockdown of these genes did not cause any morphological defects in larval ensheathing glia. Possibly, similar as in follicle cell development, different mechanisms are in place to guarantee the establishment and maintenance of the polar ensheathing glial cell phenotype91.
A hallmark of polarized cells is the asymmetric distribution of lipids with PIP2 being enriched at the apical surface62 and reduction in βH-Spectrin causes a disrupted PIP2 localization. This suggests, that a polar spectrin cytoskeleton might orchestrate lipid composition at the apical membrane domain which subsequently affects polarized distribution of transmembrane proteins such as the Na+/K+ ATPase. This might cause to a differential distribution of Na+ ions which are used by antiporters involved in metabolite transport. One of them is the Excitatory amino acid transporter 2 (EAAT2)92. Antibodies against EAAT2 are available27 but unfortunately do not allow the analysis of its subcellular localization in larvae. Together, the different transport systems expressed in the ensheathing glia will also affect extracellular environment and thus diffusion properties in and out of the neuropil, which needs to be addressed in further studies.
The synaptic neuropil is localized at a unique position within the nerve cord very close to the dorsal surface and thus, the hemolymph. Whereas the ventral as well as the lateral parts of the neuropil are flanked by neuronal cell bodies embedded in cortex glia, only few neuronal cell bodies are found at the dorsal surface of the nervous system. More importantly, no cortex glial cells are found dorsally to the neuropil20,28. Thus, metabolites can be transported from the blood–brain barrier to the neuropil only by the ensheathing glia. Upon ablation of the ensheathing glia, the blood–brain barrier forming glial cells might provide enough metabolites to ensure an almost normal function of the nervous system. In case of suppression of Nrv2 expression in the ensheathing glia, ion gradients required for directed transport are not established across the ensheathing glial plasma membrane. At the same time the barrier provided by the ensheathing glia is still in place and therefore metabolically isolates the neuropil which results in an increased mobility possibly aimed to direct the larva to new and better food sources. The neuropil itself is highly organized and its dorsal domain is dedicated to synaptic connections of motor neurons93. Possibly, the motor neurons are more sensitive to nutrient supply and thus a more direct metabolic support through the ensheathing glia is required.
Methods
Drosophila genetics
Flies were raised on food prepared according to a recipe of the Bloomington stock center (https://bdsc.indiana.edu/information/recipes/bloomfood.html). Depending on the culture conditions, fly stocks were flipped every 2–4 weeks. Experimental flies were always kept at 25 °C. For crosses flies were anesthetized with CO2. Flies of undesired genotypes were either inactivated by placing them at −30 °C for 2 days or by placing them in 70% ethanol as approved by the authorities.
The following flies were obtained from public stock centers: 83E12-Gal4 (BDSC#40363), 83E12-LexA (BDSC #54288), P{y[+t7.7] w[+mC]=13XLexAop2-mCD8::GFP}attP2 (BDSC#32203), MCFO-2: pBPhsFlp2::PEST;; HA_V5_FLAG_OLLAS (BDSC#64086), stringdsRNA (NIF-fly, 1395R-2), P{w[+mC]=UAS-htl.lambda\cI.M}40-22-2 (Kyoto Stock collection, 108191), P{w[+mC]=UAS-Lam.GFP}3-3 (BDSC#7376), P{w[+mC]=UAS-mCD8::GFP.L}LL6 (BDSC#5130), P{UAS-mCD8.ChRFP}2 (BDSC#27391), P{w[+mC]=UAS-rpr.C}27 (BDSC#5823), P{w[+mC]=UAS-hid.Z}2/CyO (BDSC#65403), P{4 C.UAS-PLCδ-PH-mCherry} (BDSC#51658), PBac{806.LOX-SVS-2}ifCPTI004152 (#4152), Mi{PT-GFSTF.0}trolMI04580-GFSTF.0 (BDSC#60214), Mi{PT-GFSTF.1}kstMI03134-GFSTF.1 (BDSC#60193), Mi{MIC}kstMI03134 (BDSC#36203), Mi{MIC}kstMI13613 (BDSC#59172), P{XP}kstd11183 (BDSC#19345), kstP{EPgy2}EY01010 (BDSC#15488), kstdsRNA (BDSC#50536), Df(3 L)ED208 (BDSC#8059), Mi{PT-GFSTF.1}nrv2MI03354-GFSTF.1 (BDSC#59407), nrv2dsRNA (VDRC#2260), P{w[+mC]=lexAop-rCD2.RFP}2; P{w[+mC]=UAS-CD4-spGFP1-10}3, P{w[+mC]=lexAop-CD4-spGFP11}3 (BDSC#58755), 55B12-Gal4 (BDSC#39103), VGlut[OK371]-Gal4 (BDSC#26160), GFPdsRNA (BDSC#9331). All other fly stocks were generated or were provided by other labs: 83E12-Gal4AD, repo -Gal4DBD, Mi{Trojan-GAL4.0} kstMI03134, dally::GFP, viking::GFP (all this study), PBac{806.LOX-SVS-2}ifCPTI004152 (FlyProt, 4152), UAS-fzr53, UAS-PH-Akt::GFP (S. Luschnig, Münster, Germany). The MCFO flies were heat-shocked for 1 h at 37 °C in a water bath at different developmental time points as indicated. The Trojan-Gal4 cassettes was exchanged by phiC31-integration protocols94.
Immunohistochemistry
Immunohistochemistry for larval, pupal and adult brains was performed using standard protocols95. The following antibodies were used: anti-Repo ([1:5], Cat# 8D12, RRID: AB_528448, Developmental Studies Hybridoma bank); anti-mCherry ([1:1,000], Cat# M11217, RRID: AB_2536611); anti-V5 ([1:1,000], Cat# R960-25, RRID: AB_2556564) and DAPI ([1:5,000], Cat# D1306, RRID: AB_2307445) (all from Invitrogen™); anti-Flag ([1:10], Cat# SAB4200071, RRID: AB_10603396, Sigma-Aldrich); anti-Ollas ([1:1,000], Cat# MAB7372, RRID: AB_11152481, Abnova), anti-HA ([1:500], Cat# 9015116, RRID: AB_2820200, BioLegend); anti-GFP ([1:1,000], Cat# A-6455, RRID: AB_221570, Life Technologies); Anti-phospho-histone H3 (phospho S28) ([1:500], RRID:AB_2295065, Cat# ab10543, Abcam); anti-HRP-Alexa Flour 647 conjugated ([1:1,000], RRID: AB_2338952, Cat# 123-005-021, Dianova); anti-Nazgul ([1:250], gift from B. Altenhein, Cologne). Rabbit anti-Rumpel [1:500] (gift of K. Yildirim, Münster). A guinea Pig anti-serum generated against a GST-EAAT2 fusion protein [1:500]27 was a gift of D. van Meyel. A C-terminally located peptide (3622LADERRRAEKQHEHRQN3639) shared by all ßH-Spectrin proteins was used to immunize rabbits (Pineda, Berlin). ßH-Spectrin was used in a dilution of [1:5,000]. For confocal imaging and analysis samples were imaged using a Zeiss LSM 880 with Zeiss imager ZEN 2.3 or a Leica SP8 confocal microscope using Leica Leica Application Suite X (LAS X) 3.5.7.23225 and Imaris 8.4.1 Oxford instruments software for 3D reconstruction.
Electron microscopic analysis
All ssTEM data were generated at the Howard Hughes Medical Institute Janelia Research Campus and processed as described31,43. The CATMAID interface44,96 was used to annotate glial cells associated with the neuropil. Glial cell bodies were identified by characteristic color of the cytoplasm and non-neuronal morphology. A cell body node was positioned at the glial soma and annotated using the following information: hemineuromere, glial subtype, serial number for cells in one hemineuromere. The tools built into CATMAID were used to generate 3D renderings and extract image data. Cells identified in RAW data were highlighted using Adobe Photoshop CS6.
Measurement of DAPI intensity
To determine the polyploidy of ensheathing glia, we stained larval or adult brains with DAPI, Repo and Elav in order to identify glial cells and neurons. We compared DAPI stained nuclei identified by 83E12-Gal4 > UAS-Lam::GFP with DAPI staining of directly neighboring neuronal nuclei identified by anti-Elav staining. The amount of DAPI staining of neighboring glial and neuronal nuclei was determined using Fiji 1.52b97. In each case the entire nuclear volume was measured.
EdU incorporation assay
First instar larvae were placed on freshly prepared fly food containing 0.2 mM EdU and were kept on the food at 25 °C until the third instar stage. Brains of the desired age were dissected and stained for EdU presence using the Click-iT Plus EdU detection kit of Thermo Fisher (# C10638) according to the instructions of the manufacture.
Dye injection assay
Brains of wandering L3 larvae were dissected on ice and immediately placed on Poly-L-lysine coated object slide and covered by 10 S Volatef halocarbon oil. An automated injection station (FemtoJet 4 L, Eppendorf) was used to inject 2.5 mM 10 kDa Texas-Red conjugated dextran (Cat#D1863, Invitrogen) diluted in H2O into the neuropil of one brain lobe in each brain. For injections, we used borosilicate glass capillaries of 1 mm outer diameter and 0.58 mm inner diameter (Harvard apparatus 30-0016 capillaries GC100-10) that were pulled on a P-1000 Sutter instrument micropipette puller to reach a tip diameter of about 10 µm as commonly used for DNA injections.
Longevity assay
Flies were raised at 25 °C. The offspring of the control and the ablation experiment were collected for up to 3 days immediately after hatching and separated between males and females. 20 mated females were put in a vial and a total of 200 animals were observed over a period of time. The living flies were counted and placed on fresh food without anesthesia every two days.
Larval locomotion analysis
Larvae for behavioral experiments were raised at 25 °C according. Larval locomotion of wandering third instar larvae was tracked by the FTIR-based Imaging Method (FIM) based on frustrated total internal reflection (FTIR) at room temperature74,75. 10-15 larvae per genotype were placed on the tracking arena and after a 60 s accommodation phase recording started for 3 minutes using FIMtrack v3.1.28.5 and analyzed suing FIMAnalytics v0.1.1.2.
Quantification, statistical analysis and reproducibility
All confocal analyses were repeated independently at least three times with at least 5 specimens each and similar results were obtained. Fiji was used to investigate polar distribution. Cells were divided in two ROIs and the fluorescence intensity ratio between apical and basal was compared. Counting of the nuclei and the astrocytes was performed in a 3D model in Imaris 8.4.1 Oxford Instruments. Fluorescence intensities for the Dextran Assay were measured using Fiji 1.52b97 using the normalized fluorescence intensity and the ratio between neuropil and cortex. Statistical analysis and calculation were obtained by Prism 6.0 and Excel 2016.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The fly stocks and datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request. Source data are provided with this paper. The processed single-cell RNA-sequencing data in Supplementary Fig. 6 was generated from publicly available data11. The raw data is stored in a loom format (https://github.com/linnarsson-lab/loompy) and visualized by SCope (http://scope.aertslab.org). Source data are provided with this paper.
Change history
01 February 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41467-022-28258-z
References
Zuchero, J. B. & Barres, B. A. Glia in mammalian development and disease. Development 142, 3805–3809 (2015).
Yildirim, K., Petri, J., Kottmeier, R. & Klämbt, C. Drosophila glia: few cell types and many conserved functions. Glia 21, 276 (2018).
Bundgaard, M. & Abbott, N. J. All vertebrates started out with a glial blood-brain barrier 4-500 million years ago. Glia 56, 699–708 (2008).
Carlson, S. D., Juang, J. L., Hilgers, S. L. & Garment, M. B. Blood barriers of the insect. 45, 151–174 (2000).
Schirmeier, S. & Klämbt, C. The Drosophila blood-brain barrier as interface between neurons and hemolymph. Mechanisms Dev. 138, 50–55 (2015).
Freeman, M. R. Specification and morphogenesis of astrocytes. Science 330, 774–778 (2010).
Tsacopoulos, M. & Magistretti, P. J. Metabolic coupling between glia and neurons. J. Neurosci. 16, 877–885 (1996).
Magistretti, P. J. & Allaman, I. Lactate in the brain: from metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 19, 235–249 (2018).
Simons, M. & Nave, K.-A. Oligodendrocytes: myelination and axonal support. Cold Spring Harb. Perspect. Biol. 8, a020479 (2016).
Fernandez-Castaneda, A. & Gaultier, A. Adult oligodendrocyte progenitor cells - Multifaceted regulators of the CNS in health and disease. Brain Behav. Immun. 57, 1–7 (2016).
Davie, K. et al. A single-cell transcriptome Atlas of the aging Drosophila brain. Cell 174, 982–998.e20 (2018).
Sheng, L. et al. Social reprogramming in ants induces longevity-associated glia remodeling. Sci. Adv. 6, eaba9869 (2020).
Limmer, S., Weiler, A., Volkenhoff, A., Babatz, F. & Klämbt, C. The Drosophila blood-brain barrier: development and function of a glial endothelium. Front. Neurosci. 8, 365 (2014).
Zhang, S. L., Yue, Z., Arnold, D. M., Artiushin, G. & Sehgal, A. A circadian clock in the blood-brain barrier regulates xenobiotic efflux. Cell 173, 130–139.e10 (2018).
Hindle, S. J. et al. Evolutionarily conserved roles for blood-brain barrier xenobiotic transporters in endogenous steroid partitioning and behavior. Cell Rep. 21, 1304–1316 (2017).
Volkenhoff, A. et al. Glial glycolysis is essential for neuronal survival in Drosophila. Cell Metab. 22, 437–447 (2015).
Liu, L. et al. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell 160, 177–190 (2015).
Liu, L., MacKenzie, K. R., Putluri, N., Maletić-Savatić, M. & Bellen, H. J. The glia-neuron lactate shuttle and elevated ROS promote lipid synthesis in neurons and lipid droplet accumulation in Glia via APOE/D. Cell Metab. 26, 719–737.e6 (2017).
Cabirol-Pol, M.-J., Khalil, B., Rival, T., Faivre-Sarrailh, C. & Besson, M.-T. Glial lipid droplets and neurodegeneration in a Drosophila model of complex I deficiency. Glia (2017). https://doi.org/10.1002/glia.23290.
Coutinho-Budd, J. C., Sheehan, A. E. & Freeman, M. R. The secreted neurotrophin Spätzle 3 promotes glial morphogenesis and supports neuronal survival and function. Genes Dev. 31, 2023–2038 (2017).
Kis, V., Barti, B., Lippai, M. & Sass, M. Specialized cortex glial cells accumulate lipid droplets in Drosophila melanogaster. PLoS ONE 10, e0131250 (2015).
Nakano, R. et al. Cortex glia clear dead young neurons via Drpr/dCed-6/Shark and Crk/Mbc/dCed-12 signaling pathways in the developing Drosophila optic lobe. Dev. Biol. 453, 68–85 (2019).
Etchegaray, J. I. et al. Defective phagocytic corpse processing results in neurodegeneration and can be rescued by TORC1 activation. J. Neurosci. 36, 3170–3183 (2016).
Hilu-Dadia, R., Hakim-Mishnaevski, K., Levy-Adam, F. & Kurant, E. Draper-mediated JNK signaling is required for glial phagocytosis of apoptotic neurons during Drosophila metamorphosis. Glia 117, 29 (2018).
Ito, K., Urban, J. & Technau, G. M. Distribution, classification, and development ofDrosophila glial cells in the late embryonic and early larval ventral nerve cord. Roux’s Arch. Dev. Biol. 204, 284–307 (1995).
Stacey, S. M. et al. Drosophila glial glutamate transporter Eaat1 is regulated by fringe-mediated notch signaling and is essential for larval locomotion. J. Neurosci. 30, 14446–14457 (2010).
Peco, E. et al. Drosophila astrocytes cover specific territories of the CNS neuropil and are instructed to differentiate by Prospero, a key effector of Notch. Development 143, 1170–1181 (2016).
Otto, N. et al. The sulfite oxidase Shopper controls neuronal activity by regulating glutamate homeostasis in Drosophila ensheathing glia. Nat. Commun. 9, 3514 (2018).
Stork, T., Sheehan, A., Tasdemir-Yilmaz, O. E. & Freeman, M. R. Neuron-glia interactions through the heartless FGF receptor signaling pathway mediate morphogenesis of Drosophila astrocytes. Neuron 83, 388–403 (2014).
Ma, Z., Stork, T., Bergles, D. E. & Freeman, M. R. Neuromodulators signal through astrocytes to alter neural circuit activity and behaviour. Nature 539, 428–432 (2016).
MacNamee, S. E. et al. Astrocytic glutamate transport regulates a Drosophila CNS synapse that lacks astrocyte ensheathment. J. Comp. Neurol. 524, 1979–1998 (2016).
Kremer, M. C., Jung, C., Batelli, S., Rubin, G. M. & Gaul, U. The glia of the adult Drosophila nervous system. Glia 65, 606–638 (2017).
Hartenstein, V., Spindler, S., Pereanu, W. & Fung, S. The development of the Drosophila larval brain. Adv. Exp. Med. Biol. 628, 1–31 (2008).
Zheng, Z. et al. A complete electron microscopy volume of the brain of adult Drosophila melanogaster. Cell 174, 730–743.e22 (2018).
Omoto, J. J., Yogi, P. & Hartenstein, V. Origin and development of neuropil glia of the Drosophila larval and adult brain: two distinct glial populations derived from separate progenitors. Dev. Biol. 404, 2–20 (2015).
Doherty, J., Logan, M. A., Taşdemir, O. E. & Freeman, M. R. Ensheathing glia function as phagocytes in the adult Drosophila brain. J. Neurosci. 29, 4768–4781 (2009).
Lu, T.-Y. et al. Axon degeneration induces glial responses through Draper-TRAF4-JNK signalling. Nat. Commun. 8, 14355 (2017).
Stahl, B. A. et al. The taurine transporter Eaat2 functions in ensheathing glia to modulate sleep and metabolic rate. Curr. Biol. 28, 3700–3708.e4 (2018).
Hill, A. S., Jain, P., Folan, N. E. & Ben-Shahar, Y. The Drosophila ERG channel seizure plays a role in the neuronal homeostatic stress response. PLoS Genet. 15, e1008288 (2019).
Beckervordersandforth, R. M., Rickert, C., Altenhein, B. & Technau, G. M. Subtypes of glial cells in the Drosophila embryonic ventral nerve cord as related to lineage and gene expression. Mechanisms Dev. 125, 542–557 (2008).
Li, H.-H. et al. A GAL4 driver resource for developmental and behavioral studies on the larval CNS of Drosophila. Cell Rep. 8, 897–908 (2014).
Awasaki, T., Lai, S.-L., Ito, K. & Lee, T. Organization and postembryonic development of glial cells in the adult central brain of Drosophila. J. Neurosci. 28, 13742–13753 (2008).
Ohyama, T. et al. A multilevel multimodal circuit enhances action selection in Drosophila. Nature 520, 633–639 (2015).
Saalfeld, S., Cardona, A., Hartenstein, V. & Tomancak, P. CATMAID: collaborative annotation toolkit for massive amounts of image data. Bioinformatics 25, 1984–1986 (2009).
Valdes-Aleman, J. et al. Comparative connectomics reveals how partner identity, location, and activity specify synaptic connectivity in Drosophila. Neuron 109, 105–122.e7 (2021).
Kato, K., Orihara-Ono, M. & Awasaki, T. Multiple lineages enable robust development of the neuropil-glia architecture in adult Drosophila. Development 147, (2020).
Pereanu, W., Shy, D. & Hartenstein, V. Morphogenesis and proliferation of the larval brain glia in Drosophila. Dev. Biol. 283, 191–203 (2005).
Franzdóttir, S. R. et al. Switch in FGF signalling initiates glial differentiation in the Drosophila eye. Nature 460, 758–761 (2009).
Avet-Rochex, A., Kaul, A. K., Gatt, A. P., McNeill, H. & Bateman, J. M. Concerted control of gliogenesis by InR/TOR and FGF signalling in the Drosophila post-embryonic brain. Development 139, 2763–2772 (2012).
Edgar, B. A. & O’Farrell, P. H. Genetic-control of cell-division patterns in the Drosophila embryo. Cell 57, 177–187 (1989).
Grosskortenhaus, R. & Sprenger, F. Rca1 inhibits APC-Cdh1(Fzr) and is required to prevent cyclin degradation in G2. Dev. Cell 2, 29–40 (2002).
Silies, M. & Klämbt, C. APC/C(Fzr/Cdh1)-dependent regulation of cell adhesion controls glial migration in the Drosophila PNS. Nat. Neurosci. 13, 1357–1364 (2010).
Sigrist, S. J. & Lehner, C. F. Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell 90, 671–681 (1997).
Unhavaithaya, Y. & Orr-Weaver, T. L. Polyploidization of glia in neural development links tissue growth to blood-brain barrier integrity. Genes Dev. 26, 31–36 (2012).
Nern, A., Pfeiffer, B. D. & Rubin, G. M. Optimized tools for multicolor stochastic labeling reveal diverse stereotyped cell arrangements in the fly visual system. Proc. Natl Acad. Sci. USA 112, E2967–E2976 (2015).
Pfeiffer, B. D. et al. Refinement of tools for targeted gene expression in Drosophila. Genetics 186, 735–755 (2010).
Luan, H., Peabody, N. C., Vinson, C. R. & White, B. H. Refined spatial manipulation of neuronal function by combinatorial restriction of transgene expression. 52, 425–436 (2006).
Ryglewski, S., Duch, C. & Altenhein, B. Tyramine actions on Drosophila flight behavior are affected by a glial dehydrogenase/reductase. Front Syst. Neurosci. 11, 68 (2017).
Stork, T. et al. Organization and function of the blood-brain barrier in Drosophila. J. Neurosci. 28, 587–597 (2008).
Babatz, F., Naffin, E. & Klämbt, C. The Drosophila blood-brain barrier adapts to cell growth by unfolding of pre-existing septate junctions. Developmental Cell 47, 697–710.e3 (2018).
Shewan, A., Eastburn, D. J. & Mostov, K. Phosphoinositides in cell architecture. Cold Spring Harb. Perspect. Biol. 3, a004796 (2011).
Krahn, M. P. Phospholipids of the Plasma Membrane - Regulators or Consequence of Cell Polarity? Front Cell Dev Biol. 8, (2020).
Khuong, T. M., Habets, R. L. P., Slabbaert, J. R. & Verstreken, P. WASP is activated by phosphatidylinositol-4,5-bisphosphate to restrict synapse growth in a pathway parallel to bone morphogenetic protein signaling. Proc. Natl Acad. Sci. USA 107, 17379–17384 (2010).
Ivetac, I. et al. Regulation of PI(3)K/Akt signalling and cellular transformation by inositol polyphosphate 4-phosphatase-1. 10, 487–493 (2009).
Dubreuil, R. R., Wang, P., Dahl, S., Lee, J. & Goldstein, L. Drosophila beta spectrin functions independently of alpha spectrin to polarize the Na,K ATPase in epithelial cells. J. Cell Biol. 149, 647–656 (2000).
Morin, X., Daneman, R., Zavortink, M. & Chia, W. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc. Natl Acad. Sci. USA 98, 15050–15055 (2001).
Bökel, C. & Brown, N. H. Integrins in development: moving on, responding to, and sticking to the extracellular matrix. Dev. Cell 3, 311–321 (2002).
Lowe, N. et al. Analysis of the expression patterns, subcellular localisations and interaction partners of Drosophila proteins using a pigP protein trap library. Development 141, 3994–4005 (2014).
Thomas, G. H. & Kiehart, D. P. Beta(Heavy)-spectrin has a restricted tissue and subcellular-distribution during Drosophila embryogenesis. Development 120, 2039–2050 (1994).
Hülsmeier, J. et al. Distinct functions of alpha-Spectrin and beta-Spectrin during axonal pathfinding. Development 134, 713–722 (2007).
Dubreuil, R. R. Functional links between membrane transport and the spectrin cytoskeleton. J. Membr. Biol. 211, 151–161 (2006).
Knust, E. Control of epithelial cell shape and polarity. Curr. Opin. Genet. Dev. 10, 471–475 (2000).
Liem, R. K. H. Cytoskeletal Integrators: The Spectrin Superfamily. Cold Spring Harb Perspect Biol 8, (2016).
Risse, B., Berh, D., Otto, N., Klämbt, C. & Jiang, X. FIMTrack: An open source tracking and locomotion analysis software for small animals. PLoS Comp. Biol. 13, e1005530 (2017).
Risse, B. et al. FIM, a novel FTIR-based imaging method for high throughput locomotion analysis. PLoS ONE 8, e53963 (2013).
Lee, B. P. & Jones, B. W. Transcriptional regulation of the Drosophila glial gene repo. Mechanisms Dev. 122, 849–862 (2005).
Ren, Q., Awasaki, T., Wang, Y.-C., Huang, Y.-F. & Lee, T. Lineage-guided Notch-dependent gliogenesis by Drosophila multi-potent progenitors. Development 145, (2018).
Omoto, J. J. et al. Neuronal constituents and putative interactions within the Drosophila ellipsoid body neuropil. Front. Neural Circuits 12, 103 (2018).
Hadeln von, J., Althaus, V., Häger, L. & Homberg, U. Anatomical organization of the cerebrum of the desert locust Schistocerca gregaria. Cell Tissue Res 374, 39–62 (2018).
Morris, J., Cardona, A., De Miguel-Bonet, M. D. M. & Hartenstein, V. Neurobiology of the basal platyhelminth Macrostomum lignano: map and digital 3D model of the juvenile brain neuropile. Dev. Genes Evol. 217, 569–584 (2007).
Spindler, S. R. & Hartenstein, V. The Drosophila neural lineages: a model system to study brain development and circuitry. Dev. Genes Evol. 220, 1–10 (2010).
Kiecker, C. & Lumsden, A. Compartments and their boundaries in vertebrate brain development. Nat. Rev. Neurosci. 6, 553–564 (2005).
Yoshida, M. & Colman, D. R. Glial-defined rhombomere boundaries in developing Xenopus hindbrain. J. Comp. Neurol. 424, 47–57 (2000).
Mayer, F. et al. Evolutionary conservation of vertebrate blood-brain barrier chemoprotective mechanisms in Drosophila. J. Neurosci. 29, 3538–3550 (2009).
Schwabe, T., Bainton, R. J., Fetter, R. D., Heberlein, U. & Gaul, U. GPCR signaling is required for blood-brain barrier formation in Drosophila. Cell 123, 133–144 (2005).
Daneman, R. & Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 7, a020412 (2015).
Wodarz, A. & Näthke, I. Cell polarity in development and cancer. - PubMed - NCBI. Nat. Cell Biol. 9, 1016–1024 (2007).
Tepass, U. The apical polarity protein network in Drosophila epithelial cells: regulation of polarity, junctions, morphogenesis, cell growth, and survival. Annu. Rev. Cell. Dev. Biol. 28, 655–685 (2012).
Riga, A., Castiglioni, V. G. & Boxem, M. New insights into apical-basal polarization in epithelia. Curr. Opin. Cell Biol. 62, 1–8 (2020).
Humbert, P., Russell, S. & Richardson, H. Dlg, Scribble and Lgl in cell polarity, cell proliferation and cancer. Bioessays 25, 542–553 (2003).
Shahab, J., Tiwari, M. D., Honemann-Capito, M., Krahn, M. P. & Wodarz, A. Bazooka/PAR3 is dispensable for polarity in Drosophila follicular epithelial cells. Biol. Open 4, 528–U137 (2015).
Featherstone, D. E. Glial solute carrier transporters in Drosophila and mice. Glia 59, 1351–1363 (2011).
Landgraf, M., Sánchez-Soriano, N., Technau, G. M., Urban, J. & Prokop, A. Charting the Drosophila neuropile: a strategy for the standardised characterisation of genetically amenable neurites. Dev. Biol. 260, 207–225 (2003).
Bischof, J., Maeda, R. K., Hediger, M., Karch, F. & Basler, K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl Acad. Sci. USA 104, 3312–3317 (2007).
Bauke, A.-C., Sasse, S., Matzat, T. & Klämbt, C. A transcriptional network controlling glial development in the Drosophila visual system. Development 142, 2184–2193 (2015).
Schneider-Mizell, C. M. et al. Quantitative neuroanatomy for connectomics in Drosophila. eLife Sci. 5, e12059 (2016).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Meth 9, 676–682 (2012).
Acknowledgements
We are grateful to B. Altenhein, S. Luschnig, E. Peco and D. van Meyel, and K. Yildirim for generously providing us with antibodies and flies. E. Contreras, M. Krahn, R. Stanewsky, S. Luschnig, S. Schirmeier and S. Rumpf for comments on the manuscript. We thank the Fly EM Project Team at HHMI Janelia and Richard D. Fetter for the Drosophila larval CNS ssTEM volume. We are grateful for all the support of all lab members. This work was supported by the Deutsche Forschungsgemeinschaft through funds to C.K. (SFB 1348, B5).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
N.P. and C.K. designed all experiments. N.P. conducted all experiments. H.K. identified polar expression of Karst, performed initial experiments and together with A.C. analyzed the TEM volume, A.C. contributed data and software, S.Re. and S.Ro. generated constructs used in this study. N.P. and C.K. together with input from all authors wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pogodalla, N., Kranenburg, H., Rey, S. et al. Drosophila ßHeavy-Spectrin is required in polarized ensheathing glia that form a diffusion-barrier around the neuropil. Nat Commun 12, 6357 (2021). https://doi.org/10.1038/s41467-021-26462-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-021-26462-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.