Abstract
The simple ABO3 and A-site-ordered AA′3B4O12 perovskites represent two types of classical perovskite functional materials. There are well-known simple perovskites with ferroelectric properties, while there is still no report of ferroelectricity due to symmetry breaking transition in A-site-ordered quadruple perovskites. Here we report the high pressure synthesis of an A-site-ordered perovskite PbHg3Ti4O12, the only known quadruple perovskite that transforms from high-temperature centrosymmetric paraelectric phase to low-temperature non-centrosymmetric ferroelectric phase. The coordination chemistry of Hg2+ is changed from square planar as in typical A-site-ordered quadruple perovskite to a rare stereo type with 8 ligands in PbHg3Ti4O12. Thus PbHg3Ti4O12 appears to be a combinatory link from simple ABO3 perovskites to A-site-ordered AA′3Ti4O12 perovskites, sharing both displacive ferroelectricity with former and structure coordination with latter. This is the only example so far showing ferroelectricity due to symmetry breaking phase transition in AA′3B4O12-type A-site-ordered perovskites, and opens a direction to search for ferroelectric materials.
Similar content being viewed by others
Introduction
Perovskites and their derivatives show many interesting physical, chemical and mineral properties such as ferromagnetism, ferroelectricity, piezoelectricity, ion conductivity, photocatalysis and superconductivity1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18 that can be modified dramatically by the coordination chemistry for a given composition. There is a special class of perovskite-type materials with the general chemical formula AA′3B4O12, named A-site-ordered, or quadruple perovskites, which received much attention owing to their fascinating structural and wide varieties of physical properties10, including colossal magnetoresistance under weak field11, charge disproportionation12 and giant dielectric constant over a wide temperature range13,14. For the simple ABO3 perovskite, the 12-fold coordinated A-site is often occupied by large size ions such as alkali metal, alkaline earth or lanthanide cations and the 6-fold coordinated B-site is often occupied by transition metals (TMs) to satisfy the so called tolerance factor t = (rA + rO)/√2(rB + rO) (rA, rB, & rO represent for the ion radius of A, B, and O, respectively) with t usually in the range of 0.75 to 1.05 for stable perovskite compounds1,18. However in the A-site-ordered perovskite AA′3B4O12, three quarters of the A-site is substituted by a TM, A′ with much smaller ionic radius16,17,18,19. Generally, TM ions with strong Jahn-Teller distortions like Mn3+ and Cu2+ preferentially occupy the A′-site with square-planar coordination20,21,22,23,24. The small TM ion at the A′-site causes the distortion of BO6 octahedron in AA′3B4O12 perovskite, usually resulting in a cubic crystal structure with space group Im-325. From a structural point of view, the flexibility for tilting the BO6 octahedra in AA′3B4O12 perovskite is limited in order to maintain the square-planar coordination at the originally 12-fold coordinated A-site in the simple perovskite structure21.
Ferroelectricity is one of the most important properties for application in actuators, sensors, and memory storage devices, etc. Large numbers of ferroelectric materials possess simple perovskite structures, such as BaTiO3, PbTiO3, Pb(Ti, Zr)O3, BiFeO326,27,28,29,30. The origin of ferroelectricity is due to either the lone pair 6s2 effect of cations (Pb2+, Bi3+), or the second-order Jahn-Teller active cations with electron configuration of d0 (Ti4+, Zr4+); these compounds are usually referred to as displacive-type ferroelectrics. However, ferroelectricity is seldom observed in AA′3B4O12 type A-site-ordered compounds, even when they contain second-order Jahn-Teller active cations or lone pair effect cations28. One reason for the absence of ferroelectricity in this series of compounds is the usual presence of a centrosymmetric structure, and the variation of temperature generally causes isostructural or centrosymmetric - to - centrosymmetric phase transition12,21,25, hence there cannot be spontaneous polarization. Currently the rare examples in this series that show ferroelectricity are AMn7O12 (A = Ca, Bi or Pb)31,32,33 and AMn3Cr4O12 (A = La or Bi)20,22. Although these compounds have centrosymmetric structures, all are spin-driven multiferroic systems, due to strong magnetoelectric coupling effects20,22,32. Up to now, there is still no report of ferroelectricity from symmetry breaking transition in A-site-ordered perovskite compounds.
In this work, an AA′3B4O12 type A-site-ordered perovskite oxide, PbHg3Ti4O12 (henceforth PHTO) was designed and synthesized at high pressure and high temperature conditions. It is found that the Hg2+ ion occupies the A′-site in AA′3B4O12 type perovskite in sharp contrast to previous reports where 3d TM ions (Cu2+, Mn3+) usually locate at the A′-site. Moreover, ferroelectricity was observed in PHTO, when its ambient centrosymmetric phase transformed to a non-centrosymmetric phase at 250 K. The origins of ferroelectricity are discussed based on the comprehensive characterizations of crystal structure using both neutron and synchrotron x-ray diffractions and density functional theory (DFT) calculation.
Results and Discussion
Crystal structure
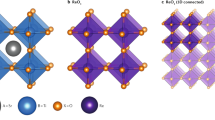
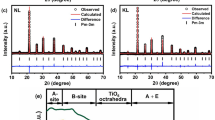
The NPD refinements are shown in Fig. 1a, which confirm that PHTO crystallizes in the AA′3B4O12 type A-site-ordered perovskite. The refined structure parameters of PHTO based on NPD data collected at 295 K are listed in Table 1. No anomaly is observed in the occupation parameters at any site, including full occupancy for the oxygen site as well. Thus, PHTO should have stoichiometric composition. Bond valence sum (BVS) calculations based on the refined structure from NPD data give valences of +2.26 for Pb ions, +1.99 for Hg ions and +3.88 for Ti ions (see Table 1), which are consistent with x-ray absorption spectroscopy (XAS) results discussed later. Hence the obtained sample is an A-site-ordered perovskite with stoichiometric Pb2+Hg2+3Ti4+4O12 formula and space group Im-3 as shown in Fig. 1b. The structure model is consistent with the refinements of SXRD data as shown in Supplementary Fig. S1 and Table S1. Moreover, for Hg ions, if one considers the four nearest - neighbor O atoms (Hg-O(×4) = 2.320(5) Å), the BVS value is only 1.56. However, if one takes into accounts the four next - nearest - neighbor O atoms (Hg-O(×4) = 2.798(1) Å) additionally, the BVS value is 1.99. Therefore, Hg ions at A′-site are close to 8 -coordinated by O, in sharp contrast to typical A-site-ordered perovskite where the A′-site is usually 4-coordinated by O (See Fig. 1c). Note that, the PHTO sample is single phase of high quality, no detectable diffraction peak belonging to PbTiO3 or HgTiO3 was found in either NPD or SXRD patterns.
a Rietveld refinements based on NPD data at 295 K. Observed (crosses), calculated (red), difference (blue) are shown in the figure, respectively. The ticks indicate the allowed Bragg reflections with space group Im-3. b Crystal structure of PHTO. c, Schematic coordination of A, A′ and B-site for ABO3, AA′3B4O12 and PbHg3Ti4O12 respectively.
As aforementioned, usually the strong Jahn-Teller active ions, like Cu2+ and Mn3+, are preferentially accommodated into the square-coordinated A′-site in the A-site-ordered perovskites, as in LaCu3Fe4O12 and BiMn3Cr4O1220,21. Some other TM ions have been recently introduced at the A′-site such as in CaCo3V4O1234, CaFe3Ti4O1235, CaPd3Ti4O1236. Thus PHTO is an AA′3B4O12 type perovskites with the A′-site fully occupied by Hg2+, other than 3d TM, or Pd as shown in Table 2. The lattice parameter (7.72 Å) of PHTO is larger compared to those of other AA′3B4O12 type perovskites (7.3–7.5 Å), while the ∠Ti–O–Ti angle (155.8°) of PHTO is less distorted from the ideal 180° than those for other AA′3B4O12 type perovskites (∠B–O–B ≈ 140°). The larger ∠Ti–O–Ti angle and correspondingly smaller ∠A′−O−Ti angle in PHTO imply the less tilting of TiO6 octahedron, which is attributed to the large ionic size of Hg2+ about 0.96 Å. Figure 2 shows the tolerance factor t versus average ionic radius of A-site ions for simple perovskites ATiO3 and A-site-ordered perovskites AA′3Ti4O12 where the t factor is calculated assuming a simple ABO3 form with the average ionic size at A site. It is found that from CaTiO3 to BaTiO3, the ionic radius of A-site increases from 1.34 Å to 1.61 Å while the tolerance factor t increases from 0.97 to 1.07. The simple perovskites ATiO3 are located at the upper right panel of Fig. 2. For most of the AA′3Ti4O12 compounds, the average ionic radius of A-site is about 0.8 Å and the tolerance factor t is around 0.77. Therefore, AA′3Ti4O12 perovskites are located at the lower left panel of the Fig. 2. However, different from these ATiO3 and AA′3Ti4O12 compounds, the average ionic radius of A-site ion for PHTO is large, about 1.1 Å and the tolerance factor t is about 0.88. Moreover as aforementioned, the coordination number for Hg ions at the A′-site in PHTO is eight, not square planar as in A′-TM quadruple perovskites AA′3Ti4O12, and it is located in the middle area of Fig. 2, between those of simple ATiO3 (coordination number 12) and AA′3Ti4O12 quadruple perovskites (coordination number 4). Thus, PHTO appears to be a link from simple ATiO3 perovskite to AA′3Ti4O12 type A-site-ordered perovskite (Fig. 2). The A-site coordination number for ABO3 perovskite is 12 while the A-site and A’-site coordination numbers in the A-site-ordered quadruple perovskite AA'3B4O12 compound are 12 and 4 respectively. However in PHTO, the coordination number of A′ (Hg2+) is 8, which is just in between that for simple ATiO3 and AA'3Ti4O12. This result provides further evidence supporting the scenario aforementioned. Considering that the ionic size of Sr2+ (1.45 Å) is similar to that Pb2+ (1.49 Å), we also fabricated the A-site-ordered perovskite compound SrHg3Ti4O12, which is isostructural with PHTO, but without the observation of ferroelectricity. The result about SrHg3Ti4O12 will be published elsewhere. According to the previous report37, the low energy level of d10 orbital prefers a relatively high coordination number owing to the weak d-s and d-p hybridization in perovskite compounds. As to PHTO system, the first principles calculation result indicates that the energy level of d10 for Hg2+ has a lower energy for 8 coordination than that for 4 coordination (See Supplementary Fig. S2a). Moreover PHTO also shows a low total energy in the state of 8 coordination of Hg2+ (See Supplementary Fig. S2b). Thus 8-fold coordination of Hg2+ is more stable than 4-fold in the PHTO system.
Valence state analysis
Soft X-ray absorption spectroscopy at the L2,3 edge of TM 3d ions38 is a highly sensitive method to determine the valence state39,40, local environment41, and orbital occupation42 of the ions. Figure 3a shows the Ti-L2,3 XAS spectra of PHTO together with SrTiO3 as a pure Ti4+ reference. The very similar multiplet spectral features in PHTO and SrTiO3 clearly correspond to the Ti 2p63d0 → 2p53d1 transition43,44, demonstrating the Ti4+ valence state in PHTO. Note that the PHTO spectrum is shifted by about 0.25 eV to lower energy with respect to the SrTiO3 spectrum, reflecting the different Ti local environments. Actually the Ti-O distance is 1.974(7) Å in PHTO and 1.951(7) Å in SrTiO345, which suggests a weaker crystal field effect in PHTO. Figure 3b shows the Pb-L3 XAS spectra of PHTO together with PbTiO3 as a Pb2+ and PbNiO3 as Pb4+ reference. The weak pre-edge feature in PbNiO3 due to excitation from a 2p core electron to the 6s orbital. It has been established that this weak pre-edge feature is a sensitive finger-print of the Pb valence state in solid state materials46. The reason is that the Pb 5d10 orbitals are fully occupied, the valence state is then reflected by the 6s occupation. However, as shown in the inset, PHTO has the Pb-L3 XAS profile similar to the Pb2+ reference PbTiO3 in detail, no 2p–6s related excitation is observed in PHTO, which indicates the valence state of Pb2+ with fully occupied 6s2 state in this compound. Figure 3c shows the Hg-L3 XAS spectra of PHTO as well as HgO. The same energy position and very similar spectral profile of both PHTO and HgO indicate the Hg2+ valence state. The XAS results show that the electronic configuration of PHTO is Pb2+Hg2+3Ti4+4O12, which is fully consistent with the BVS results based on the crystal structure.
Magnetic and transport characterizations
The magnetic susceptibility χ is almost constant and negative near zero from 300 K to 30 K, while χ increases rapidly on further decrease of temperature as shown in Supplementary Fig. S3. These results revealed that PHTO is nonmagnetic due to the 3d0, 5d10 and 6s2 electron configuration for Ti4+, Hg2+ and Pb2+, respectively, and consistent with the BVS calculations and XAS results. The resistance of PHTO at room temperature is too large to be measured, indicating the high electrical insulating property and few vacancies in the sample. Supplementary Fig. S4a shows the Ultraviolet-Visible-Near Infrared (UV-Vis-NIR) absorption spectrum of PHTO. The curve exhibits a steep decrease from 420 to 640 nm, which is a typical optical response of semiconductors. The optical band gap of the PHTO was estimated to be 2.12 eV. The first principles calculations show that PHTO is a direct gap insulator with 1.70 eV gap at the H point shown in Supplementary Fig. S4b. The gap might be underestimated in DFT calculations, nevertheless, it reveals the wide gap nature of PHTO. The obtained PHTO sample is stable at ambient up to 973 K as revealed by thermogravimetry analysis measurements shown in Supplementary Fig. S5.
Dielectricity and ferroelectricity
The permittivity and dielectric loss measured with different frequencies from 2 K to 300 K are shown in Fig. 4a, b, respectively. The relative dielectric constant, εr of PHTO is over 220 in the measured temperature range. The temperature dependence of εr shows a clear peak around 250 K at all the frequencies of measurements. The overall temperature-dependent εr strongly indicates a low-temperature ferroelectric phase transition to a high-temperature paraelectric phase at TFE ≈ 250 K. Furthermore, the transition temperature is independent of measurement frequency, indicating that PHTO is not a relaxor-type ferroelectric material. The relative increase of the dielectric loss around 250 K is consistent with the paraelectric-ferroelectric transition. Moreover the dielectric loss keeps at a very low value (<0.03) with all of frequency in the whole measurement range, which indicate the intrinsic dielectric property of PHTO. In order to further identify the ferroelectricity of PHTO, the isothermal polarizations were measured by the PUND method20. No loops of polarization versus electric field (P–E) were observed in polycrystalline PHTO at room temperature. However, canonical P–E hysteresis loops were observed below 250 K as shown in Fig. 4c, d. It is clear that polarization is enhanced at low temperature. At fixed temperature, the P–E loops significantly expand with increasing electric field. For example, the P value increases from 0.028 to 0.11 µC cm−2 as the maximum electric field changes from 17 to 32 kV cm−1 at 10 K. As known, PbTiO3 is a classic and extensively studied ferroelectric material with Curie temperature about 763 K, which excludes the possibility that the ferroelectricity of PHTO originated from PbTiO3. Thus it can be concluded that the ferroelectricity is due to PHTO.
Temperature dependent crystal structure transition
To further understand the mechanism of ferroelectricity from the structure point of view, we performed the temperature dependent SXRD, as shown in Supplementary Fig. S6. The characteristic (400) diffraction peak at different temperatures is collected in Fig. 5a. It is obvious that the diffraction peaks split into two sub-peaks between 240 K and 260 K, which indicates the occurrence of temperature induced phase transition at 250 K is due to symmetry reduction from a cubic - to - orthorhombic phase. Checking the SXRD data measured at 300 K and 90 K, all the high symmetry diffraction peaks, such as (200), (220), (222), (400) and (402) of the high temperature phase, are split into two sub-peaks on cooling to low temperature (see Supplementary Fig. S7). There is a clear long-range structural transition with decreasing temperature. The SXRD data collected at 90 K is presented in Fig. 5b. The crystal structure of PHTO can be fitted very well by an orthorhombic phase of non-centrosymmetric space group Imm2 (No.44). The inset shows the crystal structure of the low temperature phase. The Rietveld refinement results are listed in Supplementary Table S2. Figure 5c shows variation of the lattice parameters based on refinements of SXRD data. The lattice parameter a decrease lineally from 300 K to 250 K. Below the temperature of the phase transition, the lattice parameter b and c continue to decrease while parameter a increases. Figure 5d shows the evolution of TiO6 octahedron before and after phase transition. In the high temperature phase, TiO6 forms a regular octahedron with ∠Ti–O–Ti angle of 155.8° and Ti-O bond length of 1.974 Å respectively. When this cubic phase transformed to an orthorhombic non-centrosymmetric phase below 250 K, both ∠Ti–O–Ti angles and Ti-O bond lengths divergences occurred in TiO6 octahedron. These distortions originate from the relative displacements of Ti and O around all the axis directions. The polarization in the ab-plane was canceled due to the random relative displacements of Ti and O, leaving the polarization vector only along the [001] direction. As to HgO8 polyhedron, the single type HgO8 polyhedron with only two-types of Hg-O bond length in the high temperature phase evolves into three different type of HgO8 polyhedron with different bond length in the low temperature phase, but without contribution to the polarization. Supplementary Fig. S8 illustrates the evolution of HgO8 during the paraelectric-ferroelectric transition. Therefore, PHTO is a displacive-type ferroelectric. The ionic spontaneous polarization can be calculated by multiplying the effective charges and the distance between the negative and positive valence weighted mean center along the c-axis then divided by the volume of the unit cell47. Based on the refined results of NPD data collected at 5 K in Table 3, the calculated polarization value is about 13.65 μC cm−2. Although temperature-dependent phase transition is typical in A-site-ordered perovskites, it is usually isostructural or centrosymmetric - to - centrosymmetric phase transition from Im-3 to Pn-3. Most of those phase transitions are mainly induced by charge transfer between the A′-site and B-site ions or charge disproportionation at B-site ions12,21,25. PHTO is an example of AA′3B4O12 type A-site-ordered perovskite that exhibits a centrosymmetric to non-centrosymmetric phase transition.
a The characteristic diffraction peaks (400) collected at different temperatures. b Rietveld refinements based on SXRD data at 90 K. The arrow denotes the polarization direction. c Temperature dependence of the lattice constant and d schematic illustration for the changing of TiO6 octahedron along different axis.
First principles calculations
In order to get a deeper insight into the ferroelectricity of PHTO at low temperature, first principles calculations have been performed. Supplementary Fig. S9 shows the phonon spectrum for the cubic structure of PHTO: three imaginary frequency modes with irreducible representation Tu degenerate at Γ point. By moving atoms along these soft phonon modes, the non-centrosymmetric crystal structure with space group Imm2 can be obtained. Free energy per primitive cell versus moving amplitude of atoms along one of the soft modes is plotted in Fig. 6. The energy well suggests a spontaneous structure phase transition with the biggest contribution coming from the Ti-O mode. The imaginary modes at H point only consist of the Ti-O phonon modes, which are consist with the experimental conclusion. In addition, the 6s2 lone pair electrons of Pb2+ also contribute to the site instability according to the theory analysis. However, the contribution from the lone pair mechanism does not play a dominant role from the energy perspective (see Fig. 6). Using Berry phase method, the polarization of ferroelectric structure and Born effective charge have been calculated. The calculated polarization value is about 16.87 μC cm−2, which can also be obtained by multiplying the Born effective charge tensor with the corresponding atom displacements. This result is roughly in agreement with that ionic spontaneous polarization calculation result of 13.65 μC cm−2 based on neutron diffraction measurements. Both values are larger than the experimental result. This is because the calculated value is based on single crystal data, while the experimental measured value is from a polycrystalline sample of randomly distributed grains. For example, in ceramic samples of BiFeO3, much lower values of about 8.9 μC cm−2 of polarization have been measured at room temperature while in single crystals the polarization is up to 60 μC cm−2 along [012] axis and 100 μC cm−2 along [001] axis48,49. Another reason for the low experimental value of polarization may be due to the grain boundary effect and/or leaking current issue preventing the application of maximum electric field in the experiment48,50. Supplementary Fig. S10 shows the SEM image of the polycrystalline sample of PHTO. It can be seen that the grain size distributes around several microns to nearly 20 microns and generates a large amount of grain boundaries. Those grains are disorderly distributed, which will severely affect the polarization.
The A-site-ordered perovskite PbHg3Ti4O12 was synthesized at high pressure and high temperature. PbHg3Ti4O12 is a nonmagnetic insulator with a direct energy gap of ~2.12 eV and relative dielectric constant larger than 220. PbHg3Ti4O12 undergoes a transition from a high temperature centrosymmetric, cubic and paraelectric phase to a low temperature non-centrosymmetric, orthorhombic and ferroelectric phase with a record high Curie temperature at 250 K for an A-site-ordered quadruple perovskite. The ferroelectric distortion is dominated by Ti-O phonon mode anomaly. Because of the large average size of the A-site cation ~ 1.1 Å in PHTO, the coordination of Hg2+ is not square planar as in typical A-site-ordered quadruple perovskites, but closer to 8. Therefore PHTO can be considered to be structurally linking ABO3 simple and AA′3B4O12 quadruple perovskites. The established A-site-ordered perovskites AHg3B4O12 may provide a potential pathway to find a class of ferroelectric materials of high Curie temperature. The work provides one of unique examples that high pressure synthesis can lead to the compounds with unique properties that are otherwise hard to get access at ambient. Moreover perovskites are the most abundant minerals in the broad lower Mantle of the Earth where high pressure is prevailing. Hence it might shed lights to further understand the coordination chemistry of perovskites like compounds at high pressures.
Methods
Sample fabrication
The polycrystalline sample of PHTO was synthesized by a solid state reaction under high pressure of 6 GPa and high temperature of 1273 K. The starting materials HgO (Aldrich, 99.0% pure), PbO (Alfa, 99.995% pure) and TiO2 (Alfa, 99.995% pure) were mixed homogenously in a molar ratio 1:3:4 and pressed into a pellet with diameter of 6 mm in an argon gas protected glove box with oxygen and H2O level of less than 1 ppm. The pre-pressed pellet was sealed in a gold capsule. High pressure experiments were performed with the cubic anvil type high pressure apparatus51,52. After the pressure was gradually increased to 6 GPa, the sample was heated to 1273 K and maintained for 30 min. Then the temperature was quenched to ambient before the release of pressure. In this process, yellow polycrystalline PHTO was obtained.
Structure characterization
The crystal structure was characterized by neutron powder diffraction (NPD) with the BT-1 high-resolution neutron powder diffractometer with a Ge (311) monochromator at the Center for Neutron Research (NCNR) of the National Institute of Standard & Technology (NIST). The neutron wavelength was 2.0774 Å. The intensities were measured with steps of 0.05° in the 2θ range of 10°–160°. The sample was also measured by synchrotron x-ray diffraction (SXRD) at the 11-BM-B at the Advanced Photon Source (APS) in Argonne National Laboratory. The X-ray wavelength was 0.412726 Å. Diffraction data were collected in the angle (2θ) range from 0.5° to 50° with steps of 0.002°. The obtained NPD and SXRD data were analyzed by the Rietveld method with the GSAS program53.
Valences determination
The valence states of the Ti, Pb and Hg ions were determined by x-ray absorption spectroscopy (XAS). The soft XAS at the Ti-L2,3 edge was measured with total the electron yield mode at the Dragon beamline while the hard XAS at the Pb-L3 and Hg-L3 edge were measured with transmission geometry at the BL07A beamline at National Synchrotron Radiation Research Center (NSRRC) of Taiwan. SrTiO3, PbTiO3, PbNiO3 and HgO were also measured at the respective Ti-L, Pb-L and Hg-L edges as reference materials.
Permittivity and ferroelectricity characterization
The permittivity was measured with different frequency by an Agilent-4980A LCR meter on a solid pellet with 4.0 mm in diameter and 230 μm in thickness. The ferroelectric hysteresis loops were measured at 20 Hz with a Radiant Precision Premier-II Ferroelectric Test System at different temperatures based on the proposed positive-up negative-down (PUND) method. Detailed experimental descriptions can be found in Ref. 20.
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Goodenough, J. & Longo, J. Crystallographic and magnetic properties of perovskite and perovskite related compounds. Landolt-Bornstein Numerical Data and Functional Relationships in Science and Technologym, eds Hellwege, K.-H., Hellwege, A. M. (Springer, Berlin) III/4, 126–314 (1970).
Hayward, M. A. et al. The hydride anion in an extended transition metal oxide array: LaSrCoO3H0.7. Science 295, 1882–1884 (2002).
Jin, C. Q. et al. Superconductivity at 80 K in (Sr,Ca)3Cu2O4+δCl2-y induced by apical oxygen doping. Nature 375, 301–303 (1995).
Zhang, J. J. et al. Large orbital polarization in a metallic square-planar nickelate. Nat. Phys. 13, 864–870 (2017).
Li, M. R. et al. Mn2(Fe0.8Mo0.2)MoO6: a double perovskite with multiple transition metal sublattice magnetic effects. Chem. Mater. 30, 4508–4514 (2018).
Lin, J. F. et al. Spin transition zone in Earth’s lower mantle. Science 317, 1740–1743 (2007).
Kageyama, H. et al. Expanding frontiers in materials chemistry and physics with multiple anions. Nat. Commun. 9, 772 (2018).
Li, W. M. et al. Superconductivity in a unique type of copper oxide. Proc. Natl Acad. Sci. USA 116, 12156–12160 (2019).
Jin, C. Q., Adachi, S., Wu, X. J., Yamauchi, H. & Tanaka, S. 117 K Superconductivity in the Ba-Ca-Cu-O system. Phys. C. 223, 238–242 (1994).
Shimakawa, Y. A-site-ordered perovskites with intriguing physical properties. Inorg. chem. 47, 8562–8570 (2008).
Zeng, Z., Greenblatt, M., Subramanian, M. A. & Croft, M. Large low-field magnetoresistance in perovskite-type CaCu3Mn4O12 without double exchange. Phys. Rev. Lett. 82, 3164–3167 (1999).
Yamada, I. et al. A perovskite containing quadrivalent iron as a charge-disproportionated ferrimagnet. Angew. Chem. Int. Ed. 47, 7032–7035 (2008).
Ramirez, A. P. et al. Giant dielectric constant response in a copper-titanate. Solid State Commun. 115, 217–220 (2000).
Arbatti, M., Shan, X. & Cheng, Z. Y. Ceramic-polymer composites with high dielectric constant. Adv. Mater. 19, 1369–1372 (2007).
Cheng, J. G. et al. Possible Kondo physics near a metal-insulator crossover in the A-site ordered perovskite CaCu3Ir4O12. Phys. Rev. Lett. 111, 176403 (2013).
Panda, P. K. Review: environmental friendly lead-free piezoelectric materials. J. Mater. Sci. 44, 5049–5062 (2009).
Belik, A. A. et al. Low-temperature structural modulations in CdMn7O12, CaMn7O12, SrMn7O12, and PbMn7O12 perovskites studied by synchrotron X-ray powder diffraction and mossbauer spectroscopy. J. Phys. Chem. C. 120, 8278–8288 (2016).
Jin, C. Q. et al. High-pressure synthesis of the cubic perovskite BaRuO3 and evolution of ferromagnetism in ARuO3 (A = Ca, Sr, Ba) ruthenates. Proc. Natl Acad. Sci. USA 105, 7115–7119 (2008).
Deng, H. S. et al. Strong enhancement of spin ordering by A-site magnetic ions in the ferrimagnet CaCu3Fe2Os2O12. Phys. Rev. B 94, 024414 (2016).
Zhou, L. et al. Realization of large electric polarization and strong magnetoelectric coupling in BiMn3Cr4O12. Adv. Mater. 29, 1703435 (2017).
Long, Y. W. et al. Temperature-induced A-B intersite charge transfer in an A-site-ordered LaCu3Fe4O12 perovskite. Nature 458, 60–63 (2009).
Wang, X. et al. Observation of magnetoelectric multiferroicity in a cubic perovskite system: LaMn3Cr4O12. Phys. Rev. Lett. 115, 087601 (2015).
Yamada, I. et al. Control of bond-strain-induced electronic phase transitions in iron perovskites. Inorg. Chem. 52, 13751–13761 (2013).
Yin, Y. Y. et al. LaMn3Ni2Mn2O12: An A- and B-site ordered quadruple perovskite with A-site tuning orthogonal spin ordering. Chem. Mater. 28, 8988–8996 (2016).
Long, Y. et al. Intermetallic charge transfer in A-site-ordered double perovskite BiCu3Fe4O12. Inorg. Chem. 48, 8489–8492 (2009).
Choi, K. J. et al. Enhancement of ferroelectricity in strained BaTiO3 thin films. Science 306, 1005–1009 (2004).
Kubel, F. & Schmid, H. Structure of a ferroelectric and ferroelastic monodomain crystal of the perovskite BiFeO3. Acta Crystallogr. B 46, 698–702 (1990).
Aimi, A. et al. High-pressure synthesis of A-site ordered double perovskite CaMnTi2O6 and ferroelectricity driven by coupling of A-site ordering and the second-order Jahn-Teller effect. Chem. Mater. 26, 2601–2608 (2014).
Sawaguchi, E. Ferroelectricity versus antiferroelectricity in the solid solutions of PbZrO3 and PbTiO3. J. Phys. Soc. Jpn. 8, 615–629 (1953).
Yamamoto, T. Ferroelectric properties of the PbZrO3-PbTiO3 system. Jpn. J. Appl. l Phys. 35, 5104–5108 (1996).
Johnson, R. D. et al. Giant improper ferroelectricity in the ferroaxial magnet CaMn7O12. Phys. Rev. Lett. 108, 067201 (2012).
Belik, A. A. et al. Spin-driven multiferroic properties of PbMn7O12 perovskite. Inorg. Chem. 55, 6169–6177 (2016).
Mezzadri, F. et al. Synthesis and characterization of multiferroic BiMn7O12. Phys. Rev. B 79, 100106 (R) (2009).
Ovsyannikov, S. V. et al. Structural and magnetic transitions in CaCo3V4O12 perovskite at extreme conditions. Inorg. Chem. 56, 6251–6263 (2017).
Leinenweber, K., Linton, J., Navrotsky, A., Fei, Y. & Parise, J. B. High-pressure perovskites on the join CaTiO3-FeTiO3. Phys. Chem. Miner. 22, 251–258 (1995).
Shiro, K. et al. Pd2+-incorporated perovskite CaPd3B4O12 (B = Ti, V). Inorg. Chem. 52, 1604–1609 (2013).
Orgel, L. E. & Dunitz, J. D. Stereochemistry of cupric compounds. Nature 179, 462–465 (1957).
De Groot, F. X-ray absorption and dichroism of transition metals and their compounds. J. Electron. Spectrosc. 67, 529–622 (1994).
Schlappa, J. et al. Resonant soft x-ray scattering from stepped surfaces of SrTiO3. J. Phys.-Condens. Mat. 24, 035501 (2012).
Gegner, J. et al. Insulator-metal transition in TiGePt: a combined photoelectron spectroscopy, x-ray absorption spectroscopy, and band structure study. Phys. Rev. B 85, 235106 (2012).
Haverkort, M. W. et al. Determination of the orbital moment and crystal-field splitting in LaTiO3. Phys. Rev. Lett. 94, 056401 (2005).
Chang, C. F. et al. c-axis dimer and its electronic breakup: the insulator-to-metal transition in Ti2O3. Phys. Rev. X 8, 021004 (2018).
Degroot, F. M. F., Fuggle, J. C., Thole, B. T. & Sawatzky, G. A. L2,3 X-ray-absorption edges of d0 compounds: K+, Ca2+, Sc3+ and Ti4+ in Oh (octahedral) symmetry. Phys. Rev. B 41, 928–937 (1990).
Pichler, T. et al. Proof for trivalent Sc ions in Sc2 @ C84 from high-energy spectroscopy. Phys. Rev. B 62, 13196–13201 (2000).
Yamanaka, T., Hirai, N. & Komatsu, Y. Structure change of Ca1-xSrxTiO3 perovskite with composition and pressure. Am. Mineral. 87, 1183–1189 (2002).
Chen, K. et al. Valence state of Pb in transition metal perovskites PbTMO3 (TM = Ti, Ni) determined from X-ray absorption near-edge spectroscopy. Phys. Status Solidi B 255, 1800014 (2018).
Zhu, J. L. et al. Nuclear and charge density distributions in ferroelectric PbTiO3: maximum entropy method analysis of neutron and X-ray diffraction data. Powder Diffr. 28, 276–280 (2013).
Lebeugle, D., Colson, D., Forget, A. & Viret, M. Very large spontaneous electric polarization in BiFeO3 single crystals at room temperature and its evolution under cycling fields. Appl. Phys. Lett. 91, 022907 (2007).
Wang, Y. P., Yuan, G. L., Chen, X. Y., Liu, J. M. & Liu, Z. G. Electrical and magnetic properties of single-phased and highly resistive ferroelectromagnet BiFeO3 ceramic. J. Phys. D. Appl. Phys. 39, 2019–2023 (2006).
Lebeugle, D. et al. Room-temperature coexistence of large electric polarization and magnetic order in BiFeO3 single crystals. Phys. Rev. B 76, 024116 (2007).
Zhang, J. et al. A new quasi-one-dimensional compound Ba3TiTe5 and superconductivity induced by pressure. NPG Asia Mater. 11, 60 (2019).
Deng, Z. et al. A pressure-induced inverse order-disorder transition in double perovskites. Angew. Chem. Int. Ed. 59, 8240–8246 (2020).
Toby, B. H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr 34, 210–213 (2001).
Acknowledgements
The work was supported by National Science Foundation and Ministry of Science and Technology of China through the research projects (2018YFA0305700, 11921004, 11820101003, U2032220, 2017YFA0302900), and Beijing Nature Science Foundation (2202059).
Author information
Authors and Affiliations
Contributions
C.Q.J. conceived and supervised the project. C.Q.J., R.Z.Y., and H.M.W. coordinated the research. J.F.Z., X.W., W.M.L., J.Z., Z.D., L.D., C.W.R., Y.W.L., J.L.Z., R.Z.Y. and X.C.W. synthesized the samples and performed the major measurements. X.D.S., J.F.Z. and Y.W.L. carried out the dielectric and ferroelectric measurements with the help of others. J.F.Z., Y.R., R.Z.Y., C.Q.J. and C.D. performed the X-ray diffraction and crystal structure analysis. Q.Z.H. and J.F.Z. collected the powder neutron diffraction data and carried out the data analysis with the help of others. X.S. and R.C.Y. carried the TEM measurements. J.F.Z., Z.W.H., L.P.C., W.M.L., S.C.W., H.J.L., C.T.C. and L.H.T. carried out the XAS measurements. J.C.G., Y.T.Q., Z.L. and H.M.W. did the theoretical calculations. J.F.Z., R.Z.Y., Q.Q.L., C.Q.J. discussed with M.G. the experimental results in many details. J.F.Z., R.Z.Y. and C.Q.J. wrote the manuscript with comments from H.M.W., M.G. & Z.W.H. All authors contributed to the version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Shiming Zhou and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, J., Gao, J., Li, W. et al. A combinatory ferroelectric compound bridging simple ABO3 and A-site-ordered quadruple perovskite. Nat Commun 12, 747 (2021). https://doi.org/10.1038/s41467-020-20833-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-020-20833-6
This article is cited by
-
Ab Initio Study of the Effects of d-Block Metal (Mn, Re, Tc) Encapsulation on the Electronic, Phonon, Thermodynamic, and Gravimetric Hydrogen Capacity of BaXH4 Hydride Perovskites
Journal of Electronic Materials (2024)
-
Lithium-based perovskites materials for photovoltaic solar cell and protective rays window applications: a first-principle calculations
Discover Nano (2023)
-
The comparative investigations of structural, optoelectronic, and mechanical properties of AgBeX3 (X = F and Cl) metal halide-perovskites for prospective energy applications utilizing DFT approach
Optical and Quantum Electronics (2023)
-
Investigation of Properties and Application of Magneto Electro Elastic Materials and Analysis of Piezoelectric Smart Shells
Transactions of the Indian Institute of Metals (2023)
-
Quantum Chemical Study the Removal of Acetone by Using the Pristine and Si-doped C2N Monolayer
Silicon (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.