Abstract
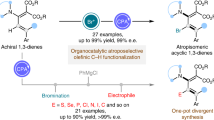
Enantioenriched 1,4-dienes are versatile building blocks in asymmetric synthesis, therefore their efficient synthesis directly from chemical feedstock is highly sought after. Here, we show an enantioselective cross-hydroalkenylation of cyclic 1,3-diene and hetero-substituted terminal olefin by using a chiral [NHC-Ni(allyl)]BArF catalyst. Using a structurally flexible chiral C2 NHC-Ni design is key to access a broad scope of chiral 1,4-diene 3 or 3′ with high enantioselectivity. This study also offers insights on how to regulate chiral C2 NHC-Ni(II) 1,3-allylic shift on cyclic diene 1 and to build sterically more hindered endocyclic chiral allylic structures on demand.
Similar content being viewed by others
Introduction
Other than asymmetric allylic substitutions and Diels-Alder reactions1,2,3, cyclic 1,3-diene chiral insertion into a transition metal catalyst represents one of the most important ways to build endocyclic chiral allylic structures for drugs synthesis4. Significant advances along this line have been made by using M-boryl species, in which notable chiral ligand designs fit at low-substitution degree have been established5,6,7,8,9,10 (Fig. 1a). Yet, chiral M-Bpin insertion is less employed or often failed in higher substituted cyclic 1,3-diene8 due to the significantly different demand in steric, 1,3-allylic shift control, and insertion reactivity. Sterically less demanding chiral M–H insertion approaches can be a good solution, indeed high ee (ee = enantiomeric excess) has been achieved by using chiral phosphorus ligand and polar hydride sources as pairs for a redox-active cycle occasionally11,12,13,14 (Fig. 1b). Non-redox-active catalytic cycle and/or those use simple alkenes directly for green advances are rare15,16,17,18,19,20,21,22,23 (Fig. 1c). Very high ee and yield were observed in vinylcyclohexenes or 1-substituted cyclic 1,3-diene with ethylene by using well-designed chiral phosphorus ligand24,25. Yet, many easily accessible and commonly observed 2- or higher substituted cyclic 1,3-diene, as well as structurally diverse terminal olefin, are not ideal substrates in those systems. Complications related to undesired steric competitions and terminal olefin consumptions by both facile isomerization and oligomerization are hard to solve26. Overall, the above factors have made the former designs inapplicable for some new challenges, like catalytic synthesis of highly substituted dienes with endocyclic chiral allylic structures at high atom economy.
Recently, we have discovered a cross-hydroalkenylation of cyclic 1,3-diene 1 with heterosubstituted terminal olefin 2 by using achiral IPr-Ni(II) as a catalyst26,27,28,29,30 (IPr = 1,3-bis(2,6-diisopropylphenyl)−1,3-dihydro-2H-imidazol-2-ylidene). It prompted us to explore the potentials of using chiral NHC for solving the above problems (NHC = N-heterocyclic carbene). Herein, we develop a [chiral C2 NHC–Ni(allyl)]BArF catalyst (BArF = [3,5-bis(trifluoromethyl)phenyl]borate) for enantioselective cross-hydroalkenylation of 1 with 2 (Fig. 1d). It provides an efficient access to synthetically valuable skipped 1,4-dienes14,24,31,32 with a chiral allylic center and endo-olefin, at high ee and high regiocontrol. Using chiral C2 NHC with low substitution on N-aryls is a key for an effective chiral induction, a 1,3-shift control and a better scope of cyclic 1,3-diene33,34,35. Compared with synthetic alternatives that are based on alkenyl metal, this work overcomes challenges related to synthesis and reactivity of different leaving groups36,37,38,39.
Results
Chiral catalyst development and optimization
Our study commenced with a screening of a set of chiral [L-Ni(allyl)]BArF] catalysts (Fig. 2, 5 mol%) by using 1a and 2a (Table 1, entries 1–8, monosubstituted dienes, entries 1–3. R2 = OTES (TESO = triethylsiloxyl), which is easily accessible from α,β-unsaturated ketones, yet a rarely explored substrate). High yield and regioselectivity of the desired chiral 1,4-diene 3aa were observed by using L1 (entry 1). It featured a C1 chiral environment, and was identified as a benchmark chiral NHC for enantioselective tail-to-tail cross-hydroalkenylation of styrene with terminal olefin before29,30. Yet, only moderate ee was obtained. Using bulkier C2 chiral NHC did not help, a drop in both ee and desired reactivity were noted (L2, entry 2). It has been suggested that there might be undesired steric interactions among 2-substituted cyclic 1,3-diene and the bulky rigid NHCs employed above. So, a less bulky and more flexible C2 chiral NHC was tested next, in hope the substituent effects on the 2-substituted cyclic 1,3-diene could work with NHC together (L3, entry 3). To our delight, it provided a good balance of ee and yield without losing the high-insertion regioselectivity or causing a faster and undesired consumption of 2a. Further study showed that the size and the place of R on cyclic 1,3-diene are both very critical for achieving a good balance of desired reactivity and selectivity by using chiral NHC (entries 3–5 and 6–8). In general, higher ee could be obtained when 1 with bulky R2 rather than R1 (entry 3 vs 6, and 3 vs 4). This set of observation suggested that could be a result of an ineffective steric effect cooperation in Ni–H insertion step, and/or a facile NHC–Ni(II) 1,3-allylic shift when R2 is smaller than OTES. Yet and notably, by comparison with the optical rotation values of related structures, an efficient 1,3-allylic shift was occurred after insertion on 1a (see Supplementary Table 4 and Supplementary Fig. 8).
Scope of the enantioselective cross-hydroalkenylation and 1,4-diene
Above findings prompted us to elucidate other factors on cyclic 1,3-diene 1 that might favor high ee and to give greater structural diversity of 1,4-diene 3 by using chiral NHC (Table 1, entries 9–12, 1,2-disubstituted dienes). Several notable features and tactics were revealed by this study. First, in sharp contrast to the above and most of the related literature, this study showed that using large OSiR3 at R2 is not mandatory for achieving high ee and 3:4 ratio at here. High selectivity also can be obtained by using 1,2-disubstituted cyclic 1,3-diene 1 (entry 9–12). The higher ee observed in 1 g vs 1a was attributed mainly to a more difficult 1,3-allylic shift and the restricted OTES rotation by the Me at R1 (entry 3 vs 9). More dramatically, ee value was doubled when 1i over 1d was used (i.e., R2 = Me vs H, 96% vs 48% ee). So, the steric effect of R1 and R2 cooperates, where the choice of R2 can be as small as a Me, and R1 can be inadequate in size to favor a high ee insertion by itself alone.
Further screening showed that the above cooperation is invaluable in expanding scope to a broader range of 1,4-diene 3 at high ee. It represents a general high ee method to avoid extensive use of very bulky R2 or R1 on cyclic 1,3-diene (Fig. 3), which is a major obstacle and limitation in many cases before. It accepts a good variety of R2 and functional groups, like higher substituted alkyls, ethers, and Bn without impairing the desired selectivity at high ee and yield (Set 1a, 88–97% ee, 3:4 > 95:5 in most cases). The success of using Br as R2 is notable since it can be a useful handle for cross-coupling and as a masked H by reduction. So, it offers a key for having greater 3 structural varieties at high ee. Heterocyclic substituent and 1,4-disubstitutions also work well with L3-Ni(II) catalyst for desired reactivity and high ee (Fig. 3, Set 2–3, 84–98% ee). At here, two new stereocenters could be obtained in 1,4-substituted diene at trans-selectivity. At this stage, cyclic 1,3-diene substituted with a ketone did not work well.
Superscript “a” indicates the standard condition employed in this figure: [L3-Ni(allyl)]BArF catalyst (5 mol%, 0.05 mmol), cyclic 1,3-diene 1: heterosubstituted terminal olefin 2 = 1:3, in 4 mL of toluene at r.t. for 6 h. Yield, chiral 1,4-diene 3:4 or 3:3’ or 3’:4 ratio were determined by 1H NMR spectroscopy, except otherwise indicated. No chiral 1,4-diene 3’ was observed, except in Set 8. Major regioisomer’s structure was confirmed by isolation and enantiomeric excesses were determined by HPLC. Superscript “b and c” indicate the amount of catalyst employed, which is 10 mol% and 20 mol%, respectively. Superscript “d” indicates an extra 1 equiv. of heterosubstituted terminal olefin 2 was added after 2 h. Superscript “e” indicates L1 was used instead of L3. Superscript “f” indicates that heterosubstituted terminal olefin 2 was added in three equal portions in 6 h. Superscript “g” indicates taht the reaction was carried out at 0 °C. Superscript “h” indicates the ratio of cyclic 1,3-diene 1 to heterosubstituted terminal olefin 2 here was 1:1.5, and reaction time was 11 h. Superscript “i” indicates that reaction time was 15 h. Superscript “j” indicates the ee value of chiral 1,4-diene 3’. Superscript “k” indicates that 4 was also observed in those entries and in 27, 17, and 8% yield, respectively.
The fine-tuned chiral L3-Ni(II) reactivity and chiral induction ability on substituted cyclic 1,3-diene came with also a broad scope of 2 (Fig. 3, Sets 4–6). High ee, 3:4 ratio and yield were observed under general condition. That is not straightforward since inefficient insertion of terminal olefin 2 by undesired steric interactions could lead to backward reaction, lower yield, and ee. Competing pathways, like isomerization/oligomerization of 2 and insertion of 2 on hydrometallated cyclic 1,3-diene minor enantiomer, could be more competitive. Yet, linear, branch, cyclic, aliphatic, and aromatic groups on heterosubstituted terminal olefin 2 were found quite effective (88–97% ee, >95% yield, 3:4 > 95:5). Only oversized NBnTs and protic NHTs (Bn = benzyl, Ts = tosyl) on heterosubstituted terminal olefin 2 were found inefficient and incompatible, respectively. Notably, using optimal size of heterosubstituted terminal olefin 2 was found useful in scope expansion of cyclic 1,3-diene and in selectivity improvement. Indeed, higher ee, yield, and 3:4 ratio were achieved by using allylNMeTs or allylOPh over 2a in Set 1b vs Table 1 entry 9 (3:4 = >95:5 vs 68:32, 92–96% ee). Such advance is mainly attributed to a relatively slower undesired 2 consumption, and a higher steric demand in heterosubstituted terminal olefin 2 insertion to the minor enantiomer of the hydrometallated cyclic 1,3-diene. Our design also allowed chemoselective insertion of terminal over internal or gem-substituted allylic ether (Set 5). It was shown that those diallyl ether 2 cycloisomerizations40 were not as competitive as our desired intermolecular skipped diene 3 formation. Heteroatom effect of 2 is important on 3:4 ratio (Set 6)26.
Higher substituted cyclic 1,3-diene with R5 or R6 were examined in hope to elucidate how those rarely explored substitution patterns in the literature might affect our chiral NHC system (Set 7). Indeed, their effects on both reactivity and ee are quite strong. Some of those patterns were found devastating to our L3-Ni(II) catalyst chiral induction effects and reactivity when cyclic 1,3-diene substituted with R1 = OTES was used (e.g., Set 7, 4–67% ee). Yet, the cooperation of R1 and R2 was found applicable, and 96% ee was achieved.
Finally, further study on the substitution patterns on cyclic 1,3-diene and properties on heterosubstituted terminal olefin have revealed a fascinating way to control NHC–Ni(II) 1,3-allylic shift on the rings (Set 8). A high selectivity of 3:3′ and vice versa were noted in several cases of 1 with R1 = H, e.g., 5–7 member cyclic dienes (3′ refers to 1,3-shifted product)28. Besides steric effect on cyclic 1,3-diene, the results showed that both steric and electronic properties of heterosubstituted terminal olefin are related to 1,3-allyl shift regulation [as reflected by 3:3′ ratio, R3 = NMeTs, O(C6H4)p-OMe vs p-CF3] on a given structure of cyclic 1,3-diene and NHC–Ni(II) catalyst. This feature is uniquely useful as the heterosubstituted terminal olefin regioselective insertion involved in forming 3′ is sterically more shielded than in 3 and 4 by simple inspection. Thus, 1,3-allylic shift on the ring with this L3-Ni(II) species may not be so highly reversible as typically assumed under this circumstances, and in turn affected the regioselective insertion of heterosubstituted terminal olefin.
Discussion
At this stage, the keys for the highly asymmetric synthesis of skipped diene 3 with broad scope are attributed mainly to (i) optimal chiral NHC–Ni(II) insertion reactivity, (ii) an effective 1,3-allylic shift control, and (iii) a highly regioselective insertion reactivity of heterosubstituted terminal olefin by the cooperation of chiral NHC–Ni and substrate, in which all of them were done under mild condition (Fig. 4, Models a–c and d, respectively). Here, the chiral C2 NHC adopts a conformation as shown in Fig. 4, Models a–c41,42,43, which is correlated to the absolute configuration of 3 obtained (see Supplementary Fig. 8). Effective steric interaction among cyclic 1,3-diene and Cy on the L3 N-aryl determines the Model a–c ratio. Due to the reversibility of cyclic 1,3-diene hydrometallation, an ineffective insertion of heterosubstituted terminal olefin to Model a and more effective insertion of heterosubstituted terminal olefin to Model b and c lower the product ee, and vice versa (e.g., NMeTs vs NBnTs).
In addition to the above, the formation of 3′ at high ee may occur when R1 = H. That is attributed to a relatively slower insertion of terminal olefin to Models a–c, and a faster L3-Ni(II) 1,3-allylic shift than backward reaction. Indeed, the allylic shift of L3-Ni(II) from 3- to 1-position on cyclic 1,3-diene is quite favorable in general, even in the absence of help from R5 and/or R6 (Fig. 5 vs Fig. 3, Set 8). In other words, the higher ee obtained in R2 = OTES (Table 1 entry 3 vs 4, 5) was not a direct result of steric suppressed NHC–Ni(II) 1,3-allylic shift. It should be attributed to the difference in insertion efficiency of heterosubstituted terminal olefin (C–C forming step) before and after the 1,3-shift, as well as the degree of the concerned 1,3-shift equilibrium, likely by creating a different steric insertion demand with the help of the chiral NHC environment. Notably, the high 3′:3 selectivity or vice versa are not controlled by steric effects only. Those are tunable also by the electronic properties of heterosubstituted terminal olefin (Set 8a, R3 = OAryl). This shows that using a more electron-rich alkene is possible to overcome the undesired steric insertion barrier at the more hindered 1-position. And the L3-Ni(II) design strongly favors 1- over the 3-position on 2-substituted cyclic 1,3-diene, particularly at lower temperature.
According to Model d, preparation of 4 might be enabled by stronger steric repulsion among substituents on heterosubstituted terminal olefin and R2 on cyclic 1,3-diene 1 vs NHC. Indeed, the high 3:4 ratio was diminished when 1i was used in place of 1d (Table 1, entry 12 vs 9, >95:5 vs 68:32). Moreover, the use of homoallylic over allylic ether as heterosubstituted terminal olefin was found useful to assist 4 formation under otherwise the same condition (Fig. 3, Set 6, >95:5 vs 71:29). At this stage, it was attributed mainly to a potential Ni-ether coordination (Model e).
Synthetically, the silyl enol ether on 3 gives us a versatile handle for post modifications (for alkylation example, see ref. 44; for aldol reaction example, see ref. 45; for Paterno-Buechi reaction example, see ref. 46. Extra chiral centers can be acquired expediently at high efficiency by those robust chemistry. For instance, both syn- and anti-products were made selectively by simple deprotection (Fig. 6).
In conclusion, an enantioselective cross-hydroalkenylation of cyclic 1,3-diene 1 and heterosubstituted terminal olefin 2 to give 1,4-diene 3 or 3′ with endocyclic chiral allylic structures was established by manipulating the relative insertion reactivity of 1 and 2 on chiral NHC–Ni(II) catalyst (up to 99% ee, 86–96% ee in general). Here, the design covers a broad range of 1 and shows a good ability in chiral NHC–Ni(II) 1,3-allylic shift regulation, where both are keys in related asymmetric NHC–Ni catalysis development in general. Selective synthesis of sterically crowded diene 3′ and the electronic effect of heterosubstituted terminal olefin 2 on regioselective insertion are unexpected, which have prompted further study in near future. Overall, this work has shed light on how to use chiral NHC–Ni for chiral allylic centers preparation and for better chiral 1,4-dienes availability from cyclic 1,3-diene 1 and heterosubstituted terminal olefin 2 directly.
Methods
General procedure for enantioselective cross-hydroalkenylation
To a [L3-Ni(Allyl)]BArF catalyst in toluene (0.05 mmol, 5 mol%, 2 mL) with 20 mol% 1-octene (for generation of NiH or equiv.)26,27,28,29,30, a toluene solution of cyclic 1,3-diene 1 and heterosubstituted terminal olefin 2 (1:3, 2 mL) was added in one-pot and stirred at r.t. for 6 h. After work up, the 1,4-diene yield, structure and selectivity (3:4 or 3:3’) were determined by 1H NMR, HPLC and isolation (average of two runs).
Data availability
All data generated during/or analyzed during the current study are included in this published article and its supplementary files.
References
Kazmaier, U. (ed.) Transition Metal Catalyzed Enantioselective Allylic Substitution in Organic Synthesis 1–345 (Springer, 2012).
Jiang, X. X. & Wang, R. Recent developments in catalytic asymmetric inverse-electron-demand Diels-Alder reaction. Chem. Rev. 113, 5515–5546 (2013).
Kagan, H. B. & Riant, O. Catalytic asymmetric Diels-Alder reactions. Chem. Rev. 92, 1007–1019 (1992).
Herrmann, N., Vogelsang, D., Behr, A. & Seidensticker, T. Homogeneously catalyzed 1,3-diene functionalization—a success story from laboratory to miniplant scale. ChemCatChem 10, 5342–5365 (2018).
Hong, K. & Morken, J. P. Catalytic enantioselective diboration of cyclic dienes. A modified ligand with general utility. J. Org. Chem. 76, 9102–9108 (2011).
Ely, R. J. & Morken, J. P. Ni(0)-catalyzed 1,4-selective diboration of conjugated dienes. Org. Lett. 12, 4348–4351 (2010).
Burks, H. E., Kliman, L. T. & Morken, J. P. Asymmetric 1,4-dihydroxylation of 1,3-dienes by catalytic enantioselective diboration. J. Am. Chem. Soc. 131, 9134–9135 (2009).
Sasaki, Y., Zhong, C. M., Sawamura, M. & Ito, H. Copper(I)-catalyzed asymmetric monoborylation of 1,3-dienes: synthesis of enantioenriched cyclic homoallyl- and allylboronates. J. Am. Chem. Soc. 132, 1226–1227 (2010).
Schuster, C. H., Li, B. & Morken, J. P. Modular monodentate oxaphospholane ligands: utility in highly efficient and enantioselective 1,4-diboration of 1,3-dienes. Angew. Chem. Int. Ed. 50, 7906–7909 (2011).
Sardini, S. R. & Brown, M. K. Catalyst controlled regiodivergent arylboration of dienes. J. Am. Chem. Soc. 139, 9823–9826 (2017).
Hayashi, T. et al. Modification of chiral monodentate phosphine ligands (MOP) for palladium-catalyzed asymmetric hydrosilylation of cyclic 1,3-dienes. Adv. Synth. Cat. 343, 279–283 (2001).
Yang, X. H., Davison, R. T. & Dong, V. M. Catalytic hydrothiolation: regio- and enantioselective coupling of thiols and dienes. J. Am. Chem. Soc. 140, 10443–10446 (2018).
Lober, O., Kawatsura, M. & Hartwig, J. F. Palladium-catalyzed hydroamination of 1,3-dienes: a colorimetric assay and enantioselective additions. J. Am. Chem. Soc. 123, 4366–4367 (2001).
Duvvuri, K. et al. Cationic Co(I)-intermediates for hydrofunctionalization reactions: regio- and enantioselective cobalt-catalyzed 1,2-hydroboration of 1,3-dienes. J. Am. Chem. Soc. 141, 7365–7375 (2019).
RajanBabu, T. V. Asymmetric hydrovinylation reaction. Chem. Rev. 103, 2845–2860 (2003).
Jing, S. M., Balasanthiran, V., Pagar, V., Gallucci, J. C. & RajanBabu, T. V. Catalytic enantioselective hetero-dimerization of acrylates and 1,3-dienes. J. Am. Chem. Soc. 139, 18034–18043 (2017).
Page, J. P. & RajanBabu, T. V. Asymmetric hydrovinylation of 1-vinylcycloalkenes. Reagent control of regio- and stereoselectivity. J. Am. Chem. Soc. 134, 6556–6559 (2012).
Timsina, Y. N., Sharma, R. K. & RajanBabu, T. V. Cobalt-catalysed asymmetric hydrovinylation of 1,3-dienes. Chem. Sci. 6, 3994–4008 (2015).
Hirano, M. et al. Mechanistic insights into catalytic linear cross-dimerization between conjugated dienes and styrenes by a ruthenium(0) complex. J. Organomet. Chem. 797, 174–184 (2015).
Hirano, M. Recent advances in the catalytic linear cross-dimerizations. Acs. Catal. 9, 1408–1430 (2019).
Hilt, G. Hydrovinylation reactions—atom-economic transformations with steadily increasing synthetic potential. Eur. J. Org. Chem. 2012, 4441–4451 (2012).
Hilt, G. Asymmetric nickel-catalysed cross-hydrovinylation of two terminal alkenes. ChemCatChem 7, 1639–1641 (2015).
Hilt, G. Cobalt(I)-catalysed reactions for the synthesis of acyclic 1,4-dienes-genesis of two synthetic methods. Synlett 2011, 1654–1659 (2011).
Zhang, A. & RajanBabu, T. V. Hydrovinylation of 1,3-dienes: a new protocol, an asymmetric variation, and a potential solution to the exocyclic side chain stereochemistry problem. J. Am. Chem. Soc. 128, 54–55 (2006).
Saha, B. & RajanBabu, T. V. Nickel(0)-catalyzed asymmetric hydrocyanation of 1,3-dienes. Org. Lett. 8, 4657–4659 (2006).
Lian, X. Y., Chen, W. H., Dang, L., Li, Y. C. & Ho, C. Y. (NHC)NiH-catalyzed intermolecular regio- and diastereoselective cross-hydroalkenylation of endocyclic dienes with alpha-olefins. Angew. Chem. Int. Ed. 56, 9048–9052 (2017).
Chen, W. H., Li, Y., Chen, Y. & Ho, C. Y. (NHC)NiH-catalyzed regiodivergent cross-hydroalkenylation of vinyl ethers with a-olefins: syntheses of 1,2-and 1,3-disubstituted allyl ethers. Angew. Chem. Int. Ed. 57, 2677–2681 (2018).
Huang, J. Q. & Ho, C. Y. [(NHC)NiIIH]-catalyzed cross-hydroalkenylation of cyclopropenes with alkynes: cyclopentadiene synthesis by [(NHC)NiII]-assisted C-C rearrangement. Angew. Chem. Int. Ed. 58, 5702–5706 (2019).
Ho, C. Y. & He, L. S. Catalytic intermolecular tail-to-tail hydroalkenylation of styrenes with alpha olefins: regioselective migratory insertion controlled by a nickel/N-heterocyclic carbene. Angew. Chem. Int. Ed. 49, 9182–9186 (2010).
Ho, C. Y., Chan, C. W. & He, L. S. Catalytic asymmetric hydroalkenylation of vinylarenes: electronic effects of substrates and chiral N-heterocyclic carbene ligands. Angew. Chem. Int. Ed. 54, 4512–4516 (2015).
Hilt, G., du Mesnil, F. X. & Luers, S. An efficient Cobalt(I) catalyst system for the selective 1,4-hydrovinylation of 1,3-dienes. Angew. Chem. Int. Ed. 40, 387–389 (2001).
Sharma, R. K. & RajanBabu, T. V. Asymmetric hydrovinylation of unactivated linear 1,3-dienes. J. Am. Chem. Soc. 132, 3295–3297 (2010).
Wang, F. J., Liu, L. J., Wang, W. F., Li, S. K. & Shi, M. Chiral NHC-metal-based asymmetric catalysis. Coord. Chem. Rev. 256, 804–853 (2012).
Janssen-Muller, D., Schlepphorst, C. & Glorius, F. Privileged chiral N-heterocyclic carbene ligands for asymmetric transition-metal catalysis. Chem. Soc. Rev. 46, 4845–4854 (2017).
Yong, X. F., Thurston, R. & Ho, C. Y. Electronic effects on chiral NHC-transition-metal catalysis. Synthesis 51, 2058–2080 (2019).
Lee, Y., Akiyama, K., Gillingham, D. G., Brown, M. K. & Hoveyda, A. H. Highly site- and enantioselective Cu-catalyzed allylic alkylation reactions with easily accessible vinylaluminum reagents. J. Am. Chem. Soc. 130, 446–447 (2008).
Hamilton, J. Y., Sarlah, D. & Carreira, E. M. Iridium-catalyzed enantioselective allylic alkynylation. Angew. Chem. Int. Ed. 52, 7532–7535 (2013).
Gao, F., Carr, J. L. & Hoveyda, A. H. A broadly applicable NHC-Cu-catalyzed approach for efficient, site-, and enantioselective coupling of readily accessible (pinacolato)alkenylboron compounds to allylic phosphates and applications to natural product synthesis. J. Am. Chem. Soc. 136, 2149–2161 (2014).
Ho, C.-Y., Ohmiya, H. & Jamison, T. F. alpha-Olefins as alkenylmetal equivalents in catalytic conjugate addition reactions. Angew. Chem. Int. Ed. 47, 1893–1895 (2008).
Ho, C.-Y. & He, L. Medium-sized heterocycle synthesis by the use of synergistic effects of Ni-NHC and gamma-coordination in cycloisomerization. J. Org. Chem. 79, 11873–11884 (2014).
Berlin, J. M., Goldberg, S. D. & Grubbs, R. H. Highly active chiral ruthenium catalysts for asymmetric cross- and ring-opening cross metathesis. Angew. Chem. Int. Ed. 45, 7591–7595 (2006).
Seiders, T. J., Ward, D. W. & Grubbs, R. H. Enantioselective ruthenium-catalyzed ring-closing metathesis. Org. Lett. 3, 3225–3228 (2001).
Chaulagain, M. R., Sormunen, G. J. & Montgomery, J. New N-heterocyclic carbene ligand and its application in asymmetric nickel-catalyzed aldehyde/alkyne reductive couplings. J. Am. Chem. Soc. 129, 9568–9569 (2007).
Stoltz, B. M. et al. in Comprehensive Organic Synthesis 2nd edn (eds Knochel, P. & Molander, G. A.) 1–55 (Elsevier, 2014).
Heathcock, C. H. in Comprehensive Organic Synthesis 2nd edn (eds Knochel, P. & Molander, G. A.) 340–395 (Elsevier, 2014).
D’Auria, M. in Comprehensive Organic Synthesis 2nd edn (eds Knochel, P. & Molander, G. A.) 159–199 (Elsevier, 2014).
Acknowledgements
L.D. thanks NSFC (21602099). C.Y.H. thanks R. H. Grubbs (Shenzhen Nobel Prize Scientists Laboratory Project C17783101), Guangdong Provincial Key Laboratory of Catalysis (2020B121201002), SZ research fund (JCYJ 20170817105041557) and SUSTech (Y01501808, Y01506014). We thank Xuran Yao for initial attempts in cycloheptadiene case.
Author information
Authors and Affiliations
Contributions
L.D. and Y.C. developed the chiral catalyst for this methodology, analyzed the results, synthesized and characterized the products obtained. L.D., Y.C., and C.Y.H. participated the discussion.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Fanke Meng and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, Y., Dang, L. & Ho, CY. NHC-Ni catalyzed enantioselective synthesis of 1,4-dienes by cross-hydroalkenylation of cyclic 1,3-dienes and heterosubstituted terminal olefins. Nat Commun 11, 2269 (2020). https://doi.org/10.1038/s41467-020-16139-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-020-16139-2
This article is cited by
-
(NHC)Pd(II) hydride-catalyzed dehydroaromatization by olefin chain-walking isomerization and transfer-dehydrogenation
Nature Communications (2022)
-
Synthesis of tri- and tetrasubstituted stereocentres by nickel-catalysed enantioselective olefin cross-couplings
Nature Catalysis (2022)
-
NHC-Ni(II)-catalyzed cyclopropene-isocyanide [5 + 1] benzannulation
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.