Abstract
Catalytic asymmetric functionalization of the N–H groups of indoles and carbazoles constitutes an important but less developed class of reactions. Herein, we describe a propargylation protocol involving the use of a lithium SPINOL phosphate as the chiral catalyst and our recently developed C-alkynyl N,O-acetals as propargylating reagents. The direct asymmetric N-propargylation of indoles and carbazoles provides hitherto inaccessible N-functionalized products. Notably, the efficiency of the system allows reactions to be run at a very low catalyst loading (as low as 0.1 mol%). Mechanistic information about the titled reaction is also disclosed. This study represents an advance in the direct asymmetric functionalization of the N–H bonds of indoles and carbazoles, and additionally expands on the application of chiral alkali metal salts of chiral phosphoric acids in asymmetric catalysis.
Similar content being viewed by others
Introduction
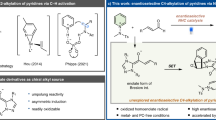
The synthesis of enantioenriched indoles is of wide interest, owing to their prevalence in natural products and pharmaceuticals, and agrochemicals. Therefore, extensive efforts have been devoted to developing catalytic asymmetric methods for the functionalization of indoles1,2. Great progress has been made in the catalytic asymmetric C-alkylation, especially at the C3 position, due to the innate nucleophilicity of C3 of the indole ring. In contrast, the catalytic asymmetric functionalization of the N–H of indole, particularly in an intermolecular manner, has remained underdeveloped3,4,5,6,7,8,9,10,11,12,13,14,15,16, owing to the mitigated nucleophilicity of this position. To avoid the regioselectivity issue, several indirect strategies have also been elegantly designed17,18,19,20,21. To date, efficient strategies for the asymmetric N-functionalization of indoles are still rather limited and most reported intermolecular reactions relied on N-allylation. Very recently, the catalytic asymmetric N-benzylation has been elegantly developed to afford chiral N-benzylic indoles22,23,24,25,26,27. Nevertheless, despite these elegant achievements, there is still no report for the direct catalytic asymmetric functionalization of the N–H of indole through a propargylation strategy.
Catalytic asymmetric propargylation has been recognized as an important class of reactions28 in organic chemistry, because they create a propargylic chiral center and introduce a synthetically versatile and biologically important alkyne functional group in one step29. Although great efforts have been devoted in this area, the potential of catalytic asymmetric propargylations has not been fully exploited and the scope of applicable nucleophiles and electrophiles is still rather limited. The direct catalytic asymmetric N-propargylation of indoles and carbazoles as nucleophiles has not yet been achieved. In addition, there are only limited types of chiral catalyst systems for the asymmetric propargylation to date.
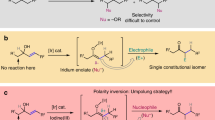
The direct catalytic asymmetric N-propargylations using 1H-indole derivatives as nucleophiles have proven far more challenging than allied reactions such as allylations and have not been successful. Indeed, You and colleagues30,31 studied the reaction of 2,3-dimethyl indole with propargylic acetates using a chiral copper–pybox complex as the catalyst, but only obtained the C-propargylated product (Fig. 1). Thus, the challenges in the development of the direct catalytic asymmetric N-propargylation of indoles and carbazoles as the nucleophiles are to find a suitable propargylating reagent and an efficient catalyst system.
We recently introduced C-alkynyl N-Boc- and N-Cbz-protected N,O-acetals as a class of coupling partners for asymmetric catalytic transformations; to date, these transformations have been limited to the formation of C–C bonds with the use of carbon-based nucleophiles (Fig. 2a)32,33,34,35. However, the corresponding asymmetric reaction of C-alkynyl N,O-acetals through carbon–heteroatom bond formation has remained an unmet task. Given the lack of direct methods available for the catalytic asymmetric N-propargylation of indoles and carbazoles, we chose to explore the possibility that 1H-indoles and carbazoles36 might serve as the first effective heteroatom-based nucleophiles to react with C-alkynyl N,O-acetals (Fig. 2b). Such reaction would provide straightforward access to chiral alkynylated acyclic N,N-aminals of indoles and carbazoles. Recent studies have shown that acyclic N,N-aminal indoles I–III have significant antibiotic properties (Fig. 2c)37,38,39,40. Thus, there is a need for organocatalytic methods that enable the enantioselective synthesis of acyclic N,N-aminals of indoles or carbazoles, biologically important yet synthetically challenging molecules bearing an acyclic N,N-substituted α-chiral carbon center on the N1-position of indoles or carbazoles. Meanwhile, incorporating alkynes into N,N-aminals is made interesting, owing to the versatile transformations of the alkyne group and the ubiquitous occurrence as a key structural motif in natural products and pharmaceuticals. However, the direct catalytic asymmetric synthesis of N,N-aminals through a propargylation method has not been reported.
On the other hand, chiral alkali and alkaline earth metal-derived salts of chiral phosphoric acids have recently emerged as a class of effective catalysts in various asymmetric transformations41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60. However, this class of chiral catalysts have not been successfully employed in the catalytic asymmetric functionalization of the N–H of indoles or carbazoles. Meanwhile, the direct catalytic asymmetric propargylation reaction catalyzed by chiral alkali or alkaline earth-derived salts of chiral phosphoric acids has remained elusive.
Besides these, there are several other challenges for the catalytic enantioselective C–N bond formation between indoles and carbazoles, and C-alkynyl N,O-acetals. C-alkynyl N,N-aminals have been reported to react with EtOH to form the corresponding C-alkynyl N,O-acetals61. Thus, unlike the products generated by C-based nucleophiles, for the products produced by N-centered nucleophiles, there is a risk of a direct conversion of newly formed C-alkynyl N,N-aminal products back to the C-alkynyl N,O-acetals (starting materials). In addition, C-alkynyl N,N-aminals reacting with EtOH would also lead to product racemization. Second, due to the low intrinsic nucleophilicity of the N–H motif of indoles and carbazoles, together with low reactivity of C-alkynyl N-Boc or N-Cbz N,O-acetals, high reaction temperature might be needed for the N-propargylation, which cause difficulties in selective control. Moreover, the C3 position of the indole could compete with the nitrogen atom as the nucleophile, as exemplified by You and colleagues30 in the catalytic asymmetric indole C-propargylation reaction. Finally, chiral alkali and alkaline earth salts of chiral phosphoric acids have been shown to efficiently catalyze the addition of alcohols to imines, to form the corresponding N,O-acetals (see Fig. 2b)57. In contrast, can this class of catalysts catalyze the elimination of alcohols from N,O-acetals to generate the corresponding C-alkynyl N-Boc or N-Cbz imines? Unlike C-aryl N-Boc- or N-Cbz-protected imines, C-alkynyl N-Boc- or N-Cbz-protected imines cannot be prepared by existing methods. Such imines have also proven more difficult to be generated by the traditional methods. Amidosulfones62,63,64,65,66,67, which are widely used imine precursors, are not suitable for this purpose. This process must overcome the potential 1,4-addition onto the alkynyl imines68.
Herein, we report the development of highly enantioselective direct catalytic asymmetric N-propargylation of indoles and carbazoles. Mechanistic investigations are also disclosed.
Results
Reaction development
We first explored the N-propargylation of carbazole 2a with our C-alkynyl N,O-acetal 1a (Table 1). Chiral bifunctional Brønsted base catalysts such as BB1 and BB2, which have been shown to be efficient in the asymmetric reaction of C-alkynyl N,O-acetals with carbon-based nucleophiles32, failed to promote the reaction, while chiral phosphoric acids33 promoted the model reaction between C-alkynyl N,O-acetal 1a and carbazole 2a but with very poor enantioselectivity (Table 1, entries 1 and 2). These results highlight the challenges of developing the proposed catalytic asymmetric N-propargylation. These results prompted us to identify an alternative organocatalyst that must be capable of both catalyzing the EtOH elimination from N,O-acetals to generate challenging N-Cbz-protected C-alkynyl imines and promoting the subsequent N-propargylation reaction, as well as imposing effective stereocontrol. After extensive investigations, we found that chiral alkali and alkaline earth metal-derived salts of chiral phosphoric acids were promising chiral catalysts for the tandem process combining the in situ generation of C-alkynyl N-Cbz imines and N-propargylation. Among them, a lithium SPINOL phosphate, Li[P2], catalyzed the EtOH elimination from N,O-acetal 1a for in situ generation of difficult accessible C-alkynyl N-Cbz imines and the subsequent asymmetric N-propargylic alkylation with 93% enantiomeric excess (ee) (Table 1, entry 3).
Mechanistic investigations
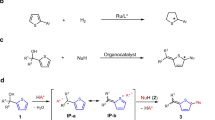
To get an understanding of the N-propargylation, we monitored the reaction of racemic 1a with 2a under the standard conditions by determining ee for both the generated product 3a and the recovered substrate 1a over time (Fig. 3a). It was found that after a reaction time of 6 h, 1a was recovered with 49% ee. With the reaction time further increasing, ee of the recovered 1a began to decrease. Meanwhile, ee of the product 3a was decreased relatively slowly (for details, see Supplementary Table 2).
a Enantioselectivity of the product 3a and the recovered substrate 1a over time. b The reaction of racemic 1a with EtOH under the chiral catalysis of Li[P2]. c The reaction of chiral 1a with EtOH under the chiral catalysis of Li[P2]. d Crossover experiment. e The reaction of chiral 3a with 2a under the chiral catalysis of Li[P2]. f The reaction of chiral 3a with EtOH under the chiral catalysis of Li[P2]. g Proposed reaction mechanism.
To investigate whether the enantioriched 1a arised from the reaction of racemic 1a with EtOH under the chiral catalysis of Li[P2], we performed the reaction between racemic 1a and EtOH in the presence of (R)-Li[P2]. The recovered 1a was still racemic and no chiral induction was observed (Fig. 3b), thus excluding such possibility. This result combined with the experiment shown in Fig. 3a implied there might be a kinetic resolution during the process between racemic 1a and 2a in the presence of Li[P2]. To further confirm this issue, we conducted kinetic studies. The (+)-1a (97% ee) and (−)-1a (97% ee) were reacted with 2a in the presence of (R)-Li[P2] as the catalyst, respectively. We found that both enantiomers of 1a delivered the same enantiomer of the product, (R)-3a, regardless of the original configuration of the C-alkynyl N,O-acetal, and the reaction with (+)-1a was faster than that of (−)-1a (Fig. 4. For details, see Supplementary Table 3), thus verifying that there was a moderate degree of kinetic resolution of 1a during the reaction with 2a.
To understand why the recovered 1a and the product 3a decreased gradually with the reaction time further increasing and to gain further insight into the N-propargylation, we made some control experiments. The experiment shown in Fig. 3c (for details, see Supplementary Tables 4 and 5) explained a possible reason for the decrease of enantioselectivity of the recovered 1a over the time. The crossover experiment shown in Fig. 3d (for details, see Supplementary Table 6) indicated that the chiral catalyst was discriminating the two enantiomers of the C-alkynyl N,N-aminal and (R)-3a was converted into the corresponding C-alkynyl N-Cbz imine more faster than (S)-3a in the presence of Li[P2] (there was a kinetic resolution of 3a during this process). These results combined with the control experiments in Fig. 3e (for details, see Supplementary Table 7) and Fig. 3f (for details, see Supplementary Table 8) explained a possible reason for the decrease of ee of the product 3a over the time. The experiment in Fig. 3f also suggested that the alkynylated N,N-aminal can react with EtOH to form the corresponding N,O-acetal. Pleasingly, the racemization of the product 3a was found to be effectively suppressed by increasing the amount of the C-alkynyl N,O-acetal substrate 1a to 1.6 eq. (for details, see Supplementary Table 9).
Next, we studied the relationship between the ee of the catalyst Li[P2] and that of the product 3a69, and a linear effect was observed (for details, see Supplementary Table 10). On the basis of the above observations, we proposed a possible reaction mechanism shown in Fig. 3g.
Substrates scope
With the optimized chiral catalyst and suitable propargylic alkylating reagent in hand, we investigated the scope of the direct catalytic asymmetric N-propargylation of carbazoles. Various C-alkynyl N-Cbz N,O-acetals 1 were reacted with carbazoles 2 to afford the desired N-propargylated products (3a–3h) in high enantioselectivities with good yields (Fig. 5). Moreover, this system was also applicable for the enantioselective N-propargylation of indoles (Fig. 6). The reaction displayed broad substrate scope of both C-alkynyl N,O-acetals and substituted indoles. The corresponding N-propargylic indoles 5a–z were obtained in good yields with high to excellent enantioselectivities (up to 99% ee) in all the cases examined (26 examples). Notably, in contrast to the work by You and colleagues30, our propargylation reactions occurred at the N1-position exclusively and no competing C3-propargylaed product was observed. On the other hand, impressively, the reaction at high temperature (110 °C) can still provide excellent enantioselectivity (5s). Examples with high stereocontrol at such a high temperature are scarce in asymmetric catalysis by chiral alkali and alkaline earth-derived salts of chiral phosphoric acids.
Product configuration determination and transformations
The absolute configuration of the N-propargylation products was determined by converting 5k into 6 (Fig. 7a), and the structure of 6 was unambiguously confirmed through X-ray crystal analysis. Reduction of 5a with LiAlH4 afforded 7, which is formally derived from the N-selective and 1,2-selective addition of α,β-unsaturated imine (Fig. 7b). When 5a was reduced with Pd/C under H2 atmosphere, the primary alkyl-substituted product 8 was obtained in quantitative yield.
Low catalyst-loading experiments
Promoted by the unusual stereocontrol of Li[P2], we examined the N-propargylation reaction with low catalyst loading (Table 2). We found that when the catalyst loading was decreased from 5 to 0.5 mol%, the yield and enantioselectivity were not affected (Table 2, entry 3 vs. entries 1 and 2). Further decreasing the catalyst loading to 0.2 mol% has only a slight impact on the enantioselectivity (Table 2, entry 4). Even 0.1 mol% catalyst loading can still provide high enantioselectivity and yield (Table 2, entry 5). This is the lowest catalyst loading that has been achieved so far for the asymmetric catalysis by chiral alkali and alkaline earth-derived salts of chiral phosphoric acids70.
N-benzylation of indoles
Finally, we explored the direct catalytic asymmetric N-benzylation of indoles with C-aryl N-Boc N,O-acetals in the presence of (R)-Li[P2]. Interestingly, the reaction did not occur. These results indicate the reactivity difference between C-alkynyl N-Boc N,O-acetals and C-aryl N-Boc N,O-acetals. Considering that C-aryl N-Boc imines can be prepared, we turned our attention to the use of C-aryl N-Boc imines preformed. By utilizing a complementary catalytic mode, hydrogen bonding enantiocontrol, we developed an enantioselective N-benzylation of indoles using a chiral phosphoric acid PA1 as the catalyst (Fig. 8).
Discussion
In summary, we have developed an organocatalytic strategy for the direct asymmetric N-functionalization of indoles and carbazoles through a propargylation. Specifically, we have successfully realized the direct catalytic asymmetric intermolecular N-propargylation of indoles and carbazoles, providing hitherto inaccessible acyclic N,N-aminals of indoles and carbazoles in good yields with high enantiocontrol, despite many potential challenges of this reaction. This C–N formation process is enabled by the use of our newly developed C-alkynyl N,O-acetals as the alkylating reagent and a lithium SPINOL phosphate as the chiral catalyst. The product racemization has been suppressed effectively. A chiral lithium SPINOL phosphate catalyst promotes the activation of N,O-acetals for the in situ formation of challenging accessible C-alkynyl N-Boc or N-Cbz imines, although this class of catalysts were previously employed for the addition of alcohols to imines to form N,O-acetals. Notably, the efficiency of the system allows reactions to be run at a very low catalyst loading of 0.1 mol%. The present protocol represents a significant advance in the asymmetric functionalization of the N–H of indoles and carbazoles, and in the catalytic asymmetric propargylation, as well as opens an application of chiral alkali and alkaline earth-derived salts of chiral phosphoric acids in the asymmetric catalysis and synthesis.
Methods
General procedure for the N-propargylation of carbazoles 2
To a solution of 1 (0.08 mmol) and 2 (0.05 mmol) in toluene (1.0 mL) was added the catalyst (R)-Li[P2] (1.8 mg, 5 mol %) at 90 °C. After stirring for 12 h, the mixture was directly purified by silica gel chromatography (ethyl acetate/petroleum ether = 1/30 to 1/20) to afford the products 3.
General procedure for the N-propargylation of indoles 4
To a solution of 1’ (0.05 mmol) and 4 (0.08 mmol) in toluene (1.0 mL) was added the catalyst (R)-Li[P2] (1.8 mg, 5 mol%) at the designated temperature. After stirring for 18 h, the mixture was directly purified by silica gel chromatography (ethyl acetate/petroleum ether = 1/100 to 1/50) to afford the products 5.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and the Supplementary Information, as well as from the authors upon reasonable request. The X-ray crystallographic coordinate for structure 6 reported in this study has been deposited at the Cambridge Crystallographic Data Centre (CCDC), under CCDC 1881884. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
References
Bandini, M. & Eichholzer, A. Catalytic functionalization of indoles in a new dimension. Angew. Chem. Int. Ed. 48, 9608–9644 (2009).
Bartoli, G., Bencivenni, G. & Dalpozzo, R. Organocatalytic strategies for the asymmetric functionalization of indoles. Chem. Soc. Rev. 39, 4449–4465 (2010).
Trost, B. M., Krische, M. J., Berl, V. & Grenzer, E. M. Chemo-, regio-, and enantioselective Pd-catalyzed allylic alkylation of indolocarbazole pro-aglycons. Org. Lett. 4, 2005–2008 (2002).
Bandini, M., Eichholzer, A., Tragni, M. & Umani-Ronchi, A. Enantioselective phase-transfer-catalyzed intramolecular aza-Michael reaction: effective route to pyrazino-indole compounds. Angew. Chem. Int. Ed. 47, 3238–3241 (2008).
Stanley, L. M. & Hartwig, J. F. Iridium-catalyzed regio- and enantioselective N-allylation of indoles. Angew. Chem. Int. Ed. 48, 7841–7844 (2009).
Cui, H.-L. et al. Chemoselective asymmetric N-allylic alkylation of indoles with Morita-Baylis-Hillman carbonates. Angew. Chem. Int. Ed. 48, 5737–5740 (2009).
Trost, B. M., Osipov, M. & Dong, G. Palladium-catalyzed dynamic kinetic asymmetric transformations of vinyl aziridines with nitrogen heterocycles: rapid access to biologically active pyrroles and indoles. J. Am. Chem. Soc. 48, 15800–15807 (2010).
Cai, Q., Zheng, C. & You, S.-L. Enantioselective intramolecular aza-Michael additions of indoles catalyzed by chiral phosphoric acids. Angew. Chem. Int. Ed. 49, 8666–8669 (2010).
Xie, Y. et al. Enantioselective N-H functionalization of indoles with α,β-unsaturated γ-lactams catalyzed by chiral Brønsted acids. Angew. Chem. Int. Ed. 50, 5682–5686 (2011).
Enders, D., Greb, A., Deckers, K., Selig, P. & Merkens, C. Quadruple domino organocatalysis: an asymmetric aza-Michael/Michael/Michael/aldol reaction sequence leading to tetracyclic indole structures with six stereocenters. Chem. Eur. J. 18, 10226–10229 (2012).
Cheng, H.-G. et al. Highly enantioselective Friedel-Crafts alkylation/N-hemiacetalization cascade reaction with indoles. Angew. Chem. Int. Ed. 52, 3250–3254 (2013).
Chen, L.-Y. et al. Enantioselective direct functionalization of indoles by Pd/sulfoxide-phosphine-catalyzed N-allylic alkylation. Org. Lett. 17, 1381–1384 (2015).
Ye, K.-Y., Cheng, Q., Zhuo, C.-X., Dai, L.-X. & You, S.-L. An iridium(I) N-heterocyclic carbene complex catalyzes asymmetric intramolecular allylic amination reactions. Angew. Chem. Int. Ed. 55, 8113–8116 (2016).
Jang, S. H., Kim, H. W., Jeong, W., Moon, D. & Rhee, Y. H. Palladium-catalyzed asymmetric nitrogen-selective addition reaction of indoles to alkoxyallenes. Org. Lett. 20, 1248–1251 (2018).
Kim, S. W., Schempp, T. T., Zbieg, J. R., Stivala, C. E. & Krische, M. J. Regio- and enantioselective iridium-catalyzed N-allylation of indoles and related azoles with racemic branched alkyl-substituted allylic acetates. Angew. Chem. Int. Ed. 58, 7762–7766 (2019).
Allen, J. R., Bahamonde, A., Furukawa, Y. & Sigman, M. S. Enantioselective N‑alkylation of indoles via an intermolecular aza-Wacker-type reaction. J. Am. Chem. Soc. 141, 8670–8674 (2019).
Liu, W.-B., Zhang, X., Dai, L.-X. & You, S.-L. Asymmetric N-allylation of indoles through the iridium-catalyzed allylic alkylation/oxidation of indolines. Angew. Chem. Int. Ed. 51, 5183–5187 (2012).
Dou, X., Yao, W., Jiang, C. & Lu, Y. Enantioselective N-alkylation of isatins and synthesis of chiral N-alkylated indoles. Chem. Commun. 50, 11354–11357 (2014).
Xu, K., Thomas, G. & Breit, B. Asymmetric synthesis of N-allylic indoles via regio- and enantioselective allylation of aryl hydrazines. Nat. Commun. 6, 7616–7622 (2015).
Chen, Q.-A., Chen, Z. & Dong, V. M. Rhodium-catalyzed enantioselective hydroamination of alkynes with indolines. J. Am. Chem. Soc. 137, 8392–8395 (2015).
Zi, Y., Lange, M., Schultz, C. & Vilotijevic, I. Latent nucleophiles in Lewis base catalyzed enantioselective N-allylations of N-heterocycles. Angew. Chem. Int. Ed. 58, 10727–10731 (2019).
Kainz, Q. M. et al. Asymmetric copper-catalyzed C-N cross-couplings induced by visible light. Science 351, 681–684 (2016).
Chen, M. & Sun, J. Catalytic asymmetric N-alkylation of indoles and carbazoles through 1,6-conjugate addition of aza-para-quinone methides. Angew. Chem. Int. Ed. 56, 4583–4587 (2017).
Trost, B. M., Gnanamani, E. & Hung, C.-I. J. Controlling regioselectivity in the enantioselective N-alkylation of indole analogues catalyzed by dinuclear zinc-ProPhenol. Angew. Chem. Int. Ed. 56, 10451–10456 (2017).
Mukherjee, S., Shee, S., Poisson, T., Besset, T. & Biju, A. T. Enantioselective N-heterocyclic carbene-catalyzed cascade reaction for the synthesis of pyrroloquinolines via N–H functionalization of indoles. Org. Lett. 20, 6998–7002 (2018).
Zhang, L. et al. Chiral phosphoric acid catalyzed enantioselective N-alkylation of indoles with in situ generated cyclic N-acyl ketimines. Chem. Commun. 54, 9230–9233 (2018).
Ye, Y., Kim, S.-T., Jeong, J., Baik, M.-H. & Buchwald, S. L. CuH-catalyzed enantioselective alkylation of indole derivatives with ligand-controlled regiodivergence. J. Am. Chem. Soc. 141, 3901–3909 (2019).
Ding, C.-H. & Hou, X.-L. Catalytic asymmetric propargylation. Chem. Rev. 111, 1914–1937 (2011).
Trost, B. M. & Li, C. -J. (eds) Modern Alkyne Chemistry. Catalytic and Atom-Economic Transformations (Wiley-VCH, Weinheim, 2015).
Shao, W., Li, H., Liu, C., Liu, C.-J. & You, S.-L. Copper-catalyzed intermolecular asymmetric propargylic dearomatization of indoles. Angew. Chem. Int. Ed. 54, 7684–7687 (2015).
Zhu, F.-L. & Hu, X.-P. Enantioselective N-propargylation of indoles via Cu-catalyzed propargylic alkylation/dehydrogenation of indolines. Chin. J. Catal. 36, 86–92 (2015).
Wang, Y. et al. Asymmetric synthesis of syn-propargylamines and unsaturated β-amino acids under Brønsted base catalysis. Nat. Commun. 6, 8544–8552 (2015).
Wang, Y. et al. An arylation strategy to propargylamines: catalytic asymmetric Friedel-Crafts-type arylation reactions of C-alkynyl imines. Angew. Chem. Int. Ed. 55, 15142–15146 (2016).
Meng, X. et al. Rh(II)/Brønsted acid catalyzed general and highly diastereo- and enantioselective propargylation of in situ generated oxonium ylides and C‑alkynyl N‑Boc N,O-acetals: synthesis of polyfunctional propargylamines. Org. Lett. 21, 1292–1296 (2019).
Zha, T., Tong, X., Deng, Y., Peng, F. & Shao, Z. Catalytic asymmetric and divergent synthesis of tricyclic and tetracyclic spirooxindoles: controllable site-selective electrophilic halocyclization of 1,6-enynes. Org. Lett. 21, 6068–6073 (2019).
Schmidt, A. W., Reddy, K. R. & Knölker, H.-J. Occurrence, biogenesis, and synthesis of biologically active carbazole alkaloids. Chem. Rev. 112, 3193–3328 (2012).
Nimavat, K. S., Popat, K. H., Vasoya, S. L. & Joshi, H. S. Synthesis and antimicrobial activity of some new aminobenzylated Mannich bases. J. Indian Chem. Soc. 80, 711–713 (2003).
Tiwari, R. & Chhabra, G. Synthesis, characterization and pharmacological evaluation of some novel 3, 6-dinitrocarbazole derivatives. Asian J. Chem. 22, 5987–5992 (2010).
Rowland, G. B. et al. Brønsted acid-catalyzed imine amidation. J. Am. Chem. Soc. 12, 15696–15697 (2005).
Park, S. Y. et al. Asymmetric aminalization via cation-binding catalysis. Chem. Eur. J. 24, 1020–1025 (2018).
Parra, A., Reboredo, S., Castroa, A. M. M. & Alemán, J. Metallic organophosphates as catalysts in asymmetric synthesis: a return journey. Org. Biomol. Chem. 10, 5001–5020 (2012).
Parmar, D., Sugiono, E., Raja, S. & Rueping, M. Complete field guide to asymmetric BINOL-phosphate derived Brønsted acid and metal catalysis: history and classification by mode of activation; Brønsted acidity, hydrogen bonding, ion pairing, and metal phosphates. Chem. Rev. 114, 9047–9153 (2014).
Hatano, M., Ikeno, T., Matsumura, T., Torii, S. & Ishihara, K. Chiral lithium salts of phosphoric acids as Lewis acid-base conjugate catalysts for the enantioselective cyanosilylation of ketones. Adv. Synth. Catal. 55, 1776–1780 (2008).
Shen, K., Liu, X., Cai, Y., Lin, L. & Feng, X. Facile and efficient enantioselective Strecker reaction of ketimines by chiral sodium phosphate. Chem. Eur. J. 15, 6008–6014 (2009).
Hatano, M., Moriyama, K., Maki, T. & Ishihara, K. Which is the actual catalyst: chiral phosphoric acid or chiral calcium phosphate. Angew. Chem. Int. Ed. 49, 3823–3826 (2010).
Klussmann, M. et al. Synthesis of TRIP and analysis of phosphate salt impurities. Synlett 2010, 2189–2192 (2010).
Zhang, Z., Zheng, W. & Antilla, J. C. Highly enantioselective catalytic benzoyloxylation of 3-aryloxindoles using chiral VAPOL calcium phosphate. Angew. Chem. Int. Ed. 50, 1135–1138 (2011).
Zheng, W., Zhang, Z., Kaplan, M. J. & Antilla, J. C. Chiral calcium VAPOL phosphate mediated asymmetric chlorination and Michael reactions of 3-substituted oxindoles. J. Am. Chem. Soc. 133, 3339–3341 (2011).
Drouet, F., Lalli, C., Liu, H., Masson, G. & Zhu, J. Chiral calcium organophosphate-catalyzed enantioselective electrophilic amination of enamides. Org. Lett. 13, 94–97 (2011).
Ingle, G. K. et al. Chiral magnesium BINOL phosphate-catalyzed phosphination of imines: access to enantioenriched α-amino phosphine oxides. Org. Lett. 13, 2054–2057 (2011).
Larson, S. E. et al. Catalytic asymmetric aza-Darzens reaction with a vaulted biphenanthrol magnesium phosphate salt. Org. Lett. 13, 2188–2191 (2011).
Terada, M. & Kanomata, K. Metal-free chiral phosphoric acid or chiral metal phosphate as active catalyst in the activation of N-acyl aldimines. Synlett 2011, 1255–1258 (2011).
Alix, A., Lalli, C., Retailleau, P. & Masson, G. Highly enantioselective electrophilic α-bromination of enecarbamates: chiral phosphoric acid and calcium phosphate salt catalysts. J. Am. Chem. Soc. 134, 10389–10392 (2012).
Rueping, M., Bootwicha, T., Kambutong, S. & Sugiono, E. Asymmetric calcium catalysis: highly enantioselective carbonyl-ene and Friedel-Crafts reactions for the synthesis of quaternary α-hydroxy esters bearing a trifluoromethyl group. Chem. Asian J. 7, 1195–1198 (2012).
Li, G., Liang, T., Wojtas, L. & Antilla, J. C. An asymmetric Diels–Alder reaction catalyzed by chiral phosphate magnesium complexes: highly enantioselective synthesis of chiral spirooxindoles. Angew. Chem. Int. Ed. 52, 4628–4632 (2013).
Mao, Z. et al. Enantioselective construction of dihydropyran-fused indoles through chiral calcium phosphate catalyzed oxo-hetero-Diels-Alder reactions by using 2-oxoindolin-3-ylidenes as heterodienes. Chem. Eur. J. 19, 9754–9759 (2013).
Nimmagadda, S. K., Zhang, Z. & Antilla, J. C. Asymmetric one-pot synthesis of 1,3-oxazolidines and 1,3-oxazinanes via hemiaminal intermediates. Org. Lett. 16, 4098–4101 (2014).
Ingle, G., Mormino, M. G. & Antilla, J. C. Lithium BINOL phosphate catalyzed desymmetrization of meso-epoxides with aromatic thiols. Org. Lett. 16, 5548–5551 (2014).
Lalli, C. et al. Chiral calcium-BINOL phosphate catalyzed diastereo- and enantioselective synthesis of syn-1,2-disubstituted 1,2-diamines: scope and mechanistic studies. Chem. Eur. J. 21, 1704–1712 (2015).
Mori, K., Isogai, R., Kamei, Y., Yamanaka, M. & Akiyama, T. Chiral magnesium bisphosphate-catalyzed asymmetric double C(sp3)–H bond functionalization based on sequential hydride shift/cyclization process. J. Am. Chem. Soc. 140, 6203–6207 (2015).
Yurino, T., Aota, Y., Asakawa, D., Kano, T. & Maruoka, K. N-Boc-aminals as easily accessible precursors for less accessible N-Boc-imines: facile synthesis of optically active propargylamine derivatives using Mannich-type reactions. Tetrahedron 72, 3687–3700 (2016).
Marcantoni, E., Palmieri, A. & Petrin, M. Recent synthetic applications of α-amido sulfones as precursors of N-acylimino derivatives. Org. Chem. Front. 6, 2142–2182 (2019).
Fini, F. et al. Phase-transfer-catalyzed asymmetric aza-Henry reaction using N-carbamoyl imines generated in situ from α-amido sulfones. Angew. Chem. Int. Ed. 44, 7975–7978 (2005).
Enrique, G.-B. et al. Asymmetric aza-Henry reaction under phase transfer catalysis: an experimental and theoretical study. J. Am. Chem. Soc. 130, 7955–7966 (2008).
Cassani, C., Bernardi, L., Fini, F. & Ricci, A. Catalytic asymmetric Mannich reactions of sulfonylacetates. Angew. Chem. Int. Ed. 48, 5694–5697 (2009).
Galzerano, P. et al. Controlling stereoselectivity in the aminocatalytic enantioselective Mannich reaction of aldehydes with in situ generated N-carbamoyl imines. Chem. Eur. J. 16, 6069–6076 (2010).
Bae, H. Y., Kim, M. J., Sim, J. H. & Song, C. E. Direct catalytic asymmetric Mannich reaction with dithiomalonates as excellent Mannich donors: organocatalytic synthesis of (R)-sitagliptin. Angew. Chem. Int. Ed. 55, 10825–10827 (2016).
Hachiya, I., Ogura, K. & Shimizu, M. Novel 2-pyridone synthesis via nucleophilic addition of malonic esters to alkynyl imines. Org. Lett. 4, 2755–2757 (2002).
Satyanarayana, T., Abraham, S. & Kagan, H. B. Nonlinear effects in asymmetric catalysis. Angew. Chem. Int. Ed. 48, 456–494 (2009).
Giacalone, F., Gruttadauria, M., Agrigento, P. & Noto, R. Low-loading asymmetric organocatalysis. Chem. Soc. Rev. 41, 2406–2447 (2012).
Acknowledgements
This work was supported by NSFC (21672184 and 21861042), the Program for Changjiang Scholars and Innovative Research Team in University (IRT IRT17R94), the Program for Innovative Research Team (in Science and Technology) in University of Yunnan Province, Yunnan Province Government (2018FY001(016)), and YunLing Scholar of Yunnan Province.
Author information
Authors and Affiliations
Contributions
Z.S. conceived and directed the project. Y.W. and S.W. performed the experiments. W.S. prepared some substrates. Z.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Wang, S., Shan, W. et al. Direct asymmetric N-propargylation of indoles and carbazoles catalyzed by lithium SPINOL phosphate. Nat Commun 11, 226 (2020). https://doi.org/10.1038/s41467-019-13886-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-019-13886-9
This article is cited by
-
Enantioselective synthesis of chiral α,α-dialkyl indoles and related azoles by cobalt-catalyzed hydroalkylation and regioselectivity switch
Nature Communications (2024)
-
Copper-catalysed enantioconvergent alkylation of oxygen nucleophiles
Nature (2023)
-
Enantioselective synthesis of N-alkylindoles enabled by nickel-catalyzed C-C coupling
Nature Communications (2022)
-
Photoinduced copper-catalysed asymmetric amidation via ligand cooperativity
Nature (2021)
-
Study on synthesis of some substituted N-propargyl isatins by propargylation reaction of corresponding isatins using potassium carbonate as base under ultrasound- and microwave-assisted conditions
Chemical Papers (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.