Abstract
Methods for synthesis of chiral organic compounds bearing trifluoromethyl-substituted stereocenters are of great interest for agrochemical and pharmaceutical labs and industries in their search for new bioactive materials. We report on employment of bisfunctionalized electrophiles, bearing both a trifluoromethyl and a functional group as direct substituents of the reactive center, in cross-coupling reactions. We exemplify this concept in the asymmetric synthesis of enantioenriched α-trifluoromethyl- and perfluoroalkyl-containing benzylic and allylic ethers and alcohols by nickel-catalyzed stereoconvergent Hiyama cross-coupling reaction. Substrate electrophiles are conveniently prepared in few steps from trifluoroacetic acid. The method represents a conceptually different approach to chiral CF3-substituted alcohols and ethers and allows for a rapid catalytic preparation of a wide range of these valuable compounds in high yields and enantioselectivity.
Similar content being viewed by others
Introduction
Fluorine-containing compounds have attracted significant attention over recent decades, since incorporation of the fluorine atom can profoundly affect its molecular behavior1. Fluorine incorporation is a key tool used in the pharmaceutical and agrichemical industries to alter activity, bioavailability, and metabolic stability of the organic compound2, 3. Among other fluorinated functionalities, the trifluoromethyl group is widely employed in existing drugs and drug candidates4,5,6. Therefore, methods of CF3-group introduction, especially with concomitant construction of the stereodefined trifluoromethyl-substituted stereogenic centers, are of great importance7,8,9,10,11,12. Although the development of synthetic routes for the preparation of materials possessing these asymmetric carbon centers is a vibrant field, an attractive approach for their enantioselective creation by means of the cross-coupling reactions remains mainly unexplored. The sole reported example demonstrates a catalytic synthesis of enantioenriched CF3-substituted alkanes by Negishi cross-coupling reaction13, 14. This omission is rather remarkable, given that cross-coupling transformations proved to be highly useful and diverse tool for the asymmetric catalytic synthesis of compounds bearing related fluorine-substituted stereogenic centers15, 16.
We envisioned that the utilization of electrophiles of type 1 (Fig. 1) bearing both a CF3 and a functional group at the reaction center in cross-coupling reactions would represent a desirable means to augment molecular complexity and facilitate the construction of synthetic libraries of α-CF3 substituted alcohols, ethers, amines, sulfides, etc. Moreover, employing a racemic mixture of the building block 1 in an asymmetric stereoconvergent catalytic transformation would provide important compounds 2 in enantioenriched manner.
Initiating an active program in this direction we have primarily focused our efforts to examine the feasibility of this approach with regard to the asymmetric synthesis of trifluoromethyl-substituted benzyl (and alkyl) alcohols and ethers (Figs. 1, 2a). These compounds are extremely attractive building blocks since such motifs are found in a large number of biologically active compounds17,18,19. Moreover, substructure 3 (derivatives of phenethyl alcohol or its ethers) appears in a variety of bioactive compounds and existing pharmaceuticals (for example, commercial drugs, containing fragment 3; see Crizotinib, Aprepitant, Vestipitant, Fosaprepitant). Therefore, our approach, if successful, would render practical enantioselective synthesis of trifluoromethylated analogs of these materials.
It should be noted that all current synthetic methods for the preparation of highly enantioenriched α-CF3-substituted alcohols (2a, R = H) rely on enantioselective reduction of corresponding aryltrifluoromethyl ketones, utilizing chiral iridium or rhodium catalysts or chiral boranes (Fig. 2)20,21,22. An alternative approach by addition of trifluoromethyltrimethylsilane (TMSCF3) to aldehydes is known; however, this method is reported to provide moderate enantioselectivity7, 23.
In this paper we reveal a totally different synthetic approach for the preparation of these useful compounds in high yields and enantioselectivity by means of cross-coupling chemistry. Thus, we report a nickel-catalyzed asymmetric stereoconvergent Hiyama cross-coupling reaction for the synthesis of enantioenriched α-trifluoromethyl alcohols and ethers (aryl, vinyl, and alkyl). The approach can be extended to the preparation of chiral perfluoroalkyl alcohols. Substrates for this transformation are conveniently prepared in a few steps from trifluoroacetic acid (or its anhydride), as an economical source of the trifluoromethyl group (4, see Fig. 2). To the best of our knowledge this is the first example of an employment of electrophiles bearing both a trifluoromethyl and a functional group as direct substituents of the reactive center. Such electrophiles represent an attractive entry for cross-coupling transformations for the rapid increase of molecular complexity and asymmetric preparation of a wide class of organic compounds bearing CF3-substituted stereogenic centers.
Results
Optimization of reaction conditions
For proof of concept, we explored compounds 4a and 4b (for the preparation, see Supplementary note 1) in an enantioselective Hiyama cross-coupling reaction. After comprehensive investigation of the reaction conditions, we were pleased to find that α-chloro-α-trifluoromehyl ethers 4 proved eminently suitable electrophiles in Hiyama cross-coupling reaction. Under conditions, revealed by our screening, 4a provided the desired ether 5a in excellent yield and enantioselectivity (93% yield and 95% ee) after 40 h (Table 1, entry 1). Notably, the reaction employing 4b is completed after 16 h with no compromise in yield and ee (96 and 97% correspondingly). Therefore, further reaction conditions analysis was performed with substrate 4b (entry 2).
We have found that the methylene bisoxazoline ligand 7a in combination with NiCl2·glyme was an excellent and general catalyst for this process. Interestingly, structurally similar ligands 7b and 7c gave significantly poorer results in terms of yields and enantioselectivity (entries 11 and 12). A catalytic system based on ligand 7g was as efficient as that of 7a, requiring, however, longer reaction time (entry 16). Pyridine-based oxazoline ligands 7e and 7f proved to be inefficient in this transformation (entries 14 and 15). Variation in temperature has a profound effect on the reaction outcome: cooling the reaction to 10 oC drastically decreases the reaction rate (entry 6), while heating up to 40 oC causes conversion of trimethoxy(phenyl) silane to phenyl tetrafluorosilicate, which is not active as a nucleophile under our reaction conditions (entry 7). While the reaction could proceed in darkness, irradiation with a white or blue LED lamp significantly accelerated the process (compare entry 17 with 2 and 18). Although the precise mechanism of this photoinduction is to be clarified, we presume that it facilitates an oxidative addition of electrophile 4 to an excited Ni(I) catalytic species which likely takes place by radical mechanism. Induction of radical type oxidative addition step by light excited transition metal complex was previously reported24. Small amounts of water could be tolerated with no significant deterioration of the reaction outcome (entry 19); however, the reaction should be performed under anoxic conditions (entry 20).
Substrate scope
Having optimized conditions in hand, we subjected 4b to the reaction with different aryl silanes as nucleophiles (Fig. 3). Gratifyingly, various electron-rich and electron-poor aryl trimethoxy silanes are suitable partners for this reaction, providing the corresponding cross-coupling products in excellent yields and enantioselectivity. Moreover, ortho-, meta- and para-methyl substituted aryl silanes are amenable to this process (compounds 6d, 6e, and 6f). Ortho-substituted aryl silanes require significantly longer reaction times (48 h), probably due to slower transmetallation to nickel catalyst, while the yield and ee of the product are only slightly diminished (entry 6f). Interestingly, a styrenyl group could also be incorporated by this transformation; however, the ether was isolated in comparatively diminished yield, likely due to the partial oligomerization of the styrenyl residue (entry 6j). Notably, heterocyclic silanes can be employed as nucleophiles in this reaction. The corresponding ethers were obtained in good yields and ee (6n−p). This method is scalable: cross-coupling of 2g of substrate 4a with PhSi(OMe)3 furnishes ether 6a in 95% yield and 97% ee.
Examples of the Hiyama cross-coupling reaction with aryl silanes. Various aryls and heterocycles can be introduced. Isolated yield is reported (19F NMR yield is given in brackets). Reaction conditions: 4b (1 equiv.), (hetero)aryltrimethoxy silane (1.3 equiv), TBAT (2.5 equiv.), NiCl2·glyme (0.1 equiv.), Ligand 7a (0.11 equiv.), DMA, room temperature, 10 W blue led. aReaction on 2 g scale of 4b; breaction time 48 h
Although we focused our efforts on the asymmetric catalytic synthesis of trifluoromethyl-substituted benzyl alcohols and ethers, perfluoroalkyl motifs such as −C4F9 and −C8F17 could be successfully introduced (entries 6b′ and 6b″) without any modification of the reaction conditions. As such, the current methodology gives access to higher perfluorinated homologs of compound 6.
The reaction is not limited to aryl silanes. We have found that alkenyl trimethoxy silanes are highly efficient nucleophiles, which furnish α-trifluoromethylallyl ethers 8 in practically quantitative yield and high enantioselectivity (Fig. 4). In this case, ligand 7g was used as it provided superior enatnioselectivity compared to ligand 7a. For example, compound 8a was obtained in 65 and 90% ee with ligands 7a and 7g, correspondingly. While light irradiation also has a positive effect on the rate of the reaction with alkenyl silanes, it is not necessarily required here, as the reaction is complete in ca. 4 h. Notably, incorporation of an alkenyl motif allows for further versatile synthetic modification of the resulting products.
Selected examples of the cross-coupling reaction with alkenyl silanes. Isolated yield is reported (19F NMR yield is given in brackets). Reaction conditions: electrophile (1 equiv.), alkenyl trimethoxy silane (1.3 equiv), TBAT (2.5 equiv.), NiCl2·glyme (0.1 equiv.), Ligand 7g (0.11 equiv.), DMA, room temperature. aEnantiomers are not separated by HPLC
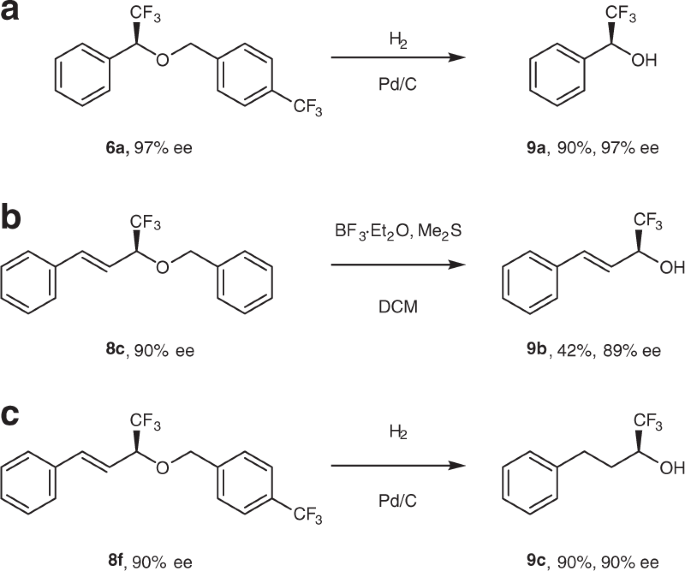
Since chiral trifluoromethylbenzyl alcohols are valuable building blocks for organic synthesis, we explored a deprotection of ethers 6 and 8 in order to examine a feasibility of preparation of these useful compounds. Notably, unprotected α-trifluoromethylbenzyl alcohols were prepared by simple hydrogenolysis of the corresponding ether in high yield and without loss of the initial ee (Fig. 5a; exemplified on ether 6a). The protective benzyl group in alkenyl ethers 8a−c was removed upon their treatment with BF3/Me2S to result in corresponding allyl alcohols in moderate yield and high ee (Fig. 5b, exemplified on ether 8c). Interestingly, when allyl ether 8f was subjected to the reductive deprotection conditions (H2, Pd/C), saturated (α-trifluoromethyl) alkyl alcohol 9c was smoothly obtained in 90% yield and with no deterioration of ee (Fig. 5c). As such, this reaction provides an entry to the preparation of (α-CF3)-alkyl-substituted alcohols in addition to their aryl and alkenyl counterparts.
Preparation of (α-trifluoromethyl) aryl, vinyl, and alkyl alcohols by deprotection (or reductive deprotection) of corresponding ethers. a Deprotection of benzylic ether 6a by palladium-on-carbon-catalyzed hydrogenation. b Deprotection of 8c with BF3 etherate and dimethylsulphide to give allylic alcohol 9b. c One-pot reductive deprotection of 8f with hydrogen and palladium on carbon to give alkyl alcohol 9c
After exploring the scope of nucleophiles, we have studied various α-chloroethers as electrophiles in our cross-coupling reaction. Gratifyingly, the method is not limited to 4-(trifluoromethyl)benzyl substituted ether 4b. Precursors bearing various alkoxy (RO-) groups, which contain ether (4c), ester (4d), protected amine (4e), benzyl (4a) or even an unfunctionalized alkyl moiety (4f) proved to be highly efficient in this cross-coupling transformation (Table 2). As such, the functionalized (trifluoromethyl)benzyl ethers 11 can be directly prepared in high yield and enantioselectivity from the corresponding substrates 4. While the reaction works with primary and secondary ethers, our attempts to use tertiary counterparts gave negligible conversion with traces of the target product. Having initiated the current work with R = benzyl group (4a) as a convenient handle for studies in methodology development, and having demonstrated diverse functionalization of the perfluoroalkyl-bearing part of the ether, we are in a position to conclude that the other half of the ether moiety could be freely modified without loss of reaction performance. This means the previously discussed deprotection to the alcohol to permit further functionalization to the corresponding ether is superfluous, although it remains a viable route where the benzyl group is desired for the orthogonal protection in multistep synthesis or for further preparation of free alcohol.
Interestingly, reaction times for various ethers could significantly vary. While 6b could be synthesized in less than 16 h with light irradiation or in 40 h in dark, reaction with 4a achieves full conversion in 30 h (60 h in the dark), but for alkyl-substituted analogs reaction times are 72 h (without light irradiation the reaction fails to reach full conversion). Presumably, an oxidative addition is the rate-limiting step and the lower the LUMO of the electrophile, the faster SET from the catalyst occurs, hence a faster reaction is observed. This hypothesis is congruent with observed reaction rates, while the reaction mechanism is likely to be similar to the previously reported for Ni-catalyzed stereoconvergent cross-coupling processes25,26,27.
Discussion
We have developed a catalytic stereoconvergent approach to the synthesis of enantioenriched α-trifluoromethyl- and perfluoroalkyl-containing benzylic and allylic ethers and alcohols. Our method is based on the utilization of a bisfunctionalized electrophile, bearing both a trifluoromethyl and a functional group as direct substituents of the reactive center, in cross-coupling reactions. Significant functional group compatibility, high yields and enantioselectivity of the reaction allows usage of this method in the late-stage synthesis for the preparation of the complex systems. The concept of the employment of such bisfunctionalized building blocks in cross-coupling reactions opens a door to the rapid preparation of numerous chiral functional organic compounds bearing trifluoromethyl (or perfluoroalkyl)-substituted stereogenic center. Utilization of this concept for the asymmetric catalytic synthesis of α-trifluoromethylthiols and amines, among others, is under studies in our labs.
Methods
General procedure for the cross-coupling reactions
In the glovebox, in 20 ml glass vial, mixture of NiCl2·glyme (22 mg, 0.1 mmol, 0.1 equiv) and ligand 7a (50.5 mg, 0.11 mmol, 0.11 equiv) in dry DMA (5 ml) were stirred for 1 h. The obtained solution of catalyst was diluted with DMA (5 ml), then TBAT (1.35 g, 2.5 mmol, 2.5 equiv.) and trimethoxy(aryl) silane (1.3 mmol, 1.3 equiv.) were added to the vial, followed by the solution of the electrophile (1 mmol, 1 equiv.) in 5 ml of DMA. The vial was tightly closed with PVC tape and stirred outside of the glovebox with additional light irradiation (household white-light 10 W LED lamp or blue-light 10 W LED lamp with λem≈460 nm) for 16 h. After completion of the reaction, a solution was poured into 15 ml 0.5 M NaOH solution and stirred for additional 10 min. The obtained mixture was diluted with 45 ml of water and extracted with ether (3 × 20 ml). Combined organic fractions were washed with 10 ml of water, 10 ml of brine and dried over Na2SO4. The residue after solvent evaporation was subjected to column chromatography (silica gel, 230–400 mesh, hexane/DCM or hexane/EtOAc) to give the product.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information file. For the experimental procedures, NMR, and HPLC analysis of the compounds in this article, see Supplementary Methods in the Supplementary Information file. The X-ray crystallographic coordinates for structure reported in this article have been deposited at the Cambridge Crystallographic Data Centre (11f: CCDC 1854431). These data could be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
References
Banks, R. E., Smart, B. E. & Tatlow, J. C. Organofluorine Chemistry: Principles and Commercial Applications (Springer US, New York US, 2013).
Müller, K., Faeh, C. & Diederich, F. Fluorine in pharmaceuticals: looking beyond intuition. Science 317, 1881–1886 (2007).
Purser, S., Moore, P. R., Swallow, S. & Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 37, 320–330 (2008).
Zhu, W. et al. Recent advances in the trifluoromethylation methodology and new CF3-containing drugs. J. Fluor. Chem. 167, 37–54 (2014).
Wang, J. et al. Fluorine in pharmaceutical industry: fluorine-containing drugs introduced to the market in the last decade (2001–2011). Chem. Rev. 114, 2432–2506 (2014).
Zhou, Y. et al. Next generation of fluorine-containing pharmaceuticals, compounds currently in phase II–III clinical trials of major pharmaceutical companies: new structural trends and therapeutic areas. Chem. Rev. 116, 422–518 (2016).
Yang, X., Wu, T., Phipps, R. J. & Toste, F. D. Advances in catalytic enantioselective fluorination, mono-, di-, and trifluoromethylation, and trifluoromethylthiolation reactions. Chem. Rev. 115, 826–870 (2015).
Brewitz, L. et al. Direct catalytic asymmetric Mannich-type reaction of α- and β-fluorinated amides. J. Am. Chem. Soc. 137, 15929–15939 (2015).
Jiang, Q., Guo, T. & Yu, Z. Copper-catalyzed asymmetric borylation: construction of a stereogenic carbon center bearing both CF3 and organoboron functional groups. J. Org. Chem. 82, 1951–1960 (2017).
Matsuzawa, A., Noda, H., Kumagai, N. & Shibasaki, M. Direct catalytic asymmetric aldol addition of an α-CF3 amide to arylglyoxal hydrates. J. Org. Chem. 82, 8304–8308 (2017).
Noda, H., Amemiya, F., Weidner, K., Kumagai, N. & Shibasaki, M. Catalytic asymmetric synthesis of CF3-substituted tertiary propargylic alcohols via direct aldol reaction of α-N3 amide. Chem. Sci. 8, 3260–3269 (2017).
Holmes, M., Nguyen, K. D., Schwartz, L. A., Luong, T. & Krische, M. J. Enantioselective formation of CF3-bearing all-carbon quaternary stereocenters via C–H functionalization of methanol: iridium catalyzed allene hydrohydroxymethylation. J. Am. Chem. Soc. 139, 8114–8117 (2017).
Liang, Y. & Fu, G. C. Stereoconvergent Negishi arylations of racemic secondary alkyl electrophiles: differentiating between a CF3 and an alkyl group. J. Am. Chem. Soc. 137, 9523–9526 (2015).
Liang, Y. & Fu, G. C. Nickel-catalyzed alkyl–alkyl cross-couplings of fluorinated secondary electrophiles: a general approach to the synthesis of compounds having a perfluoroalkyl substituent. Angew. Chem. Int. Ed. 54, 9047–9051 (2015).
Jiang, X. & Gandelman, M. Enantioselective Suzuki cross-couplings of unactivated 1-fluoro-1-haloalkanes: synthesis of chiral β-, γ-, δ-, and ε-fluoroalkanes. J. Am. Chem. Soc. 137, 2542–2547 (2015).
Liang, Y. & Fu, G. C. Catalytic asymmetric synthesis of tertiary alkyl fluorides: Negishi cross-couplings of racemic α,α-dihaloketones. J. Am. Chem. Soc. 136, 5520–5524 (2014).
O’Shea, P. D. et al. A practical enantioselective synthesis of Odanacatib, a potent cathepsin K inhibitor, via triflate displacement of an α-trifluoromethylbenzyl triflate. J. Org. Chem. 74, 1605–1610 (2009).
Goldberg, D. R. et al. Optimization of spirocyclic proline tryptophan hydroxylase-1 inhibitors. Bioorg. Med. Chem. Lett. 27, 413–419 (2017).
Wouters, J. et al. A reversible monoamine oxidase a inhibitor, befloxatone: structural approach of its mechanism of action. Biorg. Med. Chem. 7, 1683–1693 (1999).
Ohkuma, T. et al. Asymmetric hydrogenation of alkenyl, cyclopropyl, and aryl ketones. RuCl2(xylbinap)(1,2-diamine) as a precatalyst exhibiting a wide scope. J. Am. Chem. Soc. 120, 13529–13530 (1998).
Veeraraghavan Ramachandran, P., Teodorovic, A. V., & Brown, H. C. Chiral synthesis via organoboranes. 38. Selective reductions. 48. Asymmetric reduction of trifluoromethyl ketones by B-chlorodiisopinocampheylborane in high enantiomeric purity. Tetrahedron 49, 1725–1738 (1993).
Corey, E. J. & Bakshi, R. K. A new system for catalytic enantioselective reduction of achiral ketones to chiral alcohols. Synthesis of chiral α-hydroxy acids. Tetrahedron Lett. 31, 611–614 (1990).
Liu, X., Xu, C., Wang, M. & Liu, Q. Trifluoromethyltrimethylsilane: nucleophilic trifluoromethylation and Beyond. Chem. Rev. 115, 683–730 (2015).
Ahn, J. M., Peters, J. C. & Fu, G. C. Design of a photoredox catalyst that enables the direct synthesis of carbamate-protected primary amines via photoinduced, copper-catalyzed N-alkylation reactions of unactivated secondary halides. J. Am. Chem. Soc. 139, 18101–18106 (2017).
Schley, N. D. & Fu, G. C. Nickel-catalyzed Negishi arylations of propargylic bromides: a mechanistic investigation. J. Am. Chem. Soc. 136, 16588–16593 (2014).
Jones, G. D. et al. Ligand redox effects in the synthesis, electronic structure, and reactivity of an alkyl−alkyl cross-coupling catalyst. J. Am. Chem. Soc. 128, 13175–13183 (2006).
Gutierrez, O., Tellis, J. C., Primer, D. N., Molander, G. A. & Kozlowski, M. C. Nickel-catalyzed cross-coupling of photoredox-generated radicals: uncovering a general manifold for stereoconvergence in nickel-catalyzed cross-couplings. J. Am. Chem. Soc. 137, 4896–4899 (2015).
Acknowledgements
The authors acknowledge financial support from the Israel Science Foundation (grant no. 1890_/15). We are grateful to Dr. Natalia Fridman for the X-ray analysis and structure solution of compound 11f.
Author information
Authors and Affiliations
Contributions
A.V. conducted synthetic experiments and wrote the manuscript, M.G. directed the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Varenikov, A., Gandelman, M. Synthesis of chiral α-trifluoromethyl alcohols and ethers via enantioselective Hiyama cross-couplings of bisfunctionalized electrophiles. Nat Commun 9, 3566 (2018). https://doi.org/10.1038/s41467-018-05946-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-018-05946-3
This article is cited by
-
Efficient Synthesis of Chiral Aryl Alcohol with a Novel Kosakonia radicincitans Isolate in Tween 20/L-carnitine: Lysine-Containing Synergistic Reaction System
Applied Biochemistry and Biotechnology (2024)
-
Paired electrolysis-enabled nickel-catalyzed enantioselective reductive cross-coupling between α-chloroesters and aryl bromides
Nature Communications (2022)
-
Asymmetric construction of allylicstereogenic carbon center featuring atrifluoromethyl group via enantioselective reductive fluoroalkylation
Nature Communications (2022)
-
Facilitating the transmetalation step with aryl-zincates in nickel-catalyzed enantioselective arylation of secondary benzylic halides
Nature Communications (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.