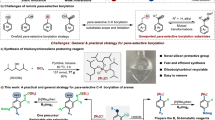
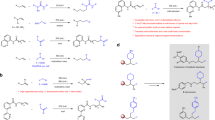
Abstract
Conventional Suzuki–Miyaura arylations use halogenated compounds as electrophilic coupling partners. Although the catalytic activation of organohalides is well established, these substrates are seldom naturally abundant and questions exist as to their metabolic and environmental friendliness. Owing to their existence as readily available chemical feedstocks, alcohols are highly desirable cross-coupling partners, although pathways to activate the strong C–O bond are rarer. Here the coupling of tertiary alcohols with boronic esters through in situ alcohol silylation is described, providing access to quaternary carbon scaffolds without needing to proceed by an activated alkyl halide or pseudohalide intermediate. A dual catalyst system is used with both Ni(0) and Bi(III) components playing a critical role, along with a mild chlorosilane reactant. This method was found to tolerate diverse functional groups including chloro, nitro, olefin, ketone, ester and phenol moieties, while also being applicable to the derivatization of heterocyclic scaffolds. Mechanistic studies suggest the combination of a Lewis acid catalyst and organosilane promote the heterolytic cleavage of the substrate C–O bond to generate an electrophilic intermediate, providing a powerful strategy for derivatizing readily available alcohols.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data supporting the findings of the study are available in the paper and its Supplementary Information.
References
Brown, D. G. & Boström, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59, 4443–4458 (2016).
Buskes, M. J. & Blanco, M.-J. Impact of cross-coupling reactions in drug discovery and development. Molecules 25, 3493 (2020).
Miyaura, N. & Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 95, 2457–2483 (1995).
Koshvandi, A. T. K., Heravi, M. M. & Momeni, T. Current applications of Suzuki-Miyaura coupling reaction in the total synthesis of natural products: an update. App. Organomet. Chem. 32, e4210 (2018).
Glasspoole, B. W. & Crudden, C. M. Cross-coupling: the final frontier. Nat. Chem. 3, 912–913 (2011).
Choi, J. & Fu, G. C. Transition metal-catalyzed alkyl-alkyl bond formation: another dimension in cross-coupling chemistry. Science 356, eaaf7230 (2017).
Yu, D.-G., Li, B.-J. & Shi, Z.-J. Exploration of new C−O electrophiles in cross-coupling reactions. Acc. Chem. Res. 43, 1486–1495 (2010).
Zhou, T. & Szostak, M. Palladium-catalyzed cross-couplings by C−O bond activation. Catal. Sci. Technol. 10, 5702–5739 (2020).
Yu, D.-G. et al. Direct application of phenolic salts to nickel-catalyzed cross-coupling reactions with aryl Grignard reagents. Angew. Chem. Int. Ed. 49, 4566–4570 (2010).
Zeng, H. et al. An adventure in sustainable cross-coupling of phenols and derivatives via carbon-oxygen bond cleavage. ACS Catal. 7, 510–519 (2017).
Yu, D.-G. et al. Direct arylation/alkylation/magnesiation of benzyl alcohols in the presence of Grignard reagents via Ni-, Fe- or Co- catalyzed sp3 C−O bond activation. J. Am. Chem. Soc. 134, 14638–14641 (2012).
Guo, P. et al. Dynamic kinetic cross-electrophile arylation of benzyl alcohols by nickel catalysis. J. Am. Chem. Soc. 143, 513–523 (2021).
Suga, T. & Ukaji, Y. Nickel-catalyzed cross-electrophile coupling between benzyl alcohols and aryl halides assisted by titanium co-reductant. Org. Lett. 20, 7846–7850 (2018).
Nazari, S. H. et al. Nickel-catalyzed Suzuki cross-couplings with unprotected allylic alcohols enabled by bidentate N-heterocyclic carbene (NHC)/phosphine ligands. ACS Catal. 8, 85–89 (2018).
Zhang, X. & MacMillan, D. W. C. Alcohols as latent coupling fragments for metallaphotoredox catalysis: sp3-sp2 cross-coupling of oxalates with aryl halides. J. Am. Chem. Soc. 138, 13862–13865 (2016).
Mills, R. J., Monteith, J. J., dos Passos Gomes, G., Aspuru-Guzik, A. & Rousseaux, S. A. L. The cyclopropane ring as a reporter of radical leaving-group reactivity for Ni-catalyzed C(sp3)−O arylation. J. Am. Chem. Soc. 142, 13246–13254 (2020).
Dong, Z. & MacMillan, D. W. C. Metallaphotoredox-enabled deoxygenative arylation of alcohols. Nature 598, 451–456 (2021).
Lackner, G. L., Quasdorf, K. W. & Overman, L. E. Direct construction of quaternary carbons from tertiary alcohols via photoredox-catalyzed fragmentation of tert-alkyl N-phthalimidoyl oxalates. J. Am. Chem. Soc. 135, 15342–15345 (2013).
Kariofillis, S. et al. Using data science to guide aryl bromide substrate scope analysis in a Ni/photoredox-catalyzed cross-coupling with acetals as alcohol-derived radical sources. J. Am. Chem. Soc. 144, 1045–1055 (2022).
Ye, Y., Chen, H., Sessler, J. L. & Gong, H. Zn-mediated fragmentation of tertiary alkyl oxalates enabling formation of alkylated and arylated quaternary carbon centers. J. Am. Chem. Soc. 141, 820–824 (2019).
Li, Z. et al. Electrochemically enabled, nickel-catalyzed dehydroxylative cross-coupling of alcohols with aryl halides. J. Am. Chem. Soc. 143, 3536–3543 (2021).
Lin, Q., Ma, G. & Gong, H. Ni-catalyzed formal cross-electrophile coupling of alcohols with aryl halides. ACS Catal. 11, 14102–14109 (2021).
Chi, B. K. et al. In-situ bromination enables formal cross-electrophile coupling of alcohols with aryl and alkenyl halides. ACS Catal. 12, 580–586 (2022).
Xie, H. et al. Radical dehydroxylative alkylation of tertiary alcohols by Ti catalysis. J. Am. Chem. Soc. 142, 16787–16794 (2020).
Sakai, H. A. & MacMillan, D. W. C. Nontraditional fragment couplings of alcohols and carboxylic acids: C(sp3)-C(sp3) cross-coupling via radical sorting. J. Am. Chem. Soc. 144, 6185–6192 (2022).
Emer, E. et al. Direct nucleophilic SN1-type reactions of alcohols. Eur. J. Org. Chem. 2011, 647–667 (2011).
Yasuda, M., Somyo, T. & Baba, A. Direct carbon-carbon bond formation from alcohols and active methylenes, alkoxyketones, or indoles catalyzed by indium trichloride. Angew. Chem. Int. Ed. 45, 793–796 (2006).
Jia, X.-G., Guo, P., Duan, J. & Shu, X.-Z. Dual nickel and Lewis acid catalysis for cross-electrophile coupling: the allylation of aryl halides with allylic alcohols. Chem. Sci. 9, 640–645 (2018).
Liu, X. et al. Lewis-acid assisted nickel-catalyzed cross-coupling of aryl methyl ethers by C−O bond-cleaving alkylation: prevention of undesired β-hydride elimination. Angew. Chem. Int. Ed. 55, 6093–6098 (2016).
Isbrandt, E. S., Sullivan, R. J. & Newman, S. G. High-throughput strategies for the discovery and optimization of catalytic reactions. Angew. Chem. Int. Ed. 58, 7180–7191 (2019).
Tulloch, A. A. D., Danopoulos, A. A., Winston, S., Kleinhenz, S. & Eastham, G. N-functionalized heterocyclic carbene complexes of silver. J. Chem. Soc. Dalton Trans. 24, 4499–4506 (2000).
Kolsi, L. E., Yli-Kauhaluoma, J. & Moreira, V. M. Catalytic, tunable, one-step bismuth (III) triflate reaction with alcohols: dehydration versus dimerization. ACS Omega 3, 8836–8842 (2018).
Zultanski, S. L. & Fu, G. C. Nickel-catalyzed carbon-carbon bond-forming reactions of unactivated tertiary alkyl halides: Suzuki arylations. J. Am. Chem. Soc. 135, 624–627 (2013).
Qin, T. et al. A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents. Science 352, 801–805 (2016).
Primer, D. N. & Molander, G. A. Enabling the cross-coupling of tertiary organoboron nucleophiles through radical-mediated alkyl transfer. J. Am. Chem. Soc. 139, 9847–9850 (2017).
Dorsheimer, J. R., Ashley, M. A. & Rovis, T. Dual nickel/photoredox-catalyzed deaminative cross-coupling of sterically hindered primary amines. J. Am. Chem. Soc. 143, 19294–19299 (2021).
Dryzhakov, M., Hellal, M., Wolf, E., Falk, F. C. & Moran, J. Nitro-assisted Bronsted acid catalysis: application to a challenging catalytic azidation. J. Am. Chem. Soc. 137, 9555–9558 (2015).
Baba, A., Yasuda, M., Nishimoto, Y., Saito, T. & Onishi, Y. Reaction of alcohols and silyl ethers in the presence of an indium/silicon-based catalyst system: deoxygenation and allyl substitution. Pure. App. Chem. 80, 845–854 (2008).
Wiensch, E., Todd, D. & Montgomery, J. Silyloxyarenes as versatile coupling substrates enabled by nickel-catalyzed C−O bond cleavage. ACS Catal. 7, 5568–5571 (2017).
Yasuda, M., Yamasaki, S., Onishi, Y. & Baba, A. Indium-catalyzed direct chlorination of alcohols using chlorodimethylsilane-benzil as a selective and mild system. J. Am. Chem. Soc. 126, 7186–7187 (2004).
Jefferies, L. R. & Cook, S. P. Iron-catalyzed arene alkylation reactions with unactivated secondary alcohols. Org. Lett. 16, 2026–2029 (2014).
Labrouillère, M., Le Roux, C., Gaspard-Iloughmane, H. & Dubac, J. Bismuth(III) chloride-catalyzed chlorination of alcohols by chlorosilanes. Synlett 9, 723–724 (1994).
Xiao, L.-J. et al. Nickel(0)-catalyzed hydroarylation of styrenes and 1,3-dienes with organoboron compounds. Angew. Chem. Int. Ed. 57, 461–464 (2017).
Lv, H., Xiao, L.-J., Zhao, D. & Zhou, Q.-L. Nickel(0)-catalyzed linear-selective hydroarylation of unactivated alkenes and styrenes with aryl boronic acids. Chem. Sci. 9, 6839–6843 (2018).
Zultanski, S. L. & Fu, G. C. Nickel-catalyzed carbon-carbon bond-forming reactions of unactivated tertiary alkyl halides: Suzuki arylations. J. Am. Chem. Soc. 135, 624–627 (2013).
Diccianni, J. B. & Diao, T. Mechanisms of nickel-catalyzed cross-coupling reactions. Trends in Chem. 9, 830–844 (2019).
Bowry, V. W., Lusztyk, J. & Ingold, K. U. Calibration of a new horologery of fast radical clocks. Ring-opening rates for ring- and α-alkyl-substituted cyclopropylcarbinyl radicals and for the bicyclo[2.1.0]pent-2-yl radical. J. Am. Chem. Soc. 113, 5687–5698 (1991).
Walling, C. & Cioffari, A. Cyclization of 5-hexenyl radicals. J. Am. Chem. Soc. 94, 6059–6064 (1972).
Hammett, L. P. Some relations between reaction rates and equilibrium constants. Chem. Rev. 17, 125–136 (1935).
Hansch, C., Leo, A. & Taft, R. W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 91, 165–195 (1991).
Vives, G., Gonzalez, A., Jaud, J., Launay, J.-P. & Rapenne, G. Synthesis of molecular motors incorporating para-phenylene-conjugated or bicyclo[2.2.2]octane-insulated electroactive groups. Chem. Eur. J. 13, 5622–5631 (2007).
Burés, J. A simple graphical method to determine the order in catalyst. Angew. Chem. Int. Ed. 55, 2028–2031 (2016).
Eyring, H. The activated complex in chemical reactions. J. Chem. Phys. 3, 107–115 (1935).
Phan, T. B., Nolte, C., Kobayashi, S., Ofial, A. R. & Mayr, H. Can one predict changes from SN1 to SN2 mechanisms? J. Am. Chem. Soc. 131, 11392–11401 (2009).
Winstein, S., Grunwald, E. & Jones, H. W. The correlation of solvolysis rates and the classification of solvolysis reactions into mechanistic categories. J. Am. Chem. Soc. 73, 2700–2707 (1951).
Krumper, J. R., Salamant, W. A. & Woerpel, K. A. Continuum of mechanisms for nucleophilic substitution of cyclic acetals. Org. Lett. 10, 4907–4910 (2008).
Danopoulos, A. A., Tulloch, A. A. D., Winston, S., Eastham, G. & Hursthouse, M. B. Chelating and ‘pincer’ dicarbene complexes of palladium; synthesis and structural studies. Dalton Trans. 5, 1009–1015 (2003).
Acknowledgements
Financial support for this work was provided by the National Science and Engineering Research Council of Canada (grant no. RGPIN-2020-05065) and the Canada Research Chair Program (grant no. 950-232650). The Canadian Foundation for Innovation and the Ontario Ministry of Research, Innovation, & Science are thanked for essential infrastructure. A.C. thanks the National Science and Engineering Research Council of Canada for a Canada Graduate Scholarship–Doctoral fellowship.
Author information
Authors and Affiliations
Contributions
A.C. and S.G.N. designed the project. A.C. discovered and developed the reaction. A.C. and P.S.O. carried out all experimental work. A.C. wrote the draft of the paper and Supplementary Information. All authors participated in revising the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Martins Oderinde, Xing-Zhong Shu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–31, Tables 1–16, Discussion, Experimental details and NMR spectra.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cook, A., St. Onge, P. & Newman, S.G. Deoxygenative Suzuki–Miyaura arylation of tertiary alcohols through silyl ethers. Nat. Synth 2, 663–669 (2023). https://doi.org/10.1038/s44160-023-00275-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00275-w