Abstract
Hypertensive disorders of pregnancy (HDP) are associated with poor maternal and neonatal prognoses. Although several studies have indicated an effect of secondhand smoke (SHS) exposure on HDP, such evidence is lacking in Japan. Therefore, we analyzed data from the Japan Environment and Children’s Study, a large-scale epidemiological investigation, to elucidate a possible link between SHS exposure and HDP risk. Data were obtained from the all-birth fixed datasets and included information on 104,062 fetuses and their parents. SHS exposure was assessed in terms of the frequency (rarely, 1–3, or 4–7 days/week) and the daily duration of exposure (<1, 1–2, or ≥2 h(s)/day). Modified Poisson regression model analyses were performed with adjustment for known risk factors for HDP. Additionally, the population attributable fractions (PAFs) of SHS exposure and maternal smoking to HDP prevalence were estimated. The relative risks of developing HDP among individuals with SHS exposures of 4–7 days/week and ≥2 h/day were 1.18 and 1.27 (95% confidence interval: 1.02–1.36 and 0.96–1.67), respectively, compared to the reference groups (rare exposure and <1 h/day). The PAFs for the risk of HDP due to SHS exposure and perinatal smoking were 3.8% and 1.8%, respectively. Japanese women with greater exposure to SHS have a higher risk of HDP after adjustment for possible confounding factors; thus, relevant measures are required to reduce SHS exposure to alleviate HDP risk.

The association between second-hand smoking exposure and hypertensive disorders of pregnancy risk was analyzed using the JECS data. The relative risks in 4–7 days/week and ≥2 h/day of SHS exposures were 1.18 and 1.27, respectively. The PAFs due to SHS exposure and maternal smoking were 3.80% and 1.81%, respectively.
Similar content being viewed by others
Introduction
Hypertensive disorders of pregnancy (HDP) are observed in ~5–10% of pregnant women and are associated with a poor maternal and neonatal prognosis due to premature delivery, stillbirth, impaired fetal growth, and maternal death [1]. HDPs are also associated with specific risk factors, including first pregnancy; higher body mass index (BMI) or age; and preexisting dyslipidemia, diabetes mellitus, or renal disease; [2, 3] and socioeconomic status [4]. Although the cause of HDP remains unknown, current hypotheses postulate a placental pathogenesis. Abnormal placentation leading to preeclampsia is marked by the failure of trophoblasts to induce physiologic dilatation and remodeling of the spiral arteries, resulting in reduced placental blood flow [5].
Smoking during pregnancy is one of the most important risk factors for various adverse birth outcomes, including low birth weight and preterm birth [6]. Furthermore, several studies have indicated an influence of secondhand smoke (SHS) exposure during pregnancy on birth outcomes. For example, Polanska et al. reported that SHS exposure during pregnancy has a negative impact on child psychomotor development within the first two years of life [7]. Additionally, Windham et al. reported that high SHS exposure (more than 7 h/day) is associated with preterm birth [8]. Studies regarding the effect of SHS exposure on HDP risk were conducted in Norway [9] and North America [10] with inconsistent results. Studies on the influence of SHS exposure on HDP risk in Japan are lacking. A recent meta-analysis in Japan revealed that smoking is a risk factor for HDP [11]. We believe it is necessary to examine the effects of secondhand smoke on HDP using Japanese data.
The Japanese Ministry of the Environment launched the Japan Environment and Children’s Study (JECS), an ongoing large-scale epidemiological investigation, in January 2011 [12]. The JECS invited ~100,000 pregnant women and their partners to participate over a period of 3 years following collection of biological samples; data on their children were subsequently obtained until the age of 13 years. Herein, we analyzed JECS data to elucidate a possible link between SHS exposure and HDP risk.
Methods
Study design
This study was part of the JECS, an ongoing nationwide birth cohort study. The JECS was approved by the Ministry of the Environment’s Institutional Review Board on Epidemiological Studies on April 6, 2010 (no. 100406001), and the ethics committees of all participating institutions. Written informed consent was obtained from all participants. Participants were recruited from January 2011 to March 2014 at 15 regional centers located in Hokkaido, Miyagi, Fukushima, Chiba, Kanagawa, Koshin, Toyama, Aichi, Kyoto, Osaka, Hyogo, Tottori, Kochi, Fukuoka, and South Kyushu and Okinawa. The analysis is based on the jecs-ta-20190930 datasets, including information on 104,062 fetuses and their parents. The baseline profiles of participants were described in a previous study [13].
Data collection
We obtained pregnancy-related information, including the weight before pregnancy, from two self-report questionnaires (MT1 and MT2). The MT1 questionnaire was completed on enrollment in the JECS, while the MT2 questionnaire comprised data pertaining to the second and third trimester. The mean (standard deviation) gestational age at the time of completion of the MT1 and MT2 questionnaires was 16.4 (8.0) and 27.9 (6.5) weeks, respectively. Data from the Dr0m questionnaire were obtained from medical records after delivery via the cooperation of health care providers or research coordinators from regional centers. The Dr0m questionnaire was designed to gather data on the outcomes of the pregnancy and offspring, including the maternal age and weight at delivery, complications during pregnancy, birth weight of child, and length of gestation. This information was transcribed by instructed physicians, midwives/nurses, and/or research coordinators from medical records. Incomplete questionnaires were completed via a face-to-face or telephone interview.
We obtained the following data from the MT1 questionnaire: parity, BMI (kg/m2), smoking and drinking habits of the participants and their partners, frequency and duration of SHS exposure, educational levels of the participants and their partners, Kessler psychological distress scale (K6) score, and complications before pregnancy (i.e., hypertension, renal diseases, dyslipidemia, and diabetes mellitus). Sodium (mg/day), potassium (mg/day), calcium (mg/day), magnesium (mg/day), salt (g/day), and total energy (kJ/day) intake were obtained from the food frequency questionnaire (FFQ) on the MT1 questionnaire. The association between nutrient intake and HDP based on the FFQ has been reported previously [14, 15]. Socioeconomic status data, such as family income and the educational level of the participants and their partners, were obtained from the MT2 questionnaire. The K6 is a widely used screening scale for psychological distress in the general population [16]. The Japanese version of the K6 was recently developed using the standard back-translation method [17]. Details regarding K6 have been previously described [18].
Participants
We excluded women who participated in the JECS twice (n = 5689), those with missing data regarding their smoking status (active smoking of participants and partners, maternal SHS exposure) (n = 6468) and those with missing baseline data (Table 1 variables) (n = 11,950). We focused on low-risk pregnancies; thus, we excluded women with multiple pregnancies (n = 1576) and those with a history of miscarriage or stillbirth (n = 120). Finally, we excluded women with specified comorbidities (n = 2433), including diagnosed hypertension (n = 359), renal diseases (n = 1572), diabetes mellitus (n = 163), and dyslipidemia (n = 401), with some overlap among the comorbidities. The records of the remaining 75,826 women were analyzed. Excluding 31,079 smokers, the main analysis included 44,747 subjects. Subanalyses were also performed for the 75,826 individuals, including smokers. Figure 1 shows the participant selection flowchart.
Secondhand smoke exposure
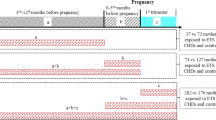
Information on SHS exposure was obtained from the self-administered MT1 questionnaire, which asked the following question: Prior to this pregnancy, how many times per week were you exposed to smoke from cigarettes smoked by others in your home, at work, or indoors when you were away from home? In the questionnaire, SHS exposure was assessed by asking how often the participant was exposed to SHS per week (rarely, 1–3, or 4–7 days/week). Exposed participants were asked to respond to a question regarding the average duration of SHS exposure per day (<1, 1–2, or ≥2 h(s)/day).
Hypertensive disorders of pregnancy
Information on HDP was obtained from the Dr0m questionnaire. This questionnaire only revealed whether the participant was diagnosed with HDP; data regarding specific HDP were not provided (i.e., gestational hypertension, preeclampsia, or superimposed preeclampsia and eclampsia). In the JECS, HDP was defined as hypertension (blood pressure [BP] ≥ 140/90 mmHg), with or without proteinuria (≥300 mg/24 h) emerging after 20 weeks gestation but resolving up to 12 weeks postpartum, or as eclampsia [19].
Statistical analysis
Statistical analyses were performed using SAS software (version 9.4, SAS Institute Inc., Cary, NC). Baseline characteristics are reported as percentages, means and standard deviations, or medians and interquartile ranges, as appropriate. Women with rare exposure to SHS were considered to be reference group in analyses of the effect of SHS exposure frequency on HDP risk, and women with SHS exposure of less than 1 h/day were considered to be the reference group in analyses of the effect of SHS exposure duration on HDP risk. We used a modified Poisson regression model to calculate crude relative risks (RR) and adjusted relative risks (aRR) [20]. In model 1, we adjusted for other risk factors for HDP, including age, parity, BMI before pregnancy, family income, maternal educational levels, drinking, smoking status, Na, K, Ca, Mg, total energy intake, and K6 score at entry. In model 2, we adjusted for a history of HDP and gestational diabetes mellitus (GDM) in addition to the factors in model 1. In model 3, the partner’s educational level was adjusted.
Additionally, we estimated the population attributable fractions (PAFs) and the 95% confidence intervals (CIs) of SHS exposure and maternal smoking to HDP prevalence using the NLEST macro. Using the aRR from model 3, the PAF was calculated as follows:
\(Estimated\;excess\;HDP\;case = pc \times \left( {aRR - 1} \right)/aRR\)
\(PAF = estimated\;excess\;HDP\;case/all\;HDP\;cases \times 100\)
\(Composite\;PAF = sum\;of\;estimated\;excess\;HDP\;cases\;of\;each\;category/allHDP\;cases \times 100\)(where pc is the proportion of HDP cases arising from each category)
Results
Table 1 shows the basic characteristics of the study participants. The distribution of SHS exposure frequency was as follows: rarely, 27,097 (60.6%); 1–3 days/week, 10,585 (23.7%); and 4–7 days/week, 7065 (15.8%). The number of primiparous women was the lowest in the rare exposure group, while those with frequent SHS exposure had less education and income. All baseline measurements were significantly different among the three groups (all p < 0.05). The prevalence of women with HDP according to SHS exposure frequency was significantly different among the three groups as follows: rarely, 2.6%; 1–3 days/week, 3.0%; and 4–7 days/week, 3.5% (p < 0.01). Supplementary Table 1 presents the results for all 75,826 participants, regardless of maternal smoking status.
Table 2 and Supplementary Table 2 show the univariate analyses on HDP risk in nonsmokers and all participants, respectively. Compared to women with rare SHS exposure, pregnant women with SHS exposure of 1–3 and 4–7 days/week showed a higher risk of developing HDP (RR: 1.15, 95% confidence interval [CI]: 1.01–1.31 and RR: 1.35, 95% CI: 1.17–1.55, respectively). Additionally, pregnant women with SHS exposure of 1–2 or ≥2 h/day had a significantly higher risk of developing HDP (RR: 1.49, 95% CI: 1.21–1.83 and RR: 1.48, 95% CI: 1.13–1.95, respectively) than those with SHS exposure of <1 h/day.
Table 3 and Supplementary Table 3 show that the relative risk for SHS exposure and HDP risk changed as a result of adjusting for possible confounding factors. In the multivariate analyses regarding the impact of SHS exposure frequency on HDP risk, after adjusting for maternal confounding factors (model 1), a previous history of GDM and HDP (model 2), and partner factors (model 3), pregnant women with SHS exposure of 4–7 days/week had a higher risk of developing HDP (aRR: 1.20, 95% CI: 1.03–1.39; aRR: 1.19, 95% CI: 1.03–1.38; aRR: 1.18, 95% CI: 1.02–1.36, respectively) than the reference group. A linear trend was observed between SHS exposure frequency and HDP risk after adjustment for all models (p < 0.05).
In the multivariate analyses regarding the impact of SHS exposure duration on HDP risk, with adjustment as per Models 1, 2, and 3, the estimated risk of HDP development among pregnant women with SHS exposure of ≥2 h/day was ~1.3 times higher than that of the reference group (aRR: 1.30, 95% CI: 0.99–1.71; aRR: 1.28, 95% CI: 0.97–1.68; aRR: 1.27, 95% CI: 0.96–1.67, respectively); this effect was not statistically significant. A linear trend was also observed between the duration of SHS exposure per day and the risk of developing HDP after adjustment for all models (p < 0.05).
Supplementary Table 4 shows the results of the association between the frequency of SHS exposure and HDP based on stratification by maternal smoking status with the same adjustment as that in Model 4 (Table 3). No significant association was found between SHS exposure and HDP in any smoking group.
Table 4 shows the PAFs and their 95% CI of SHS exposure and maternal smoking to HDP prevalence. The PAFs of SHS exposures of 1–3 and 4–7 days/week to HDP were 1.1% and 2.7%, respectively (using the rare exposure group as the reference). When a similar analysis was performed on all the participants adjusted for smoking status, the PAFs of SHS exposures of 1–3 and 4–7 days/week to HDP were 0.9% and 2.6%, respectively. In the same population, the PAFs of quitting before pregnancy, quitting during pregnancy, and continued smoking were 0.9%, 0.4%, and 0.5%, respectively (using the never-smoker group as the reference). Among nonsmokers, the composite PAF due to SHS exposure was 3.8%, which was similar (3.5%) to that obtained in the whole population using the same analysis. The PAF due to perinatal smoking was 1.8%.
Similar results were found when including women with a history of miscarriage, abortion, and diagnosed complications (hypertension, renal diseases, diabetes mellitus, and dyslipidemia) (data not shown).
Discussion
This study is the first to report an association between SHS exposure and HDP risk in Japan. Women with SHS exposure of 4–7 days/week had a significantly higher risk of developing HDP. Although it was not statistically significant, a linear trend between the duration of SHS exposure per day and the risk of developing HDP after adjustment for possible confounding factors was observed in this nationwide prospective cohort study.
Previous investigations regarding the effects of SHS exposure on HDP risk during pregnancy have shown inconsistent results. For example, Engel et al. reported insufficient evidence regarding the association between SHS exposure and HDP risk; however, SHS exposure data were obtained using a yes/no questionnaire; thus, the quantity and frequency of SHS exposure were not accessible [9]. In contrast, Luo et al. reported that previous smoking and SHS exposure (defined according to the plasma cotinine level) may increase the risk of preeclampsia [10]. Although the utilized cutoff for plasma cotinine was reasonable [21], the effects of previous smoking and SHS exposure could not be estimated separately. Thus, the present study clarifies the association between SHS exposure and HDP risk.
Socioeconomic status is an important confounding factor when considering SHS. In Japan, educational inequalities among current and heavy smokers are more apparent and larger in the younger population than in older generations [22]. Using data from the T-CHILD study, which was conducted in Japan, Jwa et al. found an association between educational levels and BP levels at early gestation, which was mediated by prepregnancy BMI [23]. In the Generation R study, maternal educational levels were significantly associated with preeclampsia and gestational hypertension [4]. In the present study, maternal smoking was considered a possible mediator of socioeconomic status. We primarily aimed to understand the association between SHS and HDP risk; thus, we adjusted for socioeconomic factors, including educational levels, income, and maternal smoking, as confounders for SHS.
Several studies have indicated an association between SHS exposure and increased hypertension risk in the nonpregnant population. For example, Yang et al. conducted a large cross-sectional study using data from over 5 million women and their husbands from the National Free Prepregnancy Checkup Projects in China. The authors observed that having husbands who smoked were significantly associated with an increased prevalence of hypertension among their wives in categorical, dose‒response, and cumulative manners [24]. Additionally, Makris et al. reported that masked hypertension (defined as the mean clinic systolic and diastolic BP of <140 and 90 mmHg, respectively, conjointly with daytime systolic or diastolic BP of >135 and 85 mmHg, respectively) was associated with SHS exposure in a dose-related manner [25]. Seki et al. reported a relationship between SHS exposure and increased BP at home in Japanese nonpregnant women; compared to that in nonpregnant women without SHS exposure, home BP measurements differed by ~3–4 mmHg in nonpregnant women exposed to SHS at home and at the workplace [26].
The present study is also the first to evaluate the PAF of SHS exposure to HDP during pregnancy. The combined estimate of PAF due to SHS was 3.8%, i.e., approximately twice 1.8% of PAF due to perinatal smoking among pregnant women; the impact of SHS on HDP is larger than that of smoking in pregnant women; therefore, it is important to prevent SHS for better health of pregnant women. The PAF of SHS exposure to cancer has been investigated in Australia [27], Spain [28], Korea [29], and Japan [30]. Each of these studies concluded that SHS exposure was a preventable risk factor for cancer incidence. In a perinatal epidemiologic study, Ojima et al. estimated PAFs of 15.6, 1.1, and 7.0 for SHS exposure, at home and at the workplace, and active smoking, respectively, to low birth weights [31]. These studies also showed that public education on the avoidance of SHS exposure is very important.
Despite relatively comparable adjusted odds ratios, we observed a higher PAF of SHS exposure to HDP than that for maternal smoking to HDP. The number of women exposed to SHS perinatally and who smoked in early pregnancy were 37,546 (49.4%) and 13,226 (17.4%), respectively. The difference between the PAF of SHS exposure to HDP and that of maternal smoking to HDP likely derives from the difference in the population exposed. The avoidance of SHS exposure has a higher impact on public health than individual smoking discontinuation.
The present study has several limitations. First, we could not classify HDP into gestational hypertension, preeclampsia, and other specific conditions due to the nature of the questionnaire employed in this study. Therefore, we could not assess the effect of SHS exposure on each subtype of HDP. Furthermore, preeclampsia is less common among smokers (smoking-preeclampsia paradox) [32]; however, we could not assess the influence of the smoking-preeclampsia paradox on SHS exposure. Second, in the present study, PAFs could not reveal the population of pregnant women in Japan because many participants were excluded due to insufficient information on baseline characteristics or complications. Subsequently, we performed the same analysis, including participants with miscarriages, abortions, or complications, and reclassified the participants with missing information into another category. Although we detected similar trends in another category, the PAFs were larger than those of the analysis performed after exclusions. Third, although we adjusted for possible confounding factors available in the datasets, unmeasured confounding factors could also affect the risk of HDP development. For example, family history of hypertension, diabetes mellitus, and HDP are important factors that influence the development of HDP; however, these were not considered. Fourth, the analysis was conducted using the questionnaire item of SHS exposure before pregnancy as an indicator of SHS up to early pregnancy. There should be careful application of our study results because the decision to avoid SHS exposure may change with the cognition of pregnancy. Furthermore, information related to SHS exposure before pregnancy was collected using self-report questionnaires, which could cause underreporting of SHS exposure and its duration, leading to misclassification. Additionally, several previous studies reported high concordance rates between self-administered questionnaires and biomarkers [33, 34], suggesting some consistency with the present results. Finally, we could not examine the genetic background of the participants.
From the Asian perspective, the effects of smoking on the population may be influenced by differences in genetic background, as discussed in previous meta-analyses [11]. The risk of hypertension is higher in people with slow nicotine metabolism than in others [35], and the allele frequency of slow nicotine metabolism was high in the Chinese and Japanese populations, at 15% and 20%, respectively, whereas it was <5% in Caucasians [36]. These genetic background differences should be taken into account when considering strategies against SHS exposure.
In conclusion, women with greater SHS exposure showed a higher risk of HDP after adjustment for possible confounding factors. Thus, relevant strategies are needed to reduce SHS exposure in the population.
References
Zhang J, Zeisler J, Hatch MC, Berkowitz G. Epidemiology of pregnancy-induced hypertension. Epidemiol Rev. 1997;19:218–32.
Kaaja R. Predictors and risk factors of pre-eclampsia. Minerva Ginecol. 2008;60:421–9.
Magnussen EB, Vatten LJ, Lund-Nilsen TI, Salvesen KA, Davey Smith G, Romundstad PR. Prepregnancy cardiovascular risk factors as predictors of pre-eclampsia: population based cohort study. BMJ. 2007;335:978.
Silva L, Coolman M, Steegers E, Jaddoe V, Moll H, Hofman A, et al. Maternal educational level and risk of gestational hypertension: the Generation R Study. J Hum Hypertens. 2008;22:483–92.
Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi JM. Pre-eclampsia: pathophysiology, diagnosis, and management. Vasc Health Risk Manag. 2011;7:467–74.
Suzuki K, Shinohara R, Sato M, Otawa S, Yamagata Z. Association Between maternal smoking During pregnancy and birth weight: an appropriately adjusted model From the Japan environment and children’s study. J Epidemiol. 2016;26:371–7.
Polanska K, Krol A, Merecz-Kot D, Ligocka D, Mikolajewska K, Mirabella F, et al. Environmental tobacco smoke exposure during pregnancy and child neurodevelopment. Int J Environ Res Public Health. 2017;14:796.
Windham GC, Hopkins B, Fenster L, Swan SH. Prenatal active or passive tobacco smoke exposure and the risk of preterm delivery or low birth weight. Epidemiology. 2000;11:427–33.
Engel SM, Scher E, Wallenstein S, Savitz DA, Alsaker ER, Trogstad L, et al. Maternal active and passive smoking and hypertensive disorders of pregnancy: risk with trimester-specific exposures. Epidemiology. 2013;24:379–86.
Luo ZC, Julien P, Wei SQ, Audibert F, Smith GN, Fraser WD, et al. Plasma cotinine indicates an increased risk of preeclampsia in previous and passive smokers. Am J Obstet Gynecol. 2014;210:232.e1–5.
Morisaki N, Obara T, Piedvache A, Kobayashi S, Miyashita C, Nishimura T, et al. Association between smoking and hypertension in pregnancy among Japanese women: a meta-analysis of birth cohort studies in the Japan Birth Cohort Consortium (JBiCC) and JECS. J Epidemiol. 2022.
Kawamoto T, Nitta H, Murata K, Toda E, Tsukamoto N, Hasegawa M, et al. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health. 2014;14:25.
Michikawa T, Nitta H, Nakayama SF, Yamazaki S, Isobe T, Tamura K, et al. Baseline profile of participants in the Japan Environment and Children’s Study (JECS). J Epidemiol. 2018;28:99–104.
Kyozuka H, Murata T, Fukuda T, Yamaguchi A, Kanno A, Yasuda S, et al. Association between pre-pregnancy calcium intake and hypertensive disorders during the first pregnancy: the Japan environment and children’s study. BMC Preg Childbirth. 2020;20:424.
Kyozuka H, Fukusda T, Murata T, Yamaguchi A, Kanno A, Yasuda S, et al. Impact of preconception sodium intake on hypertensive disorders of pregnancy: the Japan Environment and Children’s study. Preg Hypertens. 2021;23:66–72.
Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SL, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med. 2002;32:959–76.
Furukawa TA, Kawakami N, Saitoh M, Ono Y, Nakane Y, Nakamura Y, et al. The performance of the Japanese version of the K6 and K10 in the World Mental Health Survey Japan. Int J Methods Psychiatr Res. 2008;17:152–8.
Watanabe Z, Iwama N, Nishigori H, Nishigori T, Mizuno S, Sakurai K, et al. Psychological distress during pregnancy in Miyagi after the Great East Japan Earthquake: the Japan Environment and Children’s Study. J Affect Disord. 2016;190:341–8.
Watanabe K, Naruse K, Tanaka K, Metoki H, Suzuki Y. Outline of definition and classification of “Pregnancy induced hypertension (PIH)”. Hypertens Res Preg. 2013;1:3–4.
Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200.
Sasaki S, Braimoh TS, Yila TA, Yoshioka E, Kishi R. Self-reported tobacco smoke exposure and plasma cotinine levels during pregnancy—a validation study in Northern Japan. Sci Total Environ. 2011;412–413:114–8.
Tabuchi T, Kondo N. Educational inequalities in smoking among Japanese adults aged 25–94 years: nationally representative sex- and age-specific statistics. J Epidemiol. 2017;27:186–92.
Jwa SC, Fujiwara T, Hata A, Arata N, Sago H, Ohya Y. BMI mediates the association between low educational level and higher blood pressure during pregnancy in Japan. BMC Public Health. 2013;13:389.
Yang Y, Liu F, Wang L, Li Q, Wang X, Chen JC, et al. Association of husband smoking With Wife’s hypertension status in over 5 million Chinese females aged 20 to 49 years. J Am Heart Assoc. 2017;6:e004924.
Makris TK, Thomopoulos C, Papadopoulos DP, Bratsas A, Papazachou O, Massias S, et al. Association of passive smoking with masked hypertension in clinically normotensive nonsmokers. Am J Hypertens. 2009;22:853–9.
Seki M, Inoue R, Ohkubo T, Kikuya M, Hara A, Metoki H, et al. Association of environmental tobacco smoke exposure with elevated home blood pressure in Japanese women: the Ohasama study. J Hypertens. 2010;28:1814–20.
Wilson LF, Antonsson A, Green AC, Jordan SJ, Kendall BJ, Nagle CM, et al. How many cancer cases and deaths are potentially preventable? Estimates for Australia in 2013. Int J Cancer. 2018;142:691–701.
López MJ, Pérez-Ríos M, Schiaffino A, Fernández E. Mortality attributable to secondhand smoke exposure in Spain (2011). Nicotine Tob Res. 2016;18:1307–10.
Park S, Jee SH, Shin HR, Park EH, Shin A, Jung KW, et al. Attributable fraction of tobacco smoking on cancer using population-based nationwide cancer incidence and mortality data in Korea. BMC Cancer. 2014;14:406.
Inoue M, Sawada N, Matsuda T, Iwasaki M, Sasazuki S, Shimazu T, et al. Attributable causes of cancer in Japan in 2005—systematic assessment to estimate current burden of cancer attributable to known preventable risk factors in Japan. Ann Oncol. 2012;23:1362–9.
Ojima T, Uehara R, Watanabe M, Tajimi M, Oki I, Nakamura Y. Population attributable fraction of smoking to low birth weight in Japan. Pediatr Int. 2004;46:264–7.
Luque-Fernandez MA, Zoega H, Valdimarsdottir U, Williams MA. Deconstructing the smoking-preeclampsia paradox through a counterfactual framework. Eur J Epidemiol. 2016;31:613–23.
Arbuckle TE, Liang CL, Fisher M, Caron NJ, Fraser WD, and the MIREC Study Group. Exposure to tobacco smoke and validation of smoking status during pregnancy in the MIREC study. J Expo Sci Environ Epidemiol. 2018;28:461–9.
Tourangeau R, Yan T, Sun H, Hyland A, Stanton CA. Population Assessment of Tobacco and Health (PATH) reliability and validity study: selected reliability and validity estimates. Tob Control. 2019;28:663–8.
Liu T, Tyndale RF, David SP, Wang H, Yu XQ, Chen W, et al. J Hum Hypertens. 2013;27:24–30.
Raunio H, Rautio A, Gullstén H, Pelkonen O. Polymorphisms of CYP2A6 and its practical consequences. Br J Clin Pharm. 2001;52:357–63.
Acknowledgements
We would like to express our gratitude to all of the JECS study participants and to the cooperating health care providers. Members of the Japan Environment and Children’s Study (JECS) as of 2021 (principal investigator, Michihiro Kamijima, Nagoya City University, Nagoya, Japan) are as follows: Shin Yamazaki (National Institute for Environmental Studies, Tsukuba, Japan), Yukihiro Ohya (National Center for Child Health and Development, Tokyo, Japan), Reiko Kishi (Hokkaido University, Sapporo, Japan), Nobuo Yaegashi (Tohoku University, Sendai, Japan), Koichi Hashimoto (Fukushima Medical University, Fukushima, Japan), Chisato Mori (Chiba University, Chiba, Japan), Shuichi Ito (Yokohama City University, Yokohama, Japan), Zentaro Yamagata (University of Yamanashi, Chuo, Japan), Hidekuni Inadera (University of Toyama, Toyama, Japan), Takeo Nakayama (Kyoto University, Kyoto, Japan), Tomotaka Sobue (Osaka University, Suita, Japan), Masayuki Shima (Hyogo Medical University, Nishinomiya, Japan), Hiroshige Nakamura (Tottori University, Yonago, Japan), Naruhumi Sugawara (Kochi University, Nankoku, Japan), Koichi Kusuhara (University of Occupational and Environmental Health, Kitakyushu, Japan), and Takahiko Katoh (Kumamoto University, Kumamoto, Japan).
This analysis was partly supported by Grants for Scientific Research (No. 16H05243) from the Ministry of Japan Grants from Astellas Research Support, the Pfizer Academic Contributions, and Chugai Pharmaceutical Co., Ltd. This research was also supported by Research Promotion and Practical Use for Women’s Health, AMED.
The findings and conclusions of this article are solely the responsibility of the authors, and they do not represent the official views of the government. We thank Editage (www.editage.jp) for English language editing.
Funding
The Japan Environment and Children’s Study was funded by the Ministry of the Environment, Japan.
Author information
Authors and Affiliations
Consortia
Contributions
K Tanaka initiated the study and wrote the manuscript. HM and HN contributed to collecting data, evaluating strategy, analysis and discussion, and reviewing and editing the manuscript. SM, KS, MI, TO, NT, IF, SK, TA, KN, and NY contributed to collecting data and reviewing the manuscript. ZW, NI, M Satoh, and TM evaluated the strategy, analysis and discussion and reviewed the manuscript. K Tanoue, TH, and M Saito reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tanaka, K., Nishigori, H., Watanabe, Z. et al. Secondhand smoke exposure is associated with the risk of hypertensive disorders of pregnancy: the Japan Environment and Children’s Study. Hypertens Res 46, 834–844 (2023). https://doi.org/10.1038/s41440-022-01144-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-022-01144-3
Keywords
This article is cited by
-
Environments affect blood pressure in toddlers: The Japan Environment and Children’s Study
Pediatric Research (2024)
-
The impacts of secondhand smoke on future generations and the responsibility of society as a whole to protect the well-being of our future descendants
Hypertension Research (2023)
-
The importance of lifestyle modification for hypertension in Asia
Hypertension Research (2023)