Abstract
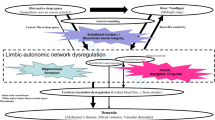
Circadian blood pressure (BP) rhythm is important for the maintenance of healthy daily life, and its disruption is associated with poor outcomes. Cardiovascular autonomic failure is often observed in older populations but has a greater impact on neurodegenerative disorders such as α-synucleinopathies. These BP abnormalities include orthostatic hypotension (OH), supine hypertension (SH), and a loss of nocturnal BP fall. OH not only causes falls or syncope but is also related to cognitive impairment in α-synucleinopathies. For example, OH doubles or triples the risk for the development of cognitive impairment in Parkinson’s disease (PD). The diffuse central and peripheral neuropathology of α-synuclein may contribute to both OH and cognitive impairment. Moreover, repeated cerebral hypoperfusion in OH is thought to be related to cerebrovascular and neuronal damage, which may cause cognitive impairment. SH, which often coexists with OH, is also associated with cognitive impairment through cerebrovascular damage, such as white matter lesions and cerebral microbleeds. The reverse-dipping (riser) pattern on ambulatory BP monitoring is commonly observed in PD (∼56%), regardless of disease duration and severity. It is also related to cognitive impairment and more pronounced when coexisting with OH. These abnormal circadian BP profiles may be synergistically associated with cognitive impairment and poor outcomes in α-synucleinopathies. Although evidence for aggressive control of BP dysregulation improving cognitive impairment and outcomes is limited, regular BP monitoring appears to be important for total management of α-synucleinopathies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Rutan GH, Hermanson B, Bild DE, Kittner SJ, LaBaw F, Tell GS. Orthostatic hypotension in older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Hypertension 1992;19:508–19.
Ooi WL, Hossain M, Lipsitz LA. The association between orthostatic hypotension and recurrent falls in nursing home residents. Am J Med. 2000;108:106–11.
Weiss A, Grossman E, Beloosesky Y, Grinblat J. Orthostatic hypotension in acute geriatric ward: is it a consistent finding? Arch Intern Med. 2002;162:2369–74.
Farrell MC, Shibao CA. Morbidity and mortality in orthostatic hypotension. Auton Neurosci. 2020;229:102717.
Ricci F, Fedorowski A, Radico F, Romanello M, Tatasciore A, Di Nicola M, et al. Cardiovascular morbidity and mortality related to orthostatic hypotension: a meta-analysis of prospective observational studies. Eur Heart J. 2015;36:1609–17.
Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72.
Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021;397:2284–303.
Fanciulli A, Wenning GK. Multiple-system atrophy. N. Engl J Med. 2015;372:249–63.
Walker Z, Possin KL, Boeve BF, Aarsland D. Lewy body dementias. Lancet. 2015;386:1683–97.
Spillantini MG, Goedert M. The alpha-synucleinopathies: Parkinson’s disease, dementia with Lewy bodies, and multiple system atrophy. Ann N. Y Acad Sci. 2000;920:16–27.
Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome). J Neurol Sci. 1989;94:79–100.
Mendoza-Velasquez JJ, Flores-Vazquez JF, Barron-Velazquez E, Sosa-Ortiz AL, Illigens BW, Siepmann T. Autonomic Dysfunction in alpha-Synucleinopathies. Front Neurol. 2019;10:363.
Norcliffe-Kaufmann L, Kaufmann H, Palma JA, Shibao CA, Biaggioni I, Peltier AC, et al. Orthostatic heart rate changes in patients with autonomic failure caused by neurodegenerative synucleinopathies. Ann Neurol. 2018;83:522–31.
Velseboer DC, de Haan RJ, Wieling W, Goldstein DS, de Bie RM. Prevalence of orthostatic hypotension in Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2011;17:724–9.
Wullner U, Schmitz-Hubsch T, Antony G, Fimmers R, Spottke A, Oertel WH, et al. Autonomic dysfunction in 3414 Parkinson’s disease patients enrolled in the German Network on Parkinson’s disease (KNP e.V.): the effect of ageing. Eur J Neurol. 2007;14:1405–8.
Senard JM, Raï S, Lapeyre-Mestre M, Brefel C, Rascol O, Rascol A, et al. Prevalence of orthostatic hypotension in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1997;63:584–9.
Stubendorff K, Aarsland D, Minthon L, Londos E. The impact of autonomic dysfunction on survival in patients with dementia with Lewy bodies and Parkinson’s disease with dementia. PLoS One. 2012;7:e45451.
Thaisetthawatkul P, Boeve BF, Benarroch EE, Sandroni P, Ferman TJ, Petersen R, et al. Autonomic dysfunction in dementia with Lewy bodies. Neurology 2004;62:1804–9.
Pavy-Le Traon A, Piedvache A, Perez-Lloret S, Calandra-Buonaura G, Cochen-De Cock V, Colosimo C, et al. New insights into orthostatic hypotension in multiple system atrophy: a European multicentre cohort study. J Neurol Neurosurg Psychiatry. 2016;87:554–61.
Fereshtehnejad SM, Lokk J. Orthostatic hypotension in patients with Parkinson’s disease and atypical parkinsonism. Parkinsons Dis. 2014;2014:475854.
Palma JA, Kaufmann H. Epidemiology, diagnosis, and management of neurogenic orthostatic hypotension. Mov Disord Clin Pr. 2017;4:298–308.
Chelban V, Catereniuc D, Aftene D, Gasnas A, Vichayanrat E, Iodice V, et al. An update on MSA: premotor and non-motor features open a window of opportunities for early diagnosis and intervention. J Neurol. 2020;267:2754–70.
Fanciulli A, Campese N, Goebel G, Ndayisaba JP, Eschlboeck S, Kaindlstorfer C, et al. Association of transient orthostatic hypotension with falls and syncope in patients with Parkinson disease. Neurology 2020;95:e2854–e65.
De Pablo-Fernandez E, Tur C, Revesz T, Lees AJ, Holton JL, Warner TT. Association of autonomic dysfunction with disease progression and survival in Parkinson's disease. JAMA Neurol. 2017;74:970–6.
Fanciulli A, Jordan J, Biaggioni I, Calandra-Buonaura G, Cheshire WP, Cortelli P, et al. Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the American Autonomic Society (AAS) and the European Federation of Autonomic Societies (EFAS): Endorsed by the European Academy of Neurology (EAN) and the European Society of Hypertension (ESH). Clin Auton Res. 2018;28:355–62.
Fanciulli A, Göbel G, Ndayisaba JP, Granata R, Duerr S, Strano S, et al. Supine hypertension in Parkinson’s disease and multiple system atrophy. Clin Auton Res. 2016;26:97–105.
Kim JS, Oh YS, Lee KS, Kim YI, Yang DW, Goldstein DS. Association of cognitive dysfunction with neurocirculatory abnormalities in early Parkinson disease. Neurology 2012;79:1323–31.
Umehara T, Matsuno H, Toyoda C, Oka H. Clinical characteristics of supine hypertension in de novo Parkinson disease. Clin Auton Res. 2016;26:15–21.
Vagaonescu TD, Saadia D, Tuhrim S, Phillips RA, Kaufmann H. Hypertensive cardiovascular damage in patients with primary autonomic failure. Lancet 2000;355:725–6.
Maule S, Milan A, Grosso T, Veglio F. Left ventricular hypertrophy in patients with autonomic failure. Am J Hypertens. 2006;19:1049–54.
Garland EM, Gamboa A, Okamoto L, Raj SR, Black BK, Davis TL, et al. Renal impairment of pure autonomic failure. Hypertension 2009;54:1057–61.
Palma JA, Redel-Traub G, Porciuncula A, Samaniego-Toro D, Millar Vernetti P, Lui YW, et al. The impact of supine hypertension on target organ damage and survival in patients with synucleinopathies and neurogenic orthostatic hypotension. Parkinsonism Relat Disord. 2020;75:97–104.
Schmidt C, Berg D, Herting, Prieur S, Junghanns S, Schweitzer K, et al. Loss of nocturnal blood pressure fall in various extrapyramidal syndromes. Mov Disord. 2009;24:2136–42.
Fanciulli A, Strano S, Ndayisaba JP, Goebel G, Gioffre L, Rizzo M, et al. Detecting nocturnal hypertension in Parkinson’s disease and multiple system atrophy: proposal of a decision-support algorithm. J Neurol. 2014;261:1291–9.
Pilleri M, Levedianos G, Weis L, Gasparoli E, Facchini S, Biundo R, et al. Heart rate circadian profile in the differential diagnosis between Parkinson disease and multiple system atrophy. Parkinsonism Relat Disord. 2014;20:217–21.
Vichayanrat E, Low DA, Iodice V, Stuebner E, Hagen EM, Mathias CJ. Twenty-four-hour ambulatory blood pressure and heart rate profiles in diagnosing orthostatic hypotension in Parkinson’s disease and multiple system atrophy. Eur J Neurol. 2017;24:90–7.
Tanaka R, Shimo Y, Yamashiro K, Ogawa T, Nishioka K, Oyama G, et al. Association between abnormal nocturnal blood pressure profile and dementia in Parkinson’s disease. Parkinsonism Relat Disord. 2018;46:24–9.
Milazzo V, Di Stefano C, Vallelonga F, Sobrero G, Zibetti M, Romagnolo A, et al. Reverse blood pressure dipping as marker of dysautonomia in Parkinson disease. Parkinsonism Relat Disord. 2018;56:82–7.
Di Stefano C, Sobrero G, Milazzo V, Vallelonga F, Romagnolo A, Zibetti M, et al. Cardiac organ damage in patients with Parkinson’s disease and reverse dipping. J Hypertens. 2020;38:289–94.
Vallelonga F, Romagnolo A, Merola A, Sobrero G, Di Stefano C, Milazzo V, et al. Detection of orthostatic hypotension with ambulatory blood pressure monitoring in Parkinson's disease. Hypertens Res. 2019;42:1552–60.
Vallelonga F, Di Stefano C, Merola A, Romagnolo A, Sobrero G, Milazzo V, et al. Blood pressure circadian rhythm alterations in alpha-synucleinopathies. J Neurol. 2019;266:1141–52.
Lodhi HA, Peri-Okonny PA, Schesing K, Phelps K, Ngo C, Evans H, et al. Usefulness of blood pressure variability indices derived from 24-hour ambulatory blood pressure monitoring in detecting autonomic failure. J Am Heart Assoc. 2019;8:e010161.
Vallelonga F, Sobrero G, Giudici M, Valente M, Milazzo V, Di Stefano C, et al. Screening indexes for cardiovascular autonomic failure in Parkinson’s disease. J Neurol Sci. 2021;428:117571.
Vallelonga F, Sobrero G, Merola A, Valente M, Giudici M, Di Stefano C, et al. Machine learning applied to ambulatory blood pressure monitoring: a new tool to diagnose autonomic failure? J Neurol. 2022;269:3833–40.
Lawson RA, Yarnall AJ, Duncan GW, Khoo TK, Breen DP, Barker RA, et al. Severity of mild cognitive impairment in early Parkinson’s disease contributes to poorer quality of life. Parkinsonism Relat Disord. 2014;20:1071–5.
Leroi I, McDonald K, Pantula H, Harbishettar V. Cognitive impairment in Parkinson disease: impact on quality of life, disability, and caregiver burden. J Geriatr Psychiatry Neurol. 2012;25:208–14.
Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson disease. J Neurol Sci. 2010;289:18–22.
Pedersen KF, Larsen JP, Tysnes OB, Alves G. Natural course of mild cognitive impairment in Parkinson disease: A 5-year population-based study. Neurology 2017;88:767–74.
Anang JB, Gagnon JF, Bertrand JA, Romenets SR, Latreille V, Panisset M, et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology 2014;83:1253–60.
Hiorth YH, Pedersen KF, Dalen I, Tysnes OB, Alves G. Orthostatic hypotension in Parkinson disease: A 7-year prospective population-based study. Neurology 2019;93:e1526–e34.
Guo Y, Liu FT, Hou XH, Li JQ, Cao XP, Tan L, et al. Predictors of cognitive impairment in Parkinson’s disease: a systematic review and meta-analysis of prospective cohort studies. J Neurol. 2021;268:2713–22.
Stankovic I, Krismer F, Jesic A, Antonini A, Benke T, Brown RG, et al. Cognitive impairment in multiple system atrophy: a position statement by the Neuropsychology Task Force of the MDS Multiple System Atrophy (MODIMSA) study group. Mov Disord. 2014;29:857–67.
Cuoco S, Carotenuto I, Cappiello A, Scannapieco S, Russillo MC, Andreozzi V, et al. Relationship between orthostatic hypotension and cognitive functions in multiple system atrophy: a longitudinal study. Front Neurol. 2021;12:711358.
Tanaka R, Yamashiro K, Ogawa T, Oyama G, Nishioka K, Umemura A, et al. The absence of orthostatic heart rate increase is associated with cognitive impairment in Parkinson’s disease. PLoS One. 2020;15:e0240491.
Verbaan D, Marinus J, Visser M, van Rooden SM, Stiggelbout AM, van Hilten JJ. Patient-reported autonomic symptoms in Parkinson disease. Neurology 2007;69:333–41.
Udow SJ, Robertson AD, MacIntosh BJ, Espay AJ, Rowe JB, Lang AE, et al. ‘Under pressure’: is there a link between orthostatic hypotension and cognitive impairment in alpha-synucleinopathies? J Neurol Neurosurg Psychiatry. 2016;87:1311–21.
Töyry JP, Kuikka JT, Länsimies EA. Regional cerebral perfusion in cardiovascular reflex syncope. Eur J Nucl Med. 1997;24:215–8.
Centi J, Freeman R, Gibbons CH, Neargarder S, Canova AO, Cronin-Golomb A. Effects of orthostatic hypotension on cognition in Parkinson disease. Neurology 2017;88:17–24.
Kim T, Mehta SL, Kaimal B, Lyons K, Dempsey RJ, Vemuganti R. Poststroke induction of α-Synuclein mediates ischemic brain damage. J Neurosci. 2016;36:7055–65.
Lohmann S, Grigoletto J, Bernis ME, Pesch V, Ma L, Reithofer S, et al. Ischemic stroke causes Parkinson’s disease-like pathology and symptoms in transgenic mice overexpressing alpha-synuclein. Acta Neuropathol Commun. 2022;10:26.
Rodriguez-Perez AI, Dominguez-Meijide A, Lanciego JL, Guerra MJ, Labandeira-Garcia JL. Dopaminergic degeneration is enhanced by chronic brain hypoperfusion and inhibited by angiotensin receptor blockage. Age. 2013;35:1675–90.
Irwin DJ, Lee VM, Trojanowski JQ. Parkinson’s disease dementia: convergence of α-synuclein, tau and amyloid-β pathologies. Nat Rev Neurosci. 2013;14:626–36.
Kleipool EEF, Trappenburg MC, Rhodius-Meester HFM, Lemstra AW, van der Flier WM, Peters MJL, et al. Orthostatic hypotension: an important risk factor for clinical progression to mild cognitive impairment or dementia. The Amsterdam Dementia Cohort. J Alzheimers Dis. 2019;71:317–25.
Xia X, Wang R, Vetrano DL, Grande G, Laukka EJ, Ding M, et al. From normal cognition to cognitive impairment and dementia: impact of orthostatic hypotension. Hypertension 2021;78:769–78.
Zhang J, Chi H, Wang T, Zhang S, Shen T, Leng B, et al. Altered Amyloid-β and Tau proteins in neural-derived plasma exosomes of Type 2 diabetes patients with orthostatic hypotension. J Alzheimers Dis. 2021;82:261–72.
Cai Z, Liu Z, Xiao M, Wang C, Tian F. Chronic cerebral hypoperfusion promotes amyloid-beta pathogenesis via activating β/γ-Secretases. Neurochem Res. 2017;42:3446–55.
de la Torre JC. Deciphering Alzheimer’s disease pathogenic pathway: role of chronic brain hypoperfusion on p-Tau and mTOR. J Alzheimers Dis. 2021;79:1381–96.
Ihara M, Washida K. Linking atrial fibrillation with Alzheimer’s disease: epidemiological, pathological, and mechanistic evidence. J Alzheimers Dis. 2018;62:61–72.
Bohnen NI, Albin RL. White matter lesions in Parkinson's disease. Nat Rev Neurol. 2011;7:229–36.
Umoto M, Miwa H, Ando R, Kajimoto Y, Kondo T. White matter hyperintensities in patients with multiple system atrophy. Parkinsonism Relat Disord. 2012;18:17–20.
Daida K, Tanaka R, Yamashiro K, Ogawa T, Oyama G, Nishioka K, et al. The presence of cerebral microbleeds is associated with cognitive impairment in Parkinson’s disease. J Neurol Sci. 2018;393:39–44.
Yamashiro K, Tanaka R, Hoshino Y, Hatano T, Nishioka K, Hattori N. The prevalence and risk factors of cerebral microbleeds in patients with Parkinson’s disease. Parkinsonism Relat Disord. 2015;21:1076–81.
Espay AJ, LeWitt PA, Hauser RA, Merola A, Masellis M, Lang AE. Neurogenic orthostatic hypotension and supine hypertension in Parkinson’s disease and related synucleinopathies: prioritisation of treatment targets. Lancet Neurol. 2016;15:954–66.
Yamashiro K, Tanaka R, Shimo Y, Oyama G, Ogawa T, Umemura A, et al. Cerebral microbleeds and blood pressure abnormalities in Parkinson’s disease. eNeurologicalSci 2018;10:5–11.
Vesely B, Rektor I. The contribution of white matter lesions (WML) to Parkinson’s disease cognitive impairment symptoms: A critical review of the literature. Parkinsonism Relat Disord. 2016;22:S166–70.
Pilotto A, Romagnolo A, Scalvini A, Masellis M, Shimo Y, Bonanni L, et al. Association of orthostatic hypotension with cerebral atrophy in patients with lewy body disorders. Neurology 2021;97:e814–e24.
Chen SW, Wang YK, Dou RH, Xie XY, Hu YB, Ding N, et al. Characteristics of the 24-h ambulatory blood pressure monitoring in patients with Parkinson’s disease—the SFC BP multicentre study in China. J Hypertens. 2020;38:2270–8.
Kanemaru A, Kanemaru K, Kuwajima I. The effects of short-term blood pressure variability and nighttime blood pressure levels on cognitive function. Hypertens Res. 2001;24:19–24.
Yano Y, Inokuchi T, Hoshide S, Kanemaru Y, Shimada K, Kario K. Association of poor physical function and cognitive dysfunction with high nocturnal blood pressure level in treated elderly hypertensive patients. Am J Hypertens. 2011;24:285–91.
Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Ambulatory blood pressure as an independent determinant of brain atrophy and cognitive function in elderly hypertension. J Hypertens. 2008;26:1636–41.
Senard JM, Brefel-Courbon C, Rascol O, Montastruc JL. Orthostatic hypotension in patients with Parkinson’s disease: pathophysiology and management. Drugs Aging. 2001;18:495–505.
Freeman R, Abuzinadah AR, Gibbons C, Jones P, Miglis MG, Sinn DI. Orthostatic hypotension: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:1294–309.
Palma JA, Gomez-Esteban JC, Norcliffe-Kaufmann L, Martinez J, Tijero B, Berganzo K, et al. Orthostatic hypotension in Parkinson disease: how much you fall or how low you go? Mov Disord. 2015;30:639–45.
Goldberger ZD, Petek BJ, Brignole M, Shen WK, Sheldon RS, Solbiati M, et al. ACC/AHA/HRS Versus ESC Guidelines for the Diagnosis and Management of Syncope: JACC guideline comparison. J Am Coll Cardiol. 2019;74:2410–23.
Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group. JAMA 1997;277:1046–51.
Kaufmann H, Freeman R, Biaggioni I, Low P, Pedder S, Hewitt LA, et al. Droxidopa for neurogenic orthostatic hypotension: a randomized, placebo-controlled, phase 3 trial. Neurology 2014;83:328–35.
Zhao S, Cheng R, Zheng J, Li Q, Wang J, Fan W, et al. A randomized, double-blind, controlled trial of add-on therapy in moderate-to-severe Parkinson’s disease. Parkinsonism Relat Disord. 2015;21:1214–8.
Gibbons CH, Schmidt P, Biaggioni I, Frazier-Mills C, Freeman R, Isaacson S, et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol. 2017;264:1567–82.
Vallelonga F, Maule S. Diagnostic and therapeutical management of supine hypertension in autonomic failure: a review of the literature. J Hypertens. 2019;37:1102–11.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RT has received honoraria from Daiichi Sankyo, Bayer, and Eisai. NH has received consulting fees from Hisamitsu Pharma and PARKINSON Laboratories Co., Ltd., received honoraria from Dai-Nippon Sumitomo Pharma, Kyowa Kirin Co., Ltd., Takeda Pharmaceutical, AbbVie GK, Nippon Boehringer Ingelheim, Otsuka Pharmaceutical, Novartis Pharma, Bristol-Myers Squibb, Ono Pharmaceutical, FP Pharmaceutical, Eisai, Kissei Pharmaceutical, Nihon Medi-physics, and Daiichi Sankyo, and received payment for expert testimony from Dai-Nippon Sumitomo Pharma, Kyowa Kirin Co., Ltd., Takeda Pharmaceutical, TEIJIN PHARMA LIMITED, Novartis Pharma, Ono Pharmaceutical, Biogen Idec Japan, Kissei Pharmaceutical, and Mitsubishi Tanabe Pharma and Expert testimony (Honoraria as a team leader) from RIKEN: Center for Brain Science, and stroke ownership and outside the submitted work from PARKINSON Laboratories Co., Ltd.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tanaka, R., Hattori, N. Abnormal circadian blood pressure regulation and cognitive impairment in α-synucleinopathies. Hypertens Res 45, 1908–1917 (2022). https://doi.org/10.1038/s41440-022-01032-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-022-01032-w