Abstract
In Parkinson’s disease (PD), cardiovascular dysautonomia accumulates with disease progression, but studies are lacking on the natural history behind each subtype except orthostatic hypotension. This study investigated the early natural history of orthostatic blood pressure (BP) subtypes in PD. Two hundred sixty-seven early PD patients were included. Their cardiovascular functions were assessed by head-up tilt-test and 123I-metaiodobenzylguanidine scintigraphy. All patients were classified as having supine hypertension (SH), orthostatic hypertension (OHT), delayed orthostatic hypotension (dOH), or orthostatic hypotension (OH) according to consensus criteria. The patients were assigned to one of three groups: extreme BP dysregulation (BPextreme), mild BP dysregulation (BPmild), and no BP dysregulation (BPnone) according to their orthostatic BP subtypes. The autonomic functions of 237 patients were re-assessed after approximately 3 years. Among initially enrolled subjects, 61.8% of the patients showed orthostatic BP dysregulation: 29.6% in the BPextreme group and 32.2% in the BPmild group. At follow-up, the BPextreme group increased in number, while the BPmild group diminished. Two-thirds of the initial BPextreme patients maintained their initial subtype at follow-up. In comparison, 40.7% of the initial BPmild patients progressed to the BPextreme group, and 32.4% and 14.7% of the initial BPnone group progressed to BPextreme and BPmild groups, respectively. Cardiac denervation was most severe in the BPextreme group, and a linear gradient of impairment was observed across the subtypes. In conclusion, various forms of positional BP dysregulation were observed during the early disease stage. SH and OH increased with disease progression, while OHT and dOH decreased, converting primarily to SH and/or OH.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is a neurocardiologic disorder that includes a spectrum of positional blood pressure (BP) dysregulations ranging from hypertensive to hypotensive states1. BP dysregulation is a continuum in its respective body position, demarcated by operational criteria. These positional BP fluctuations can take the form of supine hypertension (SH), orthostatic hypertension (OHT), delayed orthostatic hypotension (dOH), or orthostatic hypotension (OH)2,3,4,5.
The pathophysiology of orthostatic BP dysregulation is multifaceted and involves central and peripheral catecholaminergic deficits6,7, and pathobiological inter-relay between dOH and OH has been suggested3,8. Delayed OH is a mild form of OH5. These findings suggest that the range of positional hemodynamics represents different levels of adrenergic derangement. Also, each orthostatic subtype can influence the clinical outcomes negatively9,10,11,12, highlighting the need to investigate the characteristics of each.
Many studies have focused on the neurobiology and outcome of OH in PD6; dOH, SH, and OHT have received relatively little attention until recently3,5, and their inter-relationships have been ignored. This research aimed to describe the features of orthostatic subtypes in early de novo PD. We focused on the interrelationships of these subtypes through disease progression and on their early changes to minimize confounders of comorbidities and dopaminergic medication use.
Results
Clinical and autonomic characteristics
The clinical and autonomic features of the patients are summarized in Tables 1 and 2. Seventy-nine of 267 patients (29.6%) were classified as BPextreme, and 86 (32.2%) were classified as BPmild at enrollment. Overall, 61.8% of the total population had orthostatic BP dysregulation. The mean age at diagnosis was 66.9 ± 9.1 years, and 128 (47.9%) were female. Disease duration at diagnosis was 13.3 ± 10.8 months, and patients were monitored clinically through an average period of 61.4 ± 21.8 months (Supplementary Fig. 1). The age at diagnosis was oldest in the BPextreme group, and this tendency persisted during follow-up. The total follow-up period and levodopa equivalent daily dose (LEDD) did not differ across subgroups, and there were no differences among groups in the types of drugs used to treat PD symptoms at each patient’s endpoint. Antihypertensive classes were not different, with fewer than six participants in each subgroup taking beta-blockers.
At initial evaluation, 23 PD patients (8.6%) were hypertensive in the supine position; 48 (18.0%) patients were OH. Twenty-two (8.2%) were determined to have delayed OH, and 64 (24.0%) had orthostatic hypertension. The two OH groups were not different in age at diagnosis, disease duration, or H/M ratio (Supplementary Table 1). The prevalence of OH increased during the follow-up examination period. More than 95% of PD patients with OH experienced reduced cardiac baroreflex gain, indicating its neurogenic origin (initial vs. follow-up ∆HR/∆SBP3min < 0.5, 95.8% vs. 96.1%).
The initial converted MDS-UPDRS Part II score was 6.6 ± 4.3, and this did not differ among the orthostatic subgroups. This lack of difference remained at follow-up between-group comparisons. The initial motor score, part III, was 18.5 ± 9.1, and its severity was similar between groups. This score increased to 19.5 ± 11.0 at the follow-up exam, and the BPextreme group had more severe motor impairment than the BPnone group (21.1 ± 12.2 vs. 17.5 ± 7.6). The median H&Y stage was two; this grade was maintained throughout the follow-up. PIGD and AR phenotypes were the dominant forms. The motor phenotype was significantly associated with the initial orthostatic subtype, particularly BPextreme related to PIGD (TD´ vs. PIGD vs. intermediate, 16.5% vs. 69.6% vs. 13.9%).
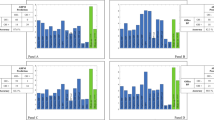
The overall H/M uptake ratios for both early and delayed groups were below the pre-defined reference. The BPextreme group had the smallest tracer uptake ratio overall and the highest washout rate (WR). Trend analyses of the tracer uptake ratio revealed a gradient of increase or decrease in the groups (Fig. 1). Early and delayed H/M ratio demonstrated a linear increase, while the WR showed decremental linearity across subtypes (BPextreme vs. BPmild vs. BPnone).
a, b represent the linear trend of cardiac denervation at initial (n = 267) and follow-up (n = 237) populations, respectively. A negative z value in Jonckheere-Terpstra test indicates an ascending linear trend, whereas a positive value represents a descending linear trend across groups. Black stars mark the central tendency of each subgroup distribution. H/M heart-to-mediastinum.
Overall changes of orthostatic subtypes and motor phenotypes over time
Positional ∆BP types progressed in different distribution patterns after 29.3 ± 9.4 months (Figs. 2 and 3). The BPextreme group (especially OH and SH + OH) increased, while patients with BPmild group decreased. About one-third of patients in the initial BPextreme group changed to an orthostatic BP dysregulation subtype, while the rest retained their subtype at follow-up (67.1% of the initial status; Fig. 2a, b). Among the BPmild patients, 40.7% progressed to BPextreme, while 22.1% remained as the initial subtype (Fig. 2a, b). Of the initial BPnone group, 32.4% and 14.7% progressed to orthostatic dysregulation (BPextreme vs. BPmild; respectively), while 42.2% remained free of orthostatic stress (Fig. 2a, b). At the follow-up, a total of 121 participants were classified as BPextreme group (fifty-three from initial BPextreme; thirty-five from initial BPmild; thirty-three from initial BPnone). A linear ascending trend of delayed H/M at both initial and follow-up was observed across turnover patterns of BPnone subtype (initial BPsubtype → follow-up BPsubtype; BPnone →BPextreme vs. BPnone →BPmild vs. BPnone →BPnone; Supplementary Fig. 2). The proportions of those either transforming into or maintaining BPnone group increased across the initial subtypes (initial BPsubtype → follow-up BPsubtype; BPextreme →BPnone vs. BPmild →BPnone vs. BPnone →BPnone; 15.2% vs. 26.7% vs. 42.2%; Cochran-Armitage test, χ2(1) = 15.9, p < 0.001, Fig. 2a).
a represents changes in orthostatic subtype; b motor PIGD versus TD phenotype; and c motor AR versus TD phenotype. Two SH+dOH patients at the follow-up are not represented in the figure because its subtype did not exist at the initial; thus, its changes could not be plotted in the radar chart. dOH delayed orthostatic hypotension, OHT orthostatic hypertension, SH supine hypertension, OH orthostatic hypotension, TD tremor dominant, PIGD postural instability/gait difficulty, AR akinetic-rigid.
The distributions of motor phenotypes also changed (Fig. 3). The PIGD prevalence increased, while that of TD´ decreased; AR type increased as TD˝ and mixed types decreased.
Sub-analyses of orthostatic subtype transformation pattern
Initial BPmild and BPnone groups were selected for sub-analyses to compare the characteristics before and after subtype transformation (Table 3). No discernible between-group differences were observed except for patients with BPmild, among whom those with status improvement to normal orthostatic response (BPmild→BPnone) showed shorter disease duration before diagnosis. There were no associations between motor phenotypes and subtype transformation patterns.
Discussion
Early PD, the diagnosis of which was re-affirmed at an average follow-up of five years, was investigated for positional adrenergic failure. Cardiovascular dysautonomia was sub-grouped into BPextreme (SH and/or OH with any other forms), BPmild (dOH or OHT), and BPnone (PD without orthostatic BP dysregulation). The BPextreme group was initially associated with PIGD but lost its relationship during follow-up. The number of patients in the BPextreme group increased, while that of those with BPmild decreased after 2–3 years. A large proportion of the BPmild group progressed to BPextreme. The transformations of orthostatic subtypes suggested a dynamic classification along its adrenergic failure: BPnone → BPmild → BPextreme.
In this study, the PD population was in the early stage of disease at enrollment. The patients were at the mid-phase of the disease when their diagnosis was reviewed for the study. The point prevalence of PD with OH (PD + OH) at initial presentation and follow-up was comparable to that in a previous study by Hiorth et al.13. That group estimated the point prevalence at the final visit to be 32.8%, but the cumulative incidence of OH was centered at a median of 1.7 years (interquartile range, 1.0–2.5 years). Therefore, most newly detected OH occurred within 2.5 years and then approached a plateau. This approximates our 32.5% prevalence at an average of 29.3 ± 9.4 months. The age of the BPextreme group was the oldest among the groups. Previous studies reported the OH group to be the oldest; this difference could be explained by the status of OH as the major constituent of the BPextreme group5,13.
The prevalence of SH and SH + OH was lower in the present study than in previous estimates2,14,15,16. This difference could be attributed to how supine BP was calculated. This study used the average of four separate measurements, which might have resulted in a lower estimate of SH. The study focused on accurately identifying SH, as it can co-occur with neurogenic OH in PD2, making its separation crucial for the study’s orthostatic subtyping. The definitions of OH and OHT were influenced by supine BP, which served as a reference for comparing standing BP. The study aimed to provide a conservative estimation of the prevalence of different subtypes, leading to a lower prevalence of SH. However, the baseline prevalence of OHT in this study was similar to that of other previous work4.
PD Patients with SH and/or OH (BPextreme) represent the extremes of hemodynamic adrenergic failure in its respective supine and upright positions by a potentially shared pathophysiology7. Delayed OH and OHT (BPmild) previously were conceptualized to be situated between PD without cardiovascular dysautonomia (BPnone) and BPextreme along a spectrum of adrenergic failure3,4,5,8. The characteristics of delayed OH and OHT also did not differ in the present study; thus, they were grouped to represent BPmild. The H/M uptake ratios and washout rates reflected postganglionic cardiac sympathetic denervation and its progressing catecholaminergic malfunction17,18,19. Trend analyses revealed increasing uptake ratios and decreasing washout rates, implying a gradient of adrenergic failure across the groups. Higher H/M uptake ratios and lower washout rates correlated with more preserved sympathetic integrity. These gradients persisted during follow-up. The initial establishment and maintenance of the adrenergic gradient during disease progression support the argument of BPmild as an intermediary phase, indicating possible temporal evolution among the subtypes (BPnone → BPmild → BPextreme).
The BPextreme group was assumed to have the most severe adrenergic failures and increased in number over time; while the BPmild group, presumably the lesser impaired, decreased. Most patients with initial BPextreme maintained in the extreme group, while many in the BPmild group deteriorated into BPextreme. This stepwise deterioration was presumed to occur because the extreme subclass lacked the reserve for progression while the milder forms allowed remnant biology to progress. In a sub-analysis of turnover patterns of BPnone to other subtypes, a linear gradient indicated that BPnone→BPextreme (the most severe form) was correlated with the most impaired sympathetic tone. In contrast, the BPnone→BPnone (the most preserved form) demonstrated the least impairment. This association between the severity of sympathetic tone and the grade of orthostatic turnovers could provide another explanation of the underlining pathophysiologic nature of BP dysregulations (BPnone → BPmild → BPextreme).
The proportion of patients resolving to BPnone increased significantly across the initial subtypes. This implies that increasing remnant sympathetic function, as indicated by increased linear H/M uptake ratio, played a role in such transformation. Within the context of PD as a neurocardiologic disease, we interpreted regression to BPnone to be a result of different resiliencies of extant sympathetic functions among subtypes, rather than actual resolution6. Some of the transformations into normal BP regulation might result from the hypotensive effect of L-DOPA, particularly in the SH + OHT group (BPextreme) which needs further clarification with a larger population in future research.
The non-tremor-dominant type was the most prevalent motor phenotype in this cohort despite the absence of a significant difference. Both PIGD and AR types increased during follow-up, while others decreased. The most impaired subtype, BPextreme, showed the largest overlap with the PIGD or AR motor phenotype. Motor impairment was greater in this extreme group than in the others at follow-up. We presumed that this was not a coincidence since BPextreme and non-tremor-dominant PD correlated with poor outcomes6,7,9,20,21, and BPextreme was suggested to demonstrate diffuse pathology22. Future longitudinal studies are warranted to assess the association between motor prognosis and orthostatic subtype.
This study has some strengths. First, we attempted to reduce selection bias by including PD patients who were re-assessed after at least two years and clinically followed for an average of five years. Another strength was the distinction of the OHT group, allowing for a hypertensive span in both supine and upright positions. The inclusion of an OHT group is rare in descriptions of cardiovascular dysautonomia in patients with PD, and the present study provides a baseline for future studies. In addition, various labile BP subgroups were studied. Finally, this study demonstrated the change in each subtype from baseline, allowing observation of the dynamic changes of BP dysregulations.
However, this study also had several limitations. One limitation is that the follow-up head-up tilt test period was relatively short. One purpose of this study was to investigate whether transformation between orthostatic subtypes could indicate the severity of neurobiology, avoiding the influence of confounders such as the administration of dopaminergic medications. Had the study design included results of patients with advanced PD, the changes of subtypes could have been significantly affected by the effects of medication. However, prolonged follow-up, including those in the advanced stage would be informative since there lacks of prospective investigation of the prevalence of diverse orthostatic subtypes that reflect clinical reality. The exclusion criteria also could have biased the results as it might prohibit the enrollment of PD patients who indeed had orthostatic adrenergic failure, regardless of confounders. In a study looking into the natural course of a disease, the excluded patients could provide precious information that mirrors the clinical circumstances; thus, offering generalizability in its interpretation. Another limitation is that clinical severity was not related to laboratory parameters. The study would be more complete if the longitudinal clinical symptoms, other than motor phenotypes, were associated with longitudinal changes. Future study encompassing both clinometric and laboratory findings is needed. Lastly, we did not include any suspected cases with clinical manifestations of atypical PD in the study. We investigated only PD patients whose diagnosis was assured by the two neurologists. Their diagnoses were confirmed by reviewing the patients’ clinical course after an average follow-up period of 61.4 ± 21.8 months. This signified that only clinically confirmed PD patients with much confidence were enrolled initially, and their early clinical course of the total follow-up (approximately the first one-third of the disease duration) was investigated. This retrospectively confirmed prospective nature of the study design prevented the authors from estimating the transition to atypical PD, such as multiple system atrophy, because they were not considered during clinical retrospection. The experimental design represents one major bias, but in our belief, it is also a strength because no single biomarker could replace the clinical follow-ups of neurologists in confirming PD. Although more extended periods of follow-ups are necessary, the average total follow-up duration allowed the neurologists to assert their re-affirmation of PD. Future prospective research, inclusive of all parkinsonism, is warranted to assess the development of multiple system atrophy.
In conclusion, various forms of positional BP dysregulation existed in the early PD patients in this study. Although the exact pathobiology of each type and their interrelationships are not fully understood, OHT and delayed OH might be a preliminary phase before progressing to SH and OH. Orthostatic subtypes might represent the severity of adrenergic dysfunction in early PD regardless of dopaminergic medication use.
Methods
Patients
This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital. All subjects provided written informed consent to participate. The research was conducted in accordance with relevant guidelines and regulations.
Two hundred sixty-seven drug-naive and de novo PD patients presenting between August 2012 and July 2020 were enrolled in this cohort. PD was diagnosed based on criteria of the UK Parkinson’s Disease Society Brain Bank23, and its diagnosis was supported by positron emission tomography studies using 18F-N-(3-fluoropropyl)-2beta-carbon ethoxy-3beta-(4-iodophenyl) nortropane24. Patients showed decreased dopamine transporter uptake in the striatum, primarily in the posterior putamen. Patients were monitored every 2–6 months for at least 24 months and a maximum of 10 years (average follow-up duration, 61.4 ± 21.8 months, Supplementary Fig. 1). Diagnoses were confirmed during follow-up by two neurologists, S.-W.Y. and J.-S.K. Thirty patients were excluded from the study because they were lost to the follow-up examinations.
Baseline characteristics of age; sex; body mass index; disease duration at diagnosis; follow-up duration; history of hypertension, diabetes mellitus, or dyslipidemia; and smoking status were investigated at the initial assessment. Patients with any of the following were excluded: (1) any symptoms or signs of atypical and/or secondary parkinsonism, (2) family history of PD among first-degree relatives, (3) history of diabetic neuropathy, (4) history of symptomatic stroke that could affect general cognition and performance, (5) history of heart failure that required cardinal symptoms and signs of exercise intolerance (such as exertional dyspnea) and fluid retention (such as edema)25, and (6) current intake of medications known to influence autonomic functions, such as alpha-blockers or tricyclic antidepressants.
UPDRS and MDS-UPDRS
The 267 patients were initially evaluated using the Unified Parkinson’s Disease Rating Scale (UPDRS), and ten were also assessed using the Movement Disorder Society (MDS)-UPDRS. At the follow-up examinations (n = 237), 175 were assessed using the UPDRS; the remainder were evaluated using the MDS-UPDRS. The Hoehn and Yahr (H&Y) stage was scored at every assessment. The subtotal scores of parts II and III of the UPDRS were scaled into the MDS-UPDRS for comparisons26.
Two motor phenotyping methods were applied to stratify the PD patients. These methods were tremor dominant (TD´) versus postural instability/gait difficulty (PIGD) or tremor dominant (TD˝) versus akinetic-rigid (AR) type27,28,29. The motor phenotypes were determined by the original scales, either UPDRS or MDS-UPDRS (Supplementary Method).
The average examination interval was 29.5 ± 9.7 months (Supplementary Fig. 1).
Head-up tilt-test
Head-up tilt-test was performed after discontinuing any antihypertensive medications for at least 7 days. Patients were also asked to abstain from drinking alcohol or coffee the day before the test. All patients were tested in the full resting state. Continuous electrocardiograph leads and non-invasive BP monitoring equipment were applied to the patients (YM6000, Mediana Tech, Redoman, WA, USA). After 20 min of supine rest, head-up tilt testing (20 min at 60°) was performed using a Manumed Special Tilt1-section tilt table (ENRAF NONIUS, Rotterdam, The Netherlands). Supine BP and heart rate were recorded every 5 min before tilting to 60°, and the same measurements were performed at 0, 3, 5, 10, 15, and 20 min during head-up tilt, and as deemed necessary to ensure subject safety.
After excluding the first supine BP (at 0 min), average supine systolic/diastolic BPs and heart rates (HR; beats per minute) were calculated from the measurements at 5, 10, 15, and 20 min. Supine hypertension (SH) was diagnosed if the average supine systolic and/or diastolic BP (SBP/DBP) was ≥ 140/90 mmHg30.
The lowest SBP/DBP (BPmin) at three or five minutes in the tilted position was selected to identify orthostatic hypotension (OH). The orthostatic BP decreases in systole (ΔSBP) and diastole (ΔDBP) were calculated from average supine BPs. When patients were supine-hypertensive, ΔSBP and/or ΔDBP ≥ 30/15 mmHg within five minutes was used to diagnose OH; otherwise, ΔSBP and/or ΔDBP ≥ 20/10 mmHg were used4,30,31. Patients were classified as delayed OH (dOH) when they met the above criteria after 10 min.
The orthostatic ΔBP was re-calculated from the highest BP (BPmax) among the tilted measures at 3, 5, 10, and 15 min (average supine BP minus highest orthostatic BPmax). Patients with PD with SH were described as having orthostatic hypertension (OHT) if ΔSBPmax and/or ΔDBPmax was ≤−20/10 mmHg. Patients with PD without SH were categorized as OHT when orthostatic BPmax was ≥140/90 mmHg or ΔBPmax was ≤−20/10 mmHg4,32. Positive ΔSBPmax and/or ΔDBPmax signified a decrease in upright ΔBP, and negative values indicated an increase in orthostatic ΔBP.
OH, dOH, and OHT diagnoses were mutually exclusive; only SH could be co-diagnosed with any of these three subtypes. For example, PD with OH patients could not be classified as dOH or OHT but could be subtyped into SH + OH.
PD patients with SH and/or OH in any combination with other forms (SH, OH, SH + OH, SH+dOH, SH + OHT) were classified as BPextreme, and PD with dOH or OHT was assigned as BPmild. If SH co-existed with either dOH or OHT, the patient was classified as BPextreme. PD with no orthostatic subtype was categorized as BPnone. These subgroups were designed to represent the grades of adrenergic failure.
Orthostatic HR at three minutes was selected to estimate the change in HR (ΔHR) from average supine HR, and its values were utilized to calculate ΔHR/ΔSBP at three minutes (ΔHR/ΔSBP3min)33,34. The measurement was only applied to those with PD with OH as the original description.
The average interval between investigations was 29.3 ± 9.4 months (Supplementary Fig. 1).
Levodopa equivalent daily dose (LEDD; mg)
The most recent dopaminergic dosage at the time of head-up tilt test was calculated as levodopa equivalent daily dose35.
123I-metaiodobenzylguanidine (123I-MIBG) myocardial scintigraphy
123I-MIBG scintigraphy was performed using a dual-head camera equipped with a low-energy, high-resolution collimator (Siemens), and data were collected at 30 minutes (early) and 120 min (delayed) after injection of 111 MBq of 123I-MIBG. A static image was obtained with a 128 × 128 matrix. Regions of interest (ROIs) were drawn manually around the heart and mediastinum. Tracer uptake was measured within each ROI to calculate the heart-to-mediastinum (H/M) ratio for early and delayed time points. The washout rate (WR) was calculated as [(early H/M ratio – late H/M ratio)/early H/M ratio] × 100. Reference values for normal H/M ratio were 1.70 for early and 1.78 for delayed ratios36.
Patients were evaluated initially at the time of diagnosis, and the changes of tracer uptake were re-assessed after 30.0 ± 9.6 months (Supplementary Fig. 1).
Statistical analyses
Statistical analyses were conducted with jamovi software (version 2.3.18; retrieved from https://www.jamovi.org) for Mac, a graphical user interface for R. Analysis of variance (ANOVA) or Kruskal-Wallis test was performed for continuous or ordinal variables when appropriate. Categorical variables were examined by Fisher’s exact test. Cochran-Armitage test and Jonckheere-Terpstra test were executed in R (version 4.2.1; PMCMRplus package). Multiple comparisons were adjusted as appropriate. Statistical significance was defined as a two-tailed p-value < 0.05.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Anonymized data generated during this study are available from the corresponding author on request from individuals affiliated with research or healthcare institutions.
References
Goldstein, D. S. & Sharabi, Y. The heart of PD: Lewy body diseases as neurocardiologic disorders. Brain Res. 1702, 74–84 (2019).
Espay, A. J. et al. Neurogenic orthostatic hypotension and supine hypertension in Parkinson’s disease and related synucleinopathies: prioritisation of treatment targets. Lancet Neurol. 15, 954–966 (2016).
Gibbons, C. H. & Freeman, R. Clinical implications of delayed orthostatic hypotension: a 10-year follow-up study. Neurology 85, 1362–1367 (2015).
Yoo, S. W. et al. Cardiac sympathetic burden reflects Parkinson disease burden, regardless of high or low orthostatic blood pressure changes. Npj. Parkinsons Dis. 7, 71 (2021).
Yoo, S. W. et al. Delayed orthostatic hypotension in Parkinson’s disease. Npj. Parkinsons Dis. 7, 37 (2021).
Jain, S. & Goldstein, D. S. Cardiovascular dysautonomia in Parkinson disease: from pathophysiology to pathogenesis. Neurobiol. Dis. 46, 572–580 (2012).
Isaacson, S. H., Dashtipour, K., Mehdirad, A. A. & Peltier, A. C. Management strategies for comorbid supine hypertension in patients with neurogenic orthostatic hypotension. Curr. Neurol. Neurosci. Rep. 21, 18 (2021).
Gibbons, C. H. & Freeman, R. Delayed orthostatic hypotension: a frequent cause of orthostatic intolerance. Neurology 67, 28–32 (2006).
Freeman, R. et al. Orthostatic Hypotension: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 72, 1294–1309 (2018).
Goldstein, D. S., Holmes, C., Sharabi, Y. & Wu, T. Survival in synucleinopathies: a prospective cohort study. Neurology 85, 1554–1561 (2015).
Magkas, N. et al. Orthostatic hypertension: from pathophysiology to clinical applications and therapeutic considerations. J. Clin. Hypertens. (Greenwich) 21, 426–433 (2019).
Palma, J. A. et al. The impact of supine hypertension on target organ damage and survival in patients with synucleinopathies and neurogenic orthostatic hypotension. Parkinsonism Relat. Disord. 75, 97–104 (2020).
Hiorth, Y. H., Pedersen, K. F., Dalen, I., Tysnes, O. B. & Alves, G. Orthostatic hypotension in Parkinson disease: a 7-year prospective population-based study. Neurology 93, e1526–e1534 (2019).
Palma, J.-A. et al. Orthostatic hypotension in Parkinson disease: how much you fall or how low you go? Mov. Disord. 30, 639–645 (2015).
Fanciulli, A. et al. Supine hypertension in Parkinson’s disease and multiple system atrophy. Clin. Auton. Res. 26, 97–105 (2016).
Umehara, T., Matsuno, H., Toyoda, C. & Oka, H. Clinical characteristics of supine hypertension in de novo Parkinson disease. Clin. Auton. Res. 26, 15–21 (2016).
Orimo, S., Yogo, M., Nakamura, T., Suzuki, M. & Watanabe, H. (123)I-meta-iodobenzylguanidine (MIBG) cardiac scintigraphy in α-synucleinopathies. Ageing Res. Rev. 30, 122–133 (2016).
Jeong, Y. J. et al. Relationship between the washout rate of I-123 MIBG scans and autonomic function in Parkinson’s disease. PLoS ONE 15, e0229860 (2020).
Orimo, S. et al. Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain 131, 642–650 (2008).
Jankovic, J. & Kapadia, A. S. Functional decline in Parkinson disease. Arch. Neurol. 58, 1611–1615 (2001).
Reijnders, J. S., Ehrt, U., Lousberg, R., Aarsland, D. & Leentjens, A. F. The association between motor subtypes and psychopathology in Parkinson’s disease. Parkinsonism Relat. Disord. 15, 379–382 (2009).
Udow, S. J. et al. ‘Under pressure’: is there a link between orthostatic hypotension and cognitive impairment in alpha-synucleinopathies? J. Neurol. Neurosurg. Psychiatry 87, 1311–1321 (2016).
Gibb, W. R. & Lees, A. J. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 51, 745–752 (1988).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601 (2015).
King, M., Kingery, J. & Casey, B. Diagnosis and evaluation of heart failure. Am. Fam. Physician 85, 1161–1168 (2012).
Goetz, C. G., Stebbins, G. T. & Tilley, B. C. Calibration of unified Parkinson’s disease rating scale scores to Movement Disorder Society-unified Parkinson’s disease rating scale scores. Mov. Disord. 27, 1239–1242 (2012).
Eggers, C., Kahraman, D., Fink, G. R., Schmidt, M. & Timmermann, L. Akinetic-rigid and tremor-dominant Parkinson’s disease patients show different patterns of FP-CIT single photon emission computed tomography. Mov. Disord. 26, 416–423 (2011).
Stebbins, G. T. et al. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: comparison with the unified Parkinson’s disease rating scale. Mov. Disord. 28, 668–670 (2013).
Jankovic, J. et al. Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology 40, 1529–1534 (1990).
Fanciulli, A. et al. Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the American Autonomic Society (AAS) and the European Federation of Autonomic Societies (EFAS): Endorsed by the European Academy of Neurology (EAN) and the European Society of Hypertension (ESH). Clin. Auton. Res. 28, 355–362 (2018).
Gibbons, C. H. et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J. Neurol. 264, 1567–1582 (2017).
Jordan, J., Ricci, F., Hoffmann, F., Hamrefors, V. & Fedorowski, A. Orthostatic hypertension: critical appraisal of an overlooked condition. Hypertension 75, 1151–1158 (2020).
Fanciulli, A. et al. Validation of the neurogenic orthostatic hypotension ratio with active standing. Ann. Neurol. 88, 643–645 (2020).
Norcliffe-Kaufmann, L. et al. Orthostatic heart rate changes in patients with autonomic failure caused by neurodegenerative synucleinopathies. Ann. Neurol. 83, 522–531 (2018).
Schade, S., Mollenhauer, B. & Trenkwalder, C. Levodopa equivalent dose conversion factors: an updated proposal including opicapone and safinamide. Mov. Disord. Clin. Pract. 7, 343–345 (2020).
Ryu, D. W. et al. Initial versus follow-up sequential myocardial 123I-MIBG scintigraphy to discriminate Parkinson disease from atypical Parkinsonian Syndromes. Clin. Nucl. Med. 44, 282–288 (2019).
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1I1A1A01050492 awarded to Sang-Won Yoo), and also by the Ministry of Science, ICT and Future Planning (NRF-2017R1D1A1B06028086 awarded to Joong-Seok Kim). This research was supported by the Korea “National Institute of Health” research project (2021-ER1008-02 awarded to Sang-Won Yoo and Joong-Seok Kim).
Author information
Authors and Affiliations
Contributions
S-.W.Y. and J-.S.K. contributed to the conception and design of the study; S-.W.Y., Y-.S.O., D-.W.R., S.H., Y.K., J-.Y.Y., and J-.S.K. contributed to the acquisition and analysis of data; S-.W.Y. and J-.S.K. contributed to the interpretation of results, drafting the text and preparing figure; S.-W.Y. drafted the manuscript Y.-S.O., D-.W.R., S.H., Y.K., J-.Y.Y., and J-.S.K. revised the manuscript. S.-W.Y. and J.-S.K. obtained funding. All authors read and approved the final version for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoo, SW., Oh, YS., Ryu, DW. et al. A 3-year natural history of orthostatic blood pressure dysregulation in early Parkinson’s disease. npj Parkinsons Dis. 9, 96 (2023). https://doi.org/10.1038/s41531-023-00546-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-023-00546-5