Abstract
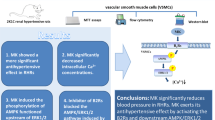
We investigated the antihypertensive effects of maximakinin (MK) on spontaneously hypertensive rats (SHRs). The effects of MK on arterial blood pressure in SHRs were observed, and flow cytometry and 4,5-diaminofluorescein-2 staining were used to examine MK-induced nitric oxide (NO) release in human umbilical vein endothelial cells (HUVECs). Western blotting was used to analyze the effects of MK on the expression of AMP-activated protein kinase (AMPK), Akt, Connexin 43, ERK1/2, p38, and p-eNOS in HUVECs. The results showed that MK induced a more significant antihypertensive effect on SHRs than bradykinin (BK). MK induced significant increases in endothelial nitric oxide synthase (eNOS) phosphorylation and NO release in HUVECs. MK also significantly increased the phosphorylation of Akt and AMPK in HUVECs. The AMPK inhibitor compound C blocked the effect of MK on the generation of NO. MK induced the phosphorylation of ERK1/2, p38, and Connexin 43. The expression of p-Connexin 43 was significantly decreased in the presence of the ERK1/2 inhibitor U0126 but not the p38 inhibitor SB203580. The effects of MK on the phosphorylation of AMPK and ERK1/2 were significantly decreased by the BK B2 receptor inhibitor HOE-140. In summary, MK can significantly reduce blood pressure in SHRs. The antihypertensive effect might be mediated through the activation of the BK B2 receptor, while the downstream AMPK/PI3K/Akt/eNOS/NO and ERK1/2/Connexin 43 signaling pathways play additional roles.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
O’Rourke M, Chen T, Hirst DG, Rao P, Shaw C. The smooth muscle pharmacology of maximakinin, a receptor-selective, bradykinin-related nonadecapeptide from the venom of the Chinese toad, Bombina maxima. Regul Pept. 2004;121:65–72.
Charest-Morin X, Bachelard H, Jean M, Marceau F. Species-specific pharmacology of maximakinin, an amphibian homologue of bradykinin: putative prodrug activity at the human B2 receptor and peptidase resistance in rats. PeerJ. 2017;5:e2911.
Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–5.
Decker B, Pumiglia K. mTORc1 activity is necessary and sufficient for phosphorylation of eNOS(S1177). Physiol Rep. 2018;6:e13733.
Feng L, Ren J, Li Y, Yang G, Kang L, Zhang S, et al. Resveratrol protects against isoproterenol induced myocardial infarction in rats through VEGF-B/AMPK/eNOS/NO signalling pathway. Free Radic Res. 2019;53:82–93.
Youn JY, Wang T, Cai H. An ezrin/calpain/PI3K/AMPK/eNOSs1179 signaling cascade mediating VEGF-dependent endothelial nitric oxide production. Circ Res. 2009;104:50–9.
Figueroa XF, Isakson BE, Duling BR. Vascular gap junctions in hypertension. Hypertension. 2006;48:804–11.
Arishiro K, Hoshiga M, Ishihara T, Kondo K, Hanafusa T. Connexin 43 expression is associated with vascular activation in human radial artery. Int J Cardiol. 2010;145:270–2.
Ogut O, Brozovich FV. The potential role of MLC phosphatase and MAPK signalling in the pathogenesis of vascular dysfunction in heart failure. J Cell Mol Med. 2008;12:2158–64.
Bawolak MT, Roy C, Gera L, Marceau F. Prolonged signalling and trafficking of the bradykinin B2 receptor stimulated with the amphibian peptide maximakinin: insight into the endosomal inactivation of kinins. Pharmacol Res. 2012;65:247–53.
Freudlsperger C, Horn D, Weissfuss S, Weichert W, Weber KJ, Saure D, et al. Phosphorylation of AKT(Ser473) serves as an independent prognostic marker for radiosensitivity in advanced head and neck squamous cell carcinoma. Int J Cancer. 2015;136:2775–85.
Le LT, Couvet M, Favier B, Coll JL, Nguyen CH, Molla A. Discovery of benzo[e]pyridoindolones as kinase inhibitors that disrupt mitosis exit while erasing AMPK-Thr172 phosphorylation on the spindle. Oncotarget. 2015;6:22152–66.
Ma J, Luo Y, Ge L, Wang L, Zhou M, Zhang Y, et al. Ranakinestatin-PPF from the skin secretion of the Fukien gold-striped pond frog, Pelophylax plancyi fukienensis: a prototype of a novel class of bradykinin B2 receptor antagonist peptide from ranid frogs. The Sci World J. 2014;2014:564839.
Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–61.
Ning WH, Zhao K. Propionyl-L-carnitine induces eNOS activation and nitric oxide synthesis in endothelial cells via PI3 and Akt kinases. Vasc Pharmacol. 2013;59:76–82.
Schmaier AH. The plasma kallikrein-kinin system counterbalances the renin-angiotensin system. J Clin Investig. 2002;109:1007–9.
Xu J, Carretero OA, Sun Y, Shesely EG, Rhaleb NE, Liu YH, et al. Role of the B1 kinin receptor in the regulation of cardiac function and remodeling after myocardial infarction. Hypertension. 2005;45:747–53.
Acknowledgements
We thank Renee Mosi, Ph.D., from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this paper.
Funding
This work was supported in part by the General Project of Liaoning Provincial Science and Technology Department (20170540844) and by the General Project of Liaoning Education Department (2017LQN16).
Author information
Authors and Affiliations
Contributions
CX and YY designed the research. YY, L-SX, YW, and F-FS performed the research. CX contributed new reagents or analytical tools. X-MZ analyzed the data. YY and CX wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, Y., Xu, LS., Wu, Y. et al. The antihypertensive effect of MK on spontaneously hypertensive rats through the AMPK/Akt/eNOS/NO and ERK1/2/Cx43 signaling pathways. Hypertens Res 44, 781–790 (2021). https://doi.org/10.1038/s41440-021-00638-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-021-00638-w
Keywords
This article is cited by
-
Not a small frog in a big pond: targeting bradykinin receptor B2 signaling in vascular smooth muscle cells for treatment of hypertension
Hypertension Research (2023)
-
Maximakinin reduced intracellular Ca2+ level in vascular smooth muscle cells through AMPK/ERK1/2 signaling pathways
Hypertension Research (2023)