Abstract
Hypertension is a global public health issue and the leading cause of premature death in humans. Despite more than a century of research, hypertension remains difficult to cure due to its complex mechanisms involving multiple interactive factors and our limited understanding of it. Hypertension is a condition that is named after its clinical features. Vascular function is a factor that affects blood pressure directly, and it is a main strategy for clinically controlling BP to regulate constriction/relaxation function of blood vessels. Vascular elasticity, caliber, and reactivity are all characteristic indicators reflecting vascular function. Blood vessels are composed of three distinct layers, out of which the endothelial cells in intima and the smooth muscle cells in media are the main performers of vascular function. The alterations in signaling pathways in these cells are the key molecular mechanisms underlying vascular dysfunction and hypertension development. In this manuscript, we will comprehensively review the signaling pathways involved in vascular function regulation and hypertension progression, including calcium pathway, NO-NOsGC-cGMP pathway, various vascular remodeling pathways and some important upstream pathways such as renin-angiotensin-aldosterone system, oxidative stress-related signaling pathway, immunity/inflammation pathway, etc. Meanwhile, we will also summarize the treatment methods of hypertension that targets vascular function regulation and discuss the possibility of these signaling pathways being applied to clinical work.
Similar content being viewed by others
Introduction
Hypertension represents a significant risk factor for cardiovascular and cerebrovascular diseases (CVDs) and remains the primary cause of premature mortality on a global scale.1 The estimated number of people aged 30–79-year-old with hypertension doubled from 648 million in 1990 to 1.27 billion in 2019.2 The prevention and control of hypertension represent a crucial global public health strategy in the effort to reduce premature mortality from CVDs.3
Blood pressure (BP) is defined as the lateral pressure exerted on the walls of blood vessels per unit area during the flow of blood. Hypertension is characterized by an increase in systolic BP and/or diastolic BP. There are two primary factors that directly affect BP: the volume of intravascular fluid and the capacity for vasodilation. The amount of fluid in the blood vessels is mainly related to the everyday intake and output volume. Vasodilatation is the basic function of blood vessels.4 The capacity for vasodilation is influenced by vascular elasticity, caliber, and reactivity. Poorer the vasodilatation capacity, higher the BP. The disturbance of vascular contraction and/or relaxation function exerts a great influence on the onset and progression of hypertension. As vasodilatation capacity plays a crucial role in regulating BP, it has garnered significant attention in the field of vascular biology research.5
Changes in signaling pathways in vascular endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) are key molecular mechanisms which trigger vascular dysfunction and promote the development of hypertension. Here, we reviewed all key signaling pathways in vascular function and hypertension, as well as their treatment application value in clinical settings.
Vascular structure and functions of each layer
Blood vessels are composed of three distinct layers, including intima, media, and adventitia (Fig. 1). The intima is mainly composed of ECs, which organize themselves into a continuous monolayer, allowing blood perfusion. On their surface, ECs form a surrounding extracellular matrix (ECM) known as the glycocalyx, which extends beyond the cell surface into the vascular lumen. This structure plays a crucial role in providing a barrier function for ECs, preventing the transmural migration of leukocytes and platelet adhesion.6,7 Intima is the “sensor” of vessels, as it can sense various stimuli (such as fluid shear force, cytokines, etc.) in the blood and actively control the degree of vascular relaxation and contraction. This active regulation is mainly achieved through the secretion of various mediators from ECs.8 ECs serve not only as a barrier-forming cell population, acting as a responsive interface, but also actively regulate their microenvironment, serving as gatekeepers of organ development, homeostasis, and tissue regeneration.9 ECs dysfunction has been regarded as a pivotal mechanism in early pathogenesis of hypertension.10 Generalized definition of ECs function or endothelial function involves barrier, secretion, sensation, etc., while in studies with respect to vascular contraction, endothelial function commonly refers specifically to ECs sensing the stimuli from blood and triggering vascular contraction.
Under the ECs is a layer of basement membrane (mainly consisting of type IV collagen and laminins 41111) that supports ECs, and under the basement membrane is the middle layer. The medial layer of arteries serves as the load-bearing unit and regulates arterial vascular tone, providing the necessary structural stability for the regulation of blood flow and delivery of oxygen to tissues.12 Generally, the farther from the heart, the thinner the middle layer in the arteries. Media layers are absent in the capillaries, which allows adequate exchange of gases and fluids through capillaries.13,14 In large (elastic) arteries, elastic fibers interweave with VSMCs, and are surrounded by other ECM.15 VSMCs are the main function performer in regulating vasoconstriction and dilation. Once accepting the external signals, VSMCs can transmit the signals by intracellular signaling pathways, thus activating the Actin in VSMCs and triggering cells contraction. Healthy ECM is responsible for maintaining the structure of the middle layer of blood vessels and directly affecting the function and status of VSMCs. Compared with the intima and media, the composition of the adventitia is more complex. The adventitia contains fibroblasts incorporated into a loose collagen extracellular matrix (ECM) enriched in hyaluronic acid. Lymphocytes, nerves, progenitors, adipocytes, and immune cells are also present in the adventitia.16,17,18 Functionally, the adventitia can sense and direct responses to a wide array of stimuli via reciprocal communication among adventitial cells, as well as with cells from neighboring tissues, acting as a biological processing center for the retrieval, integration, and storage.19
Classical signaling pathways in vascular function and hypertension
Abnormal vascular structure is an important cause of hypertension and cardiovascular events.20,21,22,23,24 An increase in vascular resistance, largely caused by a reduction in vascular diameter,25,26 is a key pathophysiological mechanism contributing to the development of hypertension. The signaling pathways underlying vascular function and hypertension are complex. There are three classical ways to regulate vascular function and BP levels, including calcium signaling pathway, the NO (nitric oxide)-NOsGC (nitric oxide-sensitive guanylate cyclase)-cGMP pathway, and vascular remodeling. Among them, calcium and NO-NOsGC-cGMP signaling pathway are reversible, and vascular remodeling is considered as a pathological change that is difficult to reverse.27
Calcium signaling pathway
The primary mechanisms regulating the contractile state of VSMCs are changes in cytosolic calcium concentration ([Ca2+]c). When vasoconstrictor stimuli are present, intracellular stores and/or the extracellular space mobilize Ca2+ to increase [Ca2+]c in VSMCs (Fig. 2). The increased [Ca2+]c will bind to calmodulin (CaM) and form a complex which can activate myosin light-chain (MLC) kinase (MLCK). Then MLCK will phosphorylate MLC to promote contraction. Conversely, myosin light chain phosphatase (MLCP) can dephosphorylate phosphorylated MLC, triggering vasodilation.28,29,30,31 One of the important procedures in calcium signaling pathways is the influx of extracellular calcium through voltage-gated Ca2+ channels. There exist two main types of Ca2+ channels in VSMCs, including the high voltage-activated (HVA) L-type and low voltage-activated (LVA) T-type channels. The primary function of L-type channels is to regulate Ca2+ entry for contraction. Nevertheless, it is generally accepted that T-type Ca2+ channels (LTCCs) do not play a significant role in arterial vasoconstriction, except possibly in the renal microcirculation.32 Many factors, including humoral or neural stimuli, can affect the function (open or close) of LTCCs. And functional regulation of Ca2+ channel relies on phosphorylation processes. Except calcium influx, Ca2+ release from the internal store (sarcoplasmic reticulum; SR) through the IP3 receptor (IP3R) and the ryanodine receptor (RyR) is also an important way to modulate cellular contraction.25,29,33
Calcium signaling pathway. CPI-17 molecular mass 17 kDa, DAG diacylglycerol, GPCR G-protein-coupled receptors, IP3 inositol trisphosphate, IP3R inositol trisphosphate receptor, MLC myosin light-chain, MLCK myosin light-chain kinase, MLCP myosin light chain phosphatase, MLC-p phosphorylated myosin light-chain, PLC phospholipase C, PKC protein kinase C
In addition to the amount of calcium ions in the cytoplasm, contraction is also regulated by calcium-sensitization mechanisms, such as the RhoA-Rho kinase pathway34,35 and the PLC (phospholipase C)–DAG (Diacylglycerol)–PKC (protein kinase C) pathway.36,37 RhoA is a small GTP-binding protein acting as molecular switcher in signaling pathway, and Rho-kinases (Rho-kinase α/ROKα/ROCK2 and Rho-kinase β/ROKβ/ROCK1) are downstream proteins of RhoA.38,39,40,41,42 Rho-kinases contribute to the contraction of VSMCs via Phosphorylation of MLC.43,44,45 Moreover, enhanced RhoA/Rho-kinase (Rho-associated kinase) signaling in VSMCs is considered to be involved in the elevated peripheral vascular resistance observed in clinical hypertension.36,46,47 PLC is a phosphodiesterase released through the action of specific phospholipases, and is a downstream product of GPCRs (G-protein-coupled receptors). PIP2 (Phosphatidylinositol-4,5-bisphosphate) is a minor phospholipid, which is generated by the hydrolysis of a minor phospholipid in the plasma membrane in response to agonist stimulation. When acted upon by a phosphoinositide-specific PLC (PI-PLC) enzyme, PIP2 can generate two intracellular second messengers:48 (1) IP3 (inositol trisphosphate), which subsequently mobilizes Ca2+ from ER stores via IP3 receptor;49 and (2) DGA, which activates PKC. Activation of PKC can lead to constriction of aorta and BP increasement, via downstream targets of PKC, such as MLCK and CPI-17 (C-kinase potentiated protein phosphatase 1 inhibitor, molecular mass 17 kDa), both of which enhance constriction.50,51 Notably, CPI-17 is a smooth-muscle-specific inhibitor of MLCP, which can bind to its catalytic subunit, impending its phosphatase activity and enabling the persistence of contraction. Both PKC and Rho/Rho kinase can induce the enhancement of contractile force via CPI-17.36,52
NO-NOsGC-cGMP pathway
The NO-NOsGC-cGMP pathway is closely linked to the contractile function of VSMCs, and its activation precedes the development of hypertension53,54,55 (Fig. 3). The generation of NO in vascular ECs is the beginning of NO-NOsGC-cGMP pathway.56 NO production can be stimulated by many chemical factors including L-arginine, nitrate, nitrite, catecholamines,57,58 bradykinin,59 serotonin,60 and physical factors such as fluid shear stress.61 And NO can be inactivated by angiotensin II.62 There are two pathways by which NO is produced in ECs, including endothelial NOS (eNOS) pathway and eNOS-independent pathway (such as nitrate, nitrite). NO produced from L-arginine by eNOS pathway is the primary source of blood NO.63,64 And eNOS knockout has been proven to lead to vascular dysfunction and hypertension.65 To be mentioned, a recent study revealed that eNOS did not only contribute to BP regulation in ECs, but also in RBCs. Both the EC and RBC eNOS KOs were significantly hypertensive, and the apply of the NO synthase inhibitor further upregulated BP in these mice.66 On the one hand, it indicated the diversity of NO sources in blood vessels. On the other hand, it also suggested that the changes in the quantity of RBCs might affect BP regulation.
NO synthesized in ECs or RBCs can diffuse into VSMCs, and this procedure is regulated by transformation of Fe2+ hemoglobin (Hb) α and oxidized Fe3+ Hb α. Endothelial Hb α haem iron in the Fe3+ state enables NO signaling, while this signaling is terminated when Hb α is converted to the Fe2+ state by endothelial CYB5R3 (cytochrome b5 reductase 3).67 In VSMCs, NO activates NOsGC, which subsequently generates the second messenger cGMP.68,69 cGMP exerts its cellular functions via cGMP-modulated cation channels (cyclic nucleotide-gated [CNG]) and cGMP-dependent protein kinases (cGKs).70 One of the cGKs substrate, cGKIα, can bind to and then phosphorylate the myosin-binding subunit of MLC phosphatase, which is crucial for the localization of cGKI near the enzyme it regulates.71,72,73,74 Besides, transfection of cGKIα could rescue defective Ca2+ regulation in cGKI-deficient VSMCs according to studies,73 and cGKI activated myosin-bound phosphatase by inhibition of RhoA/Rho-kinase pathway.75 All the evidence demonstrated that, cGKIα was also able to relax VSMCs by decreasing the cytosolic Ca2+ level and calcium-sensitization, suggesting NOsGC-cGMP pathway had a crosstalk with calcium signaling.
Vascular remodeling
Vascular remodeling presents as vascular lumen narrowing, vascular wall thickening, and elasticity loss. It can be put on a par with clinically structural arterial stiffness, which is reflected as pulse wave velocity (PWV) increasement. ECM changes in vessels are pathological conditions which lead to vascular remodeling and hypertension (Fig. 4). No method has been found to reverse the altered ECM to a healthy state currently, and researches in this direction may be the key to breakthroughs in restoring vascular function and curing hypertension. The changes of ECM mainly occur in the media, which may be related to the different SMC phenotypes and different secretory factors from SMCs. The changes of ECM include deposition of excessive collagen and glycation end-products deposition (AGEs), elastic fibers degradation, calcification etc.26,76,77,78
Vascular remodeling pathways. AGEs glycation endproducts deposition, ALPL tissue-nonspecific alkaline phosphatase, AMPK adenosine 5’-monophosphate-activated protein kinase, ANKH transmembrane protein ankylosis protein homolog, BAD bcl2-associated death promoter, BMP-2 bone morphogenetic protein-2, CBFA1 core-binding factor α-1, ECM extracellular matrix, ENPP1 ectonucleotide pyrophosphatase/phosphodiesterase, ERK extracellular-signal-regulated kinase, MMPs matrix metalloproteinases. MAPK mitogen-activated protein kinase, NADPH nicotinamide adenine dinucleotide phosphate, NF-κB nuclear factor κB, HMGB-1 high mobility box group-1, JNK JUN N-terminal kinase, RAGE AGE receptor, ROS reactive oxygen species, ox-LDL oxidized low-density lipoprotein, PI3K phosphatidylinositol-3 kinase, SOX9 chondrogenic transcription factors including SRY-Box 9
MMPs
Matrix metalloproteinases (MMPs) are a group of endopeptidases that depend on zinc and are responsible for breaking down proteins in the ECM. Activated MMPs can deposit collagen, degrade elastin, and then lead to BP increasement.26,79,80,81,82,83 At least 28 different types of MMPs are expressed in human tissue. Many types of MMPs are involved in arterial remodeling and BP regulation, including MMP-1,84,85,86 MMP-2,87,88,89 MMP-3,90,91 MMP-981,89,92,93 etc. The roles played by MMPs in regulating ECM are not entirely consistent. According to their functions, MMPs can be classified into different types, including collagenases, gelatinases, matrilysins, stromelysins etc.94,95,96,97,98 Collagenases, like MMP-1 (interstitial collagenase), can degrade collagen and cleave proMMP-9 into its active form. Gelatinases involve MMP-2 (gelatinase A), MMP-9 (gelatinase B), contributors to degradation of gelatin etc. In addition to the basic functions mentioned above, some MMPs can also function in other ways. For instance, the activation of MMP-2 can lead to calcification99,100 and collagen accumulation in the vascular wall.101 MMP-9 can cause vascular fibrolysis and enhanced collagen affinity.95,102 In addition to acting on vascular remodeling to affect structure, MMPs may also affect BP through other mechanisms. Activation of MMP-2 contributes to an increase in BP by both elevating the levels of big endothelin-1 and reducing the levels of adventitial calcitonin gene-related peptide and endothelial nitric oxide synthase.103,104,105 MMP-1/-9 decreases the density of β (2) adrenergic receptor in arterioles, leading to an increase in arteriolar tone, which also contributes to an elevation in BP.106,107
The activity of MMPs is regulated at three levels: proenzyme activation, activity inhibition (tissue inhibitors of MMPs, TIMPs), and gene transcription.101,108 During vascular remodeling, the activation of intracellular MMPs is associated with multiple stimulators, commonly including pro-inflammatory signaling molecules (cytokines, interleukins, tumor necrosis factors), growth factors, vasoactive agents (Ang II, ET-1, aldosterone) and their receptors. Signaling pathways involved in regulating MMPs transcription mainly include mitogen-activated protein kinase (MAPK),101,109,110 reactive oxygen species (ROS),111 adenosine 5’-monophosphate-activated protein kinase (AMPK),112,113 extracellular-signal-regulated kinase (ERK),114 JUN N-terminal kinase (JNK) etc.115, which can either enhance or repress the expression of MMPs. In addition to these general pathways, different types of MMPs may have specific signaling pathways. For example, MMP10 gene transcription is inhibited by HDAC7 (Histone Deacetylase 7) binding to MEF2 (myocyte enhancer factor 2), resulting in endothelial cell–cell adhesion and impaired vascular integrity.116
AGEs
Reactive byproducts resulting from nonenzymatic glucose–protein condensation reactions, as well as lipids and nucleic acids exposed to reducing sugars, form a diverse group of irreversible adducts known as “Advanced Glycation Endproducts” (AGEs).117 AGEs can be formed by glycation of proteins either within cells or in extracellular spaces. This protein glycation consists of a series of complex sequential reactions, known collectively as the Maillard reaction.118 Multiple cardiovascular risk factors can result in increase in serum and tissue AGEs, such as diabetes,119 hyperlipidemia,120 smoking,121 etc. According to the sources, AGEs can be classified into endogenous AGEs (intra- and extracellular) and exogenous AGEs taken in from certain foods. The formation of methylglyoxal (MGO) is a major precursor of endogenous AGEs, occurs spontaneously during glycolysis from the triose phosphate isomers glyceraldehyde-3 phosphate and dihydroxyacetone phosphate.118,122,123 Other crucial AGEs compounds include glyoxal (GO), 3-deoxyglucosone (3DG), Nε-carboxymethyl-lysine (CML), Nε-carboxyethyl-lysine (CEL), pentosidine, pyrraline, and glucosepane.118 Food is the primary sources of exogenous AGEs in human.124 Generally, animal fat foods contain more AGEs than plant foods. And cooking at high temperature can increase AGEs in foods.125 Moreover, studies have confirmed that controlling dietary habits is an effective way to reduce AGEs in human body.126,127,128,129
AGEs contribute a lot to vascular remodeling and hypertension. Accumulation of AGEs in ECM leads to the formation of cross-links, which can entrap other local macromolecules.130,131 There are three known receptors for AGEs: full-length AGE receptor (RAGE), N-truncated RAGE, and soluble RAGE (sRAGE). sRAGE has two isoforms, including cleaved RAGE (cRAGE) and endogenous secretory RAGE (esRAGE).132 Full-length RAGEs and N-truncated RAGEs are multiligand cell bound receptors, while sRAGEs circulate in the blood. AGEs and their receptors are closely associated with vascular function and hypertension. Plasma levels of AGEs are significantly higher in individuals with hypertension compared to those without hypertension and are associated with aortic stiffness independent of age and BP.133 Skin AGEs, an important indicator of the current level of accumulated AGEs,134 is associated with vascular stiffening independent of age and other cardiometabolic risk factors, not only in individuals with diabetes but also in those in normoglycemic and prediabetic conditions.135 Besides, studies reported an inverse correlation between sRAGE and BP,136,137 and sRAGE showed its potential in predicting cardiovascular events and/or mortality in diabetics.138
Mechanistically, the properties of collagen can be altered through AGEs–RAGE intermolecular covalent bonds or cross-links. Cross-links between AGEs and collagen or elastin increase the extracellular matrix area.139,140,141 On the other hands, cross-linking renders collagen insoluble to hydrolytic enzymes, and collagen linked with AGEs is less susceptible to hydrolytic turnover, making it stiffer.142,143 And these factors combined result in vascular remodeling and dysfunction. In intracellular pathway, studies showed interaction of AGEs with full-length RAGE via PI3K, MAPK, ERK1, and ERK2 activates NF-κB (nuclear factor κB), stimulating inflammation and various cytokines secreting.144,145 In addition to these cascades leading to inflammation, RAGE activation can increase ROS via NADPH (nicotinamide adenine dinucleotide phosphate) oxidase, and lead to oxidative stress and dysfunction in cells.146,147,148,149,150,151,152 Notably, RAGE is not only activated by AGEs but also stimulated by other factors including S100 proteins, HMGB-1 (High mobility box group-1), Mac-1 integrin, and ox-LDL (oxidized low-density lipoprotein), thus resulting in vascular dysfunction.145,151,153,154,155 On the contrary, sRAGE can fuction as a decoy receptor for RAGE ligands, effectively binding to them and exerting protective effects against the harmful consequences of the AGE-RAGE interaction on vascular function.156
Calcification
Vascular calcification refers to the process of calcium deposition in the extracellular matrix of arterial walls. Vascular calcification involves deposition of mineral in the ECM, VSMCs apoptosis and osteogenic transformations, dysregulated expression of mineralization inhibitors, and microvesicle (MV) release et al.157,158,159 There is a mutually reinforcing relationship between vascular calcification and hypertension. Hypertension is a calcification-promoting stressor,157 and vascular calcification can result in hypertension through arterial stiffness.160,161,162 Arterial stiffness can initiate media calcification through mechano-sensing pathways, which subsequently exacerbate arterial stiffness.163 Though calcification can occur in intima, media, or adventitia,164,165 it is commonly considered that medial arterial calcification (MAC) contributes the most to the dysfunction of vascular contraction.163,166,167,168 The pathobiological mechanisms of calcification can be classified into 2 categories: the loss of mineralization inhibitors and the induction of osteogenesis.
MAC is characterized as calcium phosphate depositing in the form of hydroxyapatite in media ECM. Inorganic phosphate is one of the important causes of MAC.169,170 Under normal physiological conditions, when the concentrations of calcium and phosphate exceed their solubility limits, the body relies on endogenous calcification inhibitors to prevent the ectopic precipitation of these minerals.171,172 Pyrophosphate has been identified as the strongest endogenous inhibitor of mineralization, and it is produced locally by VSMCs.173,174,175 Pyrophosphate can prevent mineralization through several mechanisms, including direct binding to growing crystals, induction of osteopontin expression, and inhibition of Tnap (an issue-nonspecific alkaline phosphatase) activity.176 VSMCs are responsible for producing and releasing pyrophosphate into the extracellular space, a process that involves the activation of two proteins: ENPP1 (ectonucleotide pyrophosphatase/phosphodiesterase) and ANKH (the transmembrane protein ankylosis protein homolog).175,177 The ENPP1 protein helps break down adenosine triphosphate into AMP (adenosine monophosphate) and pyrophosphate.178,179 ANKH protein transports pyrophosphate out of cells to ECM.180,181,182 Besides, VSMCs are also involved in taking up Fetuin-A from the extracellular space, which is a circulating protein that can bind to calcium or hydroxyapatite directly, to inhibit the growth of hydroxyapatite crystals. And VSMCs can produce several other inhibitory proteins, including matrix-Gla protein, osteopontin, and osteoprotegerin. These proteins can be loaded into extracellular vesicles to prevent vascular mineralization.172,173,183 Under pathological conditions, including high extracellular phosphate levels and exposure to uremic toxins, the production of calcification inhibitors by VSMCs can be suppressed. This can further result in the release of exosomal vesicles lacking these inhibitors and promoting vascular mineralization. On the contrary, the load of pro-calcific proteins such as ALPL (tissue-nonspecific alkaline phosphatase) is increased.184,185 All these combined can lead to the formation of microcalcifications, which can provide a site for the precipitation of calcium phosphate and the subsequent growth of crystals.185,186,187
Apoptosis and phenotypic switching of VSMCs is also critical to calcification. Apoptotic bodies released by VSMCs can potentially serve as nucleating structures for calcium crystal formation.188,189 High extracellular phosphate levels can induce apoptosis and necrosis of VSMCs.183,190 Activation of pro-apoptotic signaling pathways in VSMCs can occur due to the involvement of a multitude of upstream signaling cascades in response to phosphate. The downregulation of Gas6 (growth arrest-specific gene 6) and its receptor tyrosine kinase Axl, and activation of apoptosis-related BAD (Bcl2-associated death promoter)/Caspase-3 via PI3K/AKT pathway191,192,193,194,195 or AMPK (AMP-activated protein kinase),196,197,198 have been thought to be main pathway in apoptosis of VSMCs stimulated by phosphate. Contractile VSMCs converting into osteo-phenotype is also responsible for vascular calcification. Under high extracellular phosphate levels circumstances, VSMCs will undergo a phenotypic switch into osteo-/chondroblast-like cells, and promote vascular mineralization.199,200 These phenotypes can express osteogenic transcription factors such as MSX2 (msh homeobox 2), CBFA1 (core-binding factor α-1, also known as RUNX2)199,200,201 or osterix,202 as well as chondrogenic transcription factors including SOX9 (SRY-Box 9).203,204,205 Both CBFA1 and SOX9 play critical roles in vascular osteo-/chondrogenic transdifferentiation and calcification.204,206,207 The transcription factors, MSX2 and KLF4, have been proven to be the upstream regulators of CBFA1 and SOX9.208,209,210,211 The expression of osteogenic- and chondrogenic-specific proteins in VSMCs, such as osteocalcin, type I collagen, BMP-2 (bone morphogenetic protein-2), or ALPL, is further induced by osteo-/chondrogenic transcription factors.170,212 An increase in ALPL activity is a decisive event in vascular calcification, as ALPL is a key regulator of this process.186,213
Important upstream pathways regulating vascular function and hypertension
Renin-angiotensin-aldosterone system
Renin-angiotensin-aldosterone system (RAAS) plays a critical role in the regulation of BP. In the RAAS, angiotensinogen and downstream peptides are the main stimulators regulating vasoconstriction (Fig. 5). Angiotensinogen mainly produced by the liver is cleaved by renin into angiotensin I (Ang I). Ang I is cleaved by angiotensin-converting enzyme I (ACE1) to produce angiotensin II (Ang II) or cleaved by angiotensin-converting enzyme 2 (ACE2) to produce Ang (1–9). Ang II exerts various physiological and pathophysiological effects, including vasoconstriction and sodium/water retention, via activation of Ang II type 1 receptor (AT1R) signaling.214 Moreover, Ang II or Ang (1–9) can be converted to angiotensin 1–7 [Ang (1–7)],215 and Ang (1–7) can cause vasodilation and lower BP by binding to Mas receptor (MasR).215,216,217,218 Those peptides can have actions on endothelial cells, SMCs, and adventitia cells in vessels. Various peptides activating the receptors on different cells can result in different effects, including apoptosis and endothelial barrier damage for endothelial cells, contraction of SMCs, and fibrosis of atria.219,220,221 In addition, recent study reported the role of Ang (1–12) in the process of hypertension, the angiotensin family is still under development.222
Renin-angiotensin-aldosterone system. ACE angiotensin-converting enzyme I, ACE2 angiotensin-converting enzyme 2, Ang I angiotensin I, AT1R Ang II type 1 receptor, Ang (1–7) angiotensin (1–7), MR mineralocorticoid receptor, (P)RR (pro)renin receptor, VEGF1R vascular endothelial growth factor type 1 receptor
Angiotensin-related receptors
The signaling pathways of RAAS in cells begin with the activation of various receptors, and the diverse effects of RAAS can be attributed to the activation of different receptors.223,224 Angiotensin-related receptors are the main executors of the RAAS system in regulating vasoconstriction and BP. AT1R is a member of the GPCR (G protein-coupled receptor) family and is expressed in various cells including VSMCs, endothelium, cardiomyocytes, etc. Ang II/AT1R play a central role in regulating BP. In VSMCs, AT1R can be stimulated by Ang II and interact with a heterotrimeric G protein including Gq/11 and G12/13. Gq/11 can subsequently activate PLC-IP3-Ca2+-sensitive-MLCK signaling. While G12/13 will active PKC-RhoA/Rho kinase-mediated inhibition of MLCP.214 In addition, study showed that Ang II/AT1R inhibited the MLCK transcription by blocking the Notch signaling in VSMCs.225 On the contrary, Ang (1–7)/MasR is a bioactive peptide that can exert diverse effects, many of which are contrary to those induced by Ang II/AT1R.226,227,228 The Ang 1–7/MasR axis activation acts as a counter-regulator for the effects mediated by Ang II/AT1R, but the exact mechanism remains unclear. A recent study has found that Ang 1–7/MasR axis exerts vasodilation through two mechanisms: a telomerase-dependent manner and direct increase of telomerase activity in human endothelium.229 Since increased telomerase activity can elevate NO production and endothelial nitric oxide synthase expression,230 it is supposed that Ang 1–7/MasR-mediated vasodilation may via NO-(NOsGC)-cGMP pathway.
In addition to Ang II/AT1R and Ang 1–7/MasR, other peptides or receptors are also involved in BP regulation. Upregulation of vascular and plasma ACE2, along with increased plasma Ang 1–9 levels, can exibit a potent antihypertensive effect via RhoA/Rho kinase inhibition, without an increase in Ang 1–7 levels.231 The AT2R can promote the production of NO and cGMP by two different mechanisms, dependent or independent of the production of bradykinin (BK) via BK B2 receptors.232,233,234 ANG IV, cleaved from Ang (1–9), can bind to AT4R and cause vasorelaxation via eNOS, too.235
Angiotensin-unrelated receptors
The (pro)renin receptor ((P)RR) is thought to enhance the activity of the tissue renin–angiotensin system by binding to renin or prorenin, and it can activate intracellular tyrosine-phosphorylation-dependent pathways independently of RAAS.236 However, whether (pro)renin/(P)RR contributes to hypertension in human is controversial. A study showed that in VSMCs, prorenin can induce ERK phosphorylation via (P)RR-mediated activation of tyrosine kinase. This can subsequently lead to a vascular remodeling via MEK,237 independently of the production of angiotensin II or the activation of its receptors. Another study demonstrated that (P)RR was essential for VSMCs survival and downregulation of vascular inflammation, through maintaining normal function of the vacuolar H(+)-ATPase in Wnt signaling.238 And the deletion of (P)RR did not affect ambulatory BP levels in murine.238 To elicit intracellular signaling in vitro, much higher concentrations of (pro)renin are required than those observed in physiological plasma levels. The effects observed in animals overexpressing prorenin could be solely due to the generation of angiotensin, possibly without requiring a receptor.239 Compared with hypertension, it is now considered that (P)RR and the downstream pathway play more important roles in cell survival. (P)RR knockout, even tissue-specific, is lethal compared to other RAS components knockout, suggesting that (P)RR has an important function independent of (pro)renin.240,241 To be mentioned, in clinical studies, the addition of direct renin inhibitors (such as aliskiren) to ARB therapy did not lead to improved renal or cardiovascular outcomes. Conversely, it was associated with a higher incidence of adverse effects compared to ARB therapy alone.242 According to that, the prospect of targeting (P)RR in the treatment of hypertension is not as good as AT1R. Another angiotensin-unrelated receptor is mineralocorticoid receptor (MR). The mechanism by which MR affects BP involves its binding with aldosterone and subsequent retention of sodium. Mice with MR deficiency in VSMCs exibit decreased vascular myogenic tone, reduced contraction in response to agonists, as well as decreased expression and activity of L-type calcium channels.243 MR mediates vascular remodeling via several signalings, including RhoA/ROCK, placental growth factor (PLGF), vascular endothelial growth factor type 1 receptor (VEGF1R), and galectin signaling et al.244
Redox signaling pathway
Cellular reduction/oxidation (redox) signaling pathway is responsible for regulating a wide range of cellular functions, including but not limited to homeostasis, differentiation, proliferation, and apoptosis.245 Cellular oxidative stress occurs when reactive oxygen/nitrogen species (ROS/RNS) is produced. Generally, ROS/RNS substances include hydrogen peroxide (H2O2), hydroxyl radicals (HO), superoxide anion radicals (O2–), nitric oxide (NO), nitrogen dioxide (NO-), peroxynitrite (OONO−), dinitrogen trioxide (N2O3), and nitrous acid (HNO2) radicals.246 Redox signaling is primarily characterized by an oxidation–reduction reaction or covalent adduct formation that occurs between the sensor signaling protein and second messenger.247 Multiple transcription factors and enzymes are all redox-sensitive.248 Redox signaling has been firmly established in vascular function and hypertension (Fig. 6). Activation of redox signaling increases vascular tone by influencing the regulatory role of ECs, and by directly affecting the contractility of VSMCs.249,250,251,252 Oxidative modifications affect multiple kinases, such as Src tyrosine kinase,253 ASK-1,254 PKG (protein kinase G),255 and MAPK pathway, by which regulating vascular contraction.256,257 As mentioned above, ROS is also an important factor in the activation of MMPs, leading to vascular remodeling and BP increase. Furthermore, the expression and function of various transcription factors, including NF-κB, Nrf-2, AP-1 (activator protein 1), STATs (signal transducers and activators of transcription) etc., are affected by ROS. The activation of these factors can lead to inflammation, total antioxidant status, endothelial dysfunction, and hypertension.249,258,259,260,261
Redox signaling pathway. AP-1 activator protein 1, ECT electron transport chain, ER endoplasmic reticulum, NOXs NADPH oxidases, ROS/RNS reactive oxygen/nitrogen species, SOD superoxide dismutase, STATs signal transducers and activators of transcription, PDGF platelet-derived growth factor, PKG protein kinase G, VEGF vascular endothelial growth factor
Endogenous ROS/RNS are mainly produced through mitochondrial electron transport chain (ECT),262,263,264 enzymes NADPH oxidase,265 and other sources including xanthine oxidase,266 peroxisomes,267 endoplasmic reticulum268 etc. At the same time, there is a scavenging system in redox signaling to maintain ROS/RNS balance in the body.
Mitochondrial electron transport chain
In physiological conditions, superoxide is generated as a side product of electron transportation during oxidative phosphorylation in SMC mitochondria.269,270 Although most superoxides stay in mitochondrial matrix, some can escape to the intermembranous space and cytosol, via anion channels when the superoxides generation is excessive.271 This is one of the main sources of superoxides contributing to redox signaling activation.
NADPH oxidases
NADPH oxidases (NOXs) are enzymes that produce superoxides by transporting electrons from NADPH to molecular oxygen. NOXs are well-known as important sources of ROS in blood vessels.272 NOXs system is composed of NADPH oxidase constituents (such as Nox1, Nox2, Nox4, and Nox5) and cytosolic proteins. Activation of each constituent involves distinct regulatory mechanisms and signaling pathways, which can be attributed, in part, to the variety of cytosolic regulatory subunits, including p47phox, p67phox, Rac, Noxo1, and Noxa1.251,273,274 Hypertensive animal models, such as Ang II-induced hypertensive rats,275 SHR,276 DOCA-salt hypertensive rats, and two-kidney two-clip renovascular hypertensive rats, have been shown to exhibit increased expression and activity of NADPH oxidase or its cytosolic subunits.277,278,279 The activation of vascular NADPH oxidases through PKC, Src280, or CyPA281 dependent pathways is an important mechanism by which Ang II functions in the body. Besides, NOX1-derived reactive oxygen species regulate cell-surface AT1R expression by mechanisms like caveolin phosphorylation,282 which suggests that there is positive feedback between Ang II and NOXs. Other stimulants include PDGF, VEGF, cytokines, wounding254,283,284,285 etc.
Endoplasmic reticulum
There is growing recognition of the important role that the endoplasmic reticulum (ER) palys in redox pathophysiology, summarized in another review249 as: (i) ER enzymes, like ER oxidoreductin (Ero1) and its thiol redox partner protein disulfide isomerase (PDI), have a role in the generation of ROS, (ii) ER is responsible for the synthesis, maturation and post-translational modification (glycosylation, phosphorylation, oxidation) of p22phox and Nox, (iii) Nox activity is promoted by the interaction between Nox and the ER chaperone PDI, (iv) some Noxes, especially Nox4, are active in ER, (v) The communication between ER and mitochondria occurs through mitochondria-associated ER membranes, which facilitates the exchange of Ca2+ and ROS between the compartments, and (vi) ER plays a critical role in redox protein folding and stress responses.
ER stress results from a disruption in the ER protein-folding capacity, leading to the accumulation of unfolded and misfolded proteins.286 There is a suggestion that ER stress plays a significant role in the synergistic effects of hypertension and target organ damage.287,288,289 Expressions of key molecules increase in ER stress signaling pathway, such as ATF6 (activating transcription factor-6), IRE1 (inositol requiring enzyme 1), PERK (PKR-like eukaryotic initiating factor α kinase), XBP1s (X-box-binding protein 1), ATF4 (activating transcription factor-4), and CHOP (C/EBP homologous protein) etc., leading to activation of redox signaling and hypertension.289
Antioxidant system
In addition to increased ROS/RNS production, decreased scavenging ability also contributes to oxidative stress. The vascular system have several antioxidant systems, such as the superoxide dismutase (SOD) family, catalase, the glutathione (GSH) system, thioredoxin, peroxiredoxin, selenoproteins, and ROS scavengers such as vitamins A, C, and E.290,291,292,293 In hypertensive patients, antioxidant substances, including SOD, catalase, and GSH peroxidase etc., are significantly lower in the whole blood or peripheral mononuclear cells, when compared to normotensive individuals. After antihypertensive treatment, all these parameters can be restored.294,295,296 In animal studies, it has been observed that inhibiting GSH synthesis leads to an elevation of BP in normotensive rats,297 and knockout of extracellular-SOD (EC-SOD) in mice results in elevated baseline BP without any other treatment.298 In addition, partial deficiency of SOD2, which is the mitochondrial SOD isoform, induced spontaneous hypertension in aged mice and accelerated the development of high salt-induced hypertension.299 These results suggest that reduced antioxidant capacity alone is enough to cause vascular dysfunction and hypertension. Catalase is another important antioxidant enzyme. Catalase facilitates a two-step reaction in which it breaks down two molecules of hydrogen peroxide into one molecule of oxygen and two molecules of water. Accumulation of catalases attenuates the progression of vascular remodeling and hypertension by reducing oxidative stress.300,301,302,303
Immunity/inflammation pathway
The majority of components involved in the Immunity/inflammatory responses circulate through the blood and vasculature. And low‐grade immune response plays an important role in the initiation and maintenance of elevated BP.304,305,306 Immune responses can occur in the intima, media, and adventitia of blood vessels. The luminal and microvascular endothelial cells in the intima and adventitia play a critical role in the recruitment and activation of leukocytes, which are commonly involved in the pathophysiological processes of hypertension and target organ damage.307 The vascular media, in contrast, is often unaffected by immune-mediated disorders.307 In arteries, the inflammatory response can be initiated by humoral, vasoactive hormones, mechanical factors, metabolic factors, epigenetic dysregulation, autonomic nervous system etc.308,309,310,311 Current studies have shown that both innate and adaptive immunity are involved in the pathogenesis of hypertension306,312,313,314 (Fig. 7).
Innate immunity
The innate immunity serves as the initial defense against infectious agents and also contributes significantly to the development of sterile inflammation.315 APCs (activation of antigen presenting cells) or PRRs (pattern recognition receptors) is the initiating step of innate immunity. MHC II (major histocompatibility complex II) in APCs can initiate activation of T or B lymphocytes and lead to adaptive immunity.312 While PRRs, activated by PAMPs (Pathogen-Associated Molecular Patterns) and DAMPs (Damage-Associated Molecular Patterns), mainly including TLRs (Toll-like receptors) and NLRs (NOD-like receptors), directly activate related pathways to trigger endothelial dysfunction or vasoconstriction. TLRs are a well-characterized family of membrane-bound PRRs which are expressed on the cell membrane in macrophages, dendritic cells, and mast cells.316,317,318,319 Studies demonstrated TLR4 expression was upregulated in experimental models of Ang II and L-NAME-induced atrial hypertension.320,321,322 TLR4 inhibition contributed to alleviating vascular contractility, vascular inflammation, and oxidative stress in SHRs, preventing the development of experimental hypertension.320,323,324 Stimulation of TLR4 leads to the activation of signaling pathways or transcription factor in VSMCs such as ROS, MARK, NF-κB etc.325,326,327 TLR9, which recognizes circulating mitochondrial DNA, is also upregulated in the circulation of SHRs. Inhibition or knockout of TLR9 in mice can result in a reduction in systolic BP, which may be via the cardiac autonomic and baroreflex regulation.328,329 Another family of PRRs are intracellular NLRs, with the NLRP3 inflammasome being the most characterized. Activation of NLRP3 inflammasome (including NLRP3 sensory component, the adaptor protein ASC) is a powerful mediator of inflammatory response via the effector protein caspase-1, and plays a pivotal role in vascular diseases.330,331,332 Study showed that NLRP3 inflammasome activation contributed to VSMC phenotypic transformation and proliferation in hypertension.333 And CaSR (calcium-sensing receptor)-mediated activation of the NLRP3 inflammasome in VSMCs is an important regulator of aortic remodeling in SHRs induced by Ang II.334
Adaptive immunity
T and B lymphocytes are the characterized cell types of the adaptive immune system. Animal studies proved that, inhibition of the maturation process of T and B cells by knockout of the Rag1 (recombination activating gene1) in vivo alleviated Ang II-induced and salt-sensitive hypertension.335,336 Besides, genetic knockout of the CD247 or receptor Axl (tyrosine kinase TAM family member) gene in mice attenuated salt-sensitive hypertension by attenuating glomerular and renal tubular damage or improving endothelium-dependent vasorelaxation.337,338,339 Notably, study demonstrated that, it was CD8-/- mice but not MHCII-/- or CD4-/- mice that showed a blunted response in Ang II and DOCA/salt-Induced hypertension, which suggested that the role of specific T cell subtypes might be different in hypertension development.340 Similarly, knocking out B cell activating factor receptor (BAFF-R) also attenuated Ang II-induced BP elevation, and adoptive transfer of B cells into BAFF-R-/- mice restored Ang II-induced hypertension.341 The mechanisms by which T and B cells trigger hypertension have not been fully elucidated. A study has suggested that hypertension-induced sodium excretion via eNOS- and COX-2 (cyclooxygenase-2)-dependent pathways in kidneys is facilitated by the absence of lymphocyte activity, which may in turn protect against hypertension.342 In vascular ECs, IgG released from B cell binding to Fcγ receptors (FcγRs) and inhibiting the activity of eNOS synthase, which contributing to obesity-induced hypertension.343
In addition to T and B cells, other subtypes of T cells, mainly including T helper (Th) cells and regulatory T cells (Tregs), also contribute to the progression of hypertension by releasing pro-inflammatory factors. Th 1 cells produce IFNγ, IL-2, and TNFα; Th 2 cells produce IL-4 (interleukin-4), IL-5, IL-9, and IL-13; Th 17 cells secrete IL-17, IL-21, and IL-22. These inflammatory factors are all thought to be associated with hypertension.27,344,345,346,347,348,349,350 Tregs are a small subset of immune cells that play a crucial role in curbing excessive immune activation and maintaining immune homeostasis.351,352,353 A decreased number and impaired function of Tregs cells have been observed in various cardiovascular diseases, including hypertension.354 In contrast to T cells or Th cells, differentiation and proliferation of Tregs can increase local production of anti-inflammatory cytokines (such as IL-10 and TGF-β) and reduce plasma inflammatory cytokines (such as IFN-γ, IL-6, and TNF-α) levels, which thereby attenuates the vascular immune-inflammation, vascular oxidative stress, and endothelial dysfunction, preventing against hypertension.355,356,357,358,359 Interestingly, studies have shown that a decrease in Tregs significantly increases BP only in females, thereby eliminating the sex difference in the BP response to DOCA-salt,360 which supports the notion that the immune system contributes to sex differences in hypertension. It is worth noting that while hypertension and inflammation are physiologically connected, the impact of therapies that specifically target inflammation on BP still requires further evidence from clinic trials. IL-1β is an upstream inflammatory factor of IL-6 and CRP (C-reactive protein). IL-1β, IL-6, and CRP are considered to be closely involved in the progression of hypertension.27,361,362,363 However, a large clinical study have shown that the use of IL-1β antibody did not reduce the incidence of hypertension.364 Thus, the use of anti-inflammatory therapy to lower BP requires more in-depth study.
Sympathetic dysregulation
The regulation of bodily functions by the sympathetic system relies on the establishment and precise connections between postganglionic sympathetic neurons and peripheral organs distributed throughout the body.365,366 Sympathetic nervous system dysfunction contributes a lot to the development of hypertension367,368,369,370,371,372 (Fig. 8). The activation of muscle sympathetic nerve activity (SNA) is the key mechanism of sympathetic dysregulation leading to hypertension. Many forms of high BP are associated with an increase in muscle SNA, including essential hypertension,373,374,375 renovascular hypertension376,377 and pregnancy-induced hypertension373,378 et al.
Delivering electrical stimulation in bursts to sympathetic nerves in blood vessels can cause significant vasoconstriction. It is closely associated with norepinephrine (NE)-induced contraction of vascular smooth muscle, with NE being released by postganglionic neurons.379,380 Adrenergic receptors are distributed on the membranes of most effector cells innervated by the postganglionic fibers. Vascular SMCs have two types of adrenergic receptors, α and β. NE binding to α adrenergic receptors can cause VSMCs contraction; and it binding to β adrenergic receptors (mostly β2 receptors) can cause VSMCs relaxation. NE prefers to bind to α adrenergic receptors rather than β receptors, so it causes a vasoconstrictive effect when the vasoconstrictor fibers are excited.381 α adrenergic receptors on the vessels are divided into α1 and α2 receptors. And α-adrenoceptors can be classified into different subtypes, too.381,382,383 Both α1 and α2-adrenoceptors are crucial in the control of vascular tone. There also exists a synergistic interaction between α1 and α2-adrenergic receptors.384 Following increased muscle SNA, NE binds both α1 and α2-adrenergic receptors on vascular smooth muscle, which can lead to vasoconstriction by increased [Ca2+]c and MLC phosphorylation, as well as activation of the Rho-associated kinase calcium sensitization pathway.385,386,387
Other signaling pathways afftecting vascular function and hypertension
Signaling pathways in cell identity
With the widespread utilization of single-cell sequencing (scRNA-seq) technology, our understanding of the intrinsic properties of cells has shifted from single-dimensional descriptions to multi-dimensional and high-resolution depictions. Describing cell identity by key concepts such as phenotype, lineage, and state, we gain a deeper understanding of the mechanisms by which the cells can play different roles in different environments. In the vascular wall, phenotypic conversion, lineage origin, and cellular state of ECs and SMCs may all affect the vascular functions directly.
There are two states of ECs, activated or quiescent. Activated ECs secrete MMPs, and undergo proliferation and migration, which are often closely associated with angiogenesis.8,388,389 While quiescent ECs organize themselves into a continuous monolayer and allow perfusion with blood.8,390 The production of S-2-hydroxyglutarate (S-2HG), stimulated by activated FOXO1 (transcription factor forkhead box O1), plays a key role in promoting a quiescent endothelial state.391,392 The different states of ECs can determine the longitudinal extension of blood vessels. However, no studies have reported the role of activated or quiescent ECs in vasocontriction or BP regulation yet. In addition, there exists some condition where ECs can transform into mesenchymal cells, which is called Endothelial to Mesenchymal Transition (EndMT). A variety of cellular properties will change in ECs after EndMT, such as increased permeability, enhanced migration, upregulated expression of some mesenchymal cellular markers, and enhanced ability to secret enzymes and to change the ECM environment.393,394 TGF-β (Transforming Growth Factor-β) signaling pathtway is now considered as the most significant pathway in EndMT regulation. After binding to the receptors, TGF-β can participate in the EndMT via Smad (Small Mother against Decapentalegic) and non-Smad (MAPK, ERK, etc.) pathways.394,395 Other signaling pathways, such as NOTCH and Wnt (Wingless-Related Intergration Site) pathway, can also induce EndMT.394,395 Just a few studies reported the association of EndMT with BP alterations. It was indicated that ox-LDL could promote the production of TGF-β via Lox-1/PKC-α/MMP9, and it also could induce EndMT via SMAD2/SMAD3, resulting in increased BP.396 And this is possibly one of the crucial mechanisms by which high blood lipids induce BP increase. Some stimulators closely associated with hypertension, such as Angiotensin II397 and AGEs,398 were also confirmed to induce EndMT, thus leading to BP increase. In addition, pro-inflammatory factors, such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), have been reported to induce EndMT in human primary aortic ECs and to promote vascular calcification by downregulating BMPR2.399 A recent study used scRNA-seq to detect the heterogeneity and state of arterial cells in mice with salt-induced hypertension, and found that, EndMT was more commonly observed in arterial cells in hypertensive mice, compared with the control group. And this suggested that the occurrence of hypertension might be directly related to EndMT.400
VSMCs are classically characterized by the expression of four contractile- or contraction-associated proteins: TAGLN (smooth muscle 22 α/transgelin), ACTA2 (smooth muscle α-actin), MYH11 (smooth muscle myosin heavy chain 11), and CNN1 (H1-calponin).401 With the help of scRNA-seq, six phenotypes of VSMCs have been identified, including contractile phenotype, mesenchymal-like, fibroblast-like, macrophage-like, osteo-/chondroblast-like, and adipocyte-like phenotypes, each directly affecting the ECM with their own unique characteristics.402,403,404,405 KLF4 (Krüppel-Like Factor 4) is considered as a key transcription factor in phenotype regulation.405 Although, it is theoretically possible for VSMCs switching from a contractile phenotype to other phenotypes, which may affect the contractile function of the arteries, the role of different phenotypes in arterial function and hypertension is still unknown. At present, it is clear that the osteo-/chondroblast-like phenotype is involved in the formation of arterial calfication. In a study of salt-induced hypertensive mice,400 VSMCs were divided into 3 groups (SMC 1, SMC 2, and SMC 3) according to their markers. SMC 1 was observed to be the most expressed group in both the hypertensive and control group. The contractile phenotype-related gene expression in SMC 1 was lower in hypertensive group than that in control group. However, The expression of KLF4 was significantly higher in hypertensive group. This indicated that part of the SMCs in aorta experienced phenotypic conversion in the salt-induced hypertensive mice, which might be associated with the occurrence of hypertension. Unfortunately, the study did not further explore which phenotype is the dominant phenotype in hypertensive arteries. Nevertheless, according to our own research data (unpublished), long-term high blood lipids level can lead to arterial remodeling and increased arterial stiffness. And the pathological mechanism may be that, induced by high blood lipids, VSMCs can convert from contratile phenotype into macrophage-like phenotype, resulting in changes in ECM composition and decreased cell contractility. However, the roles of other phenotypes, such as fibroblast-like and adipocyte-like phenotype, in vascular function and BP regulation remain to be further explored.
Vascular fibrosis and TGF-β/Smad signaling pathway
Fibrosis refers to the excessive accumulation of connective tissue components in an organ or tissue. Vascular firobrosis is caused by the excessive deposition of the ECM components (especially fibronectin and collagen), which can result in a decrease in lumen diameter and thickening of arterial wall,406,407 and further lead to arterial stiffness and hypertension.101,408 Although whether the two concepts of vascular fibrosis and vascular remodeling should be unified is inconclusive for the time being, they are almost consistent from the perspective of pathological changes and diseases caused. TGF-β and downstream Smad is the key signaling pathway that promotes tissue fibrosis.407,409,410 In various cells in blood vessels, such ECs, VSMCs, and fibroblasts in adventitia, the activation of TGF-β/Smad pathway can stimulate the synthesis of fibronectin and collagens, and promote their deposition in ECM, thus resulting in vascular fibrosis.407,411,412 CTGF (connective tissue growth factor) is a chaperon protein that has a synergistic effect with TGF-β to promote fibrosis.413 Studies showed that the activation of TGF-β signaling pathway would further upregulate CTGF gene expression,414 which indicated that CTGF played an important role in regulating TGF-β pathway in a positive feedback way. PAI-1 (plasminogen activator inhibitor-1) is an inhibitor of serine protease, urinary plasminogen activator (uPA) and tissue plasminogen activator (tPA). The activation of TGF-β/Smad pathway can upregulate PAI-1 gene expression. And the increase of PAI-1 in ECM will inhibit tissue proteolytic activity and collagen degradation, thus leading to protein accumulation in ECM, vascular fibrosis, and hypertension.415,416 Moreover, as mentioned above, TGF-β/Smad pathway is also a crucial pathway which promotes EndMT. It is also worth noting that, AGEs can activate Smad via MAPK pathway, independently of TGF-β,417 which indicates that AGEs may also be one of the causes of vascular fibrosis.
Apelin/APJ signaling pathway
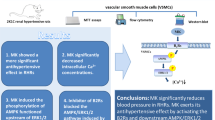
A new class of transmembrane receptor, APJ (putative receptor protein related to the angiotensin receptor), was first discovered in 1993 by O’Dowd. It has homology as high as 30% with AT1R, while it does not bind to angiotensin II.418 In 1998, an endogenous ligand for APJ, called apelin, was extracted from cow stomach by Tatemoto et al.419 Apelin is type of polypeptide hormone. APJ exists in a variety of cells such as ECs and VSMCs, and is widely expressed in large blood vessels and the vascular system of various organs.420,421,422,423 Apelin/APJ mediates vasodilation primarily by activating the eNOS/NO pathway in ECs.424,425,426 Clinical studies showed that reduced circulating Apelin was significantly associated with an increased risk of hypertension.427 And in animal studies, peripheral injection of Apelin can cause extensive vasodilation, as well as a decrease in blood pressure.428 Furthermore, Apelin/APJ also interacts with RAAS. Activation of apelin/APJ signaling pathway has an antagonistic effect towards AT1R-mediated responses.429,430,431 Apelin/APJ signaling can also upregulate ACE-2 gene expression, reinforcing the conversion from Ang II to Angiotensin 1–7.432 This inhibition of the RAAS system is an important mechanism for Apelin to exert a protective effect on the cardiovascular system.433 To be mentioned, when the vascular endothelium is damaged, Apelin can act directly on the APJ of VSMCs, increasing phosphorylation of MLC and causing vasoconstriction and increased blood pressure.434,435 It can be seen that the integrity of the vascular endothelium is very important for Apelin to play its hypotensive effect. Except Apelin, recent studies also found another endogenous polypeptide ligand for APJ, Elabela (also named Toddler).436,437 Hypertensive patients seemed to have a low level of circulating Elabela, which was strongly associated with hypertension-related vascular damage.438 It is currently believed that the function mechanism of Elabela is similar with that of Apelin, both of which exert their function by activating APJ-mediated downstream pathway.439,440 However, some studies showed that differences existed regarding the polypeptide structural characteristics and the functioning active groups between Elabela and Apelin.441 This suggested that the binding pattern of Elabela and APJ might be different from Apelin, which could be of value in pharmacology development. Both Elabela and Apelin have their latent value in antihypertensive and cardiovascular treatment.
Na+ channels and hypertension
Alterations in circulating Na+ concentration contributes a lot to the pathology of hypertension. On the one hand, increased sodium intake in the body can lead to sodium and water retention, resulting in increased pressure to the blood vessel walls. On the other hand, it can trigger vasoconstriction or relaxation via Na+ channels.
The Na+/Ca2+ exchangers (NCX) can link Na+ and Ca2+ metabolism and act as distal regulators of cytosolic Ca2+ levels. There are 2 types of NCXs. By one kind of NCXs, the Ca2+ transfer only depends on Na+ concentration (NCXs, including NCX1-3). While by the other kind, the Ca2+ transfer depends on both Na+ and K+ concentration (NCKXs, including NCKX 1-6).442,443,444 Both NCXs and NCKXs exist in VSMCs.442 The transferring direction of Ca2+ (influx or outflux) via NCXs and NCKXs depends mainly on Na+, Ca2+ (and K+) gradients, and the potential across the membrane.444,445 In salt-dependent hypertensive rats, the blockage of NCX1 could cause a reduction in cytosolic Ca2+ concentration in VSMCs, thus attenuating the Ca2+ signaling and resulting in vasodilation and BP decrease.446 This indicated that in high salt-intake condition, NCX1 could still exert its function to mediate Ca2+ influx. Besides, NCXs in VSMCs appeared to be predominantly present in the plasma membrane adjacent to SR,447 which suggested that NCXs in VSMCs might indirectly regulate the Ca2+ storage in SR.
Na+ pumps (Na, K-ATPase) can transport Na+ from intracellular to extracellular and K+ in an inverse direction against a concentration gradient by breaking down ATP, maintaining the osmotic pressure across cellular membrane. Theoretically, inhibition of the Na+ pumps activity will attenuate Na+ transporting in renal proximal tubular epithelial cells, leading to an increase in water sodium excretion and downregulating BP.448 Nevertheless, studies showed that inhibition of Na+ pumps was a main cause of increased peripheral vascular resistance in essential hypertension.449 The mechanisms by which Na+ pumps activity enhancement causes vasodilation may be related to both nitric oxide- and prostanoid-independent arterial relaxation,450,451 and Ca2+ elevation via NCXs.452,453,454 In addition, Na+ pumps can regulate inter-cell communications in vessels via cSrc-dependent Cx43 tyrosine phosphorylation, and synchronize the constriction and relaxation among VSMCs.455
Epithelial Na+ channel (ENaC), a member of DEG/ENaC family, is primarily expressed on the apical membrane of the principal cells in the aldosterone sensitive distal nephron (ASDN), where it functions as the final and rate-limiting step of renal Na+ reabsorption. Excessive ENaC activation will lead to sodium and water retention, contributing as a key mechanism to the pathology of hypertension.456 In vascular ECs, EnNaC activation by ALD or elevated Na+ concentration can lead to endothelial stiffness, reduced NO production, increased vascular tension and vascular remodeling.457,458,459,460 In CNS, over-activation of ENaC and Na+ transport may lead to elevated sympathetic activity and BP increase.461,462 In dendritic cells, increased Na+ influx mediated by ENaC can promote the release of inflammatory cytokines including IL-17, and therefore upregulate BP.463,464 In addition, a recent study demonstrated that the activation of the NLRP3 inflammasome induced by Na+ is dependent on ENaC and IsoLG(isolevuglandins), and this was an important mechanism in the pathogenesis of sensitive hypertension.463
Sodium-glucose co-transporter and vascular function
Sodium-glucose co-transporter (SGLT) family refers to carrier proteins that reabsorb filtered glucose in kidneys. It is estimated that SGLT-2 is responsible for at least 80–90% filtered glucose reabsorption.465 Meta-analyses showed that SGLT-2 inhibition lowered SBP by 2–5 mmHg and DBP by 0.5–2 mmHg, which might have a coordinated effect with other first-line antihypertensive drugs.466,467,468 The mechanisms by which SGLT-2 inhibition lowers BP include volume depletion,469,470,471 negative sodium balance,472,473 weight loss,474 renal protection, etc.475,476 Moreover, SGLT-2 inhibition can also regulate BP by affecting vascular fuctions. Both clinical RCTS and basic research have shown that SGLT-2 inhibition improves endothelial function as well as the vascular stiffness (measured by PWV, augmentation index, and degree of vascular fibrosis) in DM.477,478,479,480 Potential vascular protective effects may be attributed to its alleviation of glucotoxicity by removing excessive glucose from the body, as SGLT-2 inhibitors can reduce AGE/RAGE signaling, oxidative stress levels, and the expression of inflammatory factors.480,481,482 In addition, it has been suggested in some studies that SGLT2 inhibitors may suppress sympathetic activity, owing to improved glycemic control and insulin resistance as well as the decreased leptin levels.483 However, this mechanism remains controversial since several studies suggested that SGLT-2 inhibitors did not affect HRV or plasma adrenergic markers.479,484
Central nervous system
Initially, it was thought that the central nervous system (CNS) played a limited role in regulating BP, mainly through the baroreflexes and chemoreflexes mechanisms.485,486 In fact, the CNS also orchestrates the sympathetic outflow and integrates peripheral inputs.487 There are numerous brain nuclei involved in regulating sympathetic tone and BP, and the connections and functional interactions among these nuclei are highly complex. The brainstem and hypothalamus regions form circuits that mediate steady-state and reflex-induced changes in sympathetic activity and BP regulation.488 Studies have revealed various mechanisms involving the interactions and functional connections among different cell types in the forebrain and brainstem. Recent findings suggest that Ang-II in the brain may have an impact on bone marrow-derived hematopoietic stem and progenitor cells, potentially exacerbating hypertensive vascular pathological changes.489,490,491 Obesity and high-fat diets are thought to promote a chronic state of low-grade inflammation in the CNS. This is characterized by increased activation of microglia and astrocytes, as well as increased expression of genes encoding pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β).492 Pro-inflammatory cytokines elevation in CNS also contributes to SNA activity upregulation and the occurrence of hypertension.493
Interventions
Clinical studies have validated that targeting some vascular function-related pathways (e.g., calcium pathway, RAAS pathway, etc.) for blood pressure control is safe and effective. However, more evidence is needed to determine whether other therapeutic targets (e.g., inflammation, oxidative stress) can be utilized, too. We list below some interventions that target these pathways, both those used in clinical work already and those with potential values (Table 1).
NO-(NOsGC)-cGMP signaling
Nitrates, such as sodium nitroprusside and nitroglycerin, are widely used in clinical settings to promote NO production and vasodilation via NO-(NOsGC)-cGMP signaling. However, nitrates commonly have short-term efficacy, and the poor tolerance limits their further use.494 Some drugs, reported in animal models, such as statins and Ang II receptor blockers (ARBs) may activate eNOS or increase eNOS expression to improve endothelial dysfunction.495,496,497 In addition to these clinically used drugs, other antihypertensive drugs targeting NO-(NOsGC)-cGMP signaling pathway are still under exploration. As tetrahydrobiopterin (BH4) is a co-factor in eNOS activities, its supplementation may directly activate and regulate the eNOS signaling, which has attracted extensive attention.498 NosGC activators and stimulators are currently in clinical use or undergoing clinical development, and may potentially be tested in hypertensive patients in the near future.494 Some eNOS transcription enhancers, such as trans-resveratrol, are reported for their therapeutic potential as well.499,500 Phosphodiesterase (PDE) is an important substance that degrades intracellular cGMP. PDEs are classified into 11 primary isoenzyme subtypes, which are distinguished by their substrate affinity, selectivity, and regulation mechanisms.501 A specific and differential function in contractile VSMCs makes PDE1 inhibition an attractive novel option.502,503 Of note, ITI-214, a specific PDE1 inhibitor, has been well-tolerated by humans and a phase 2 clinical trial for heart failure has been completed.504
In addition, it is reported that lifestyle interventions should be preliminary approaches for the improvement of NO bioavailability, including a healthy diet, exercise, weight reduction, and smoking cessation. In dietary interventions, supplementation of nitrate and nitrite can exert antihypertensive effects.505 Another example is that cocoa can increase NO production, with a beneficial effect on hypertension.506 Physical activity can improve downstream NO bioavailability, both in healthy subjects and in those who are at higher cardiovascular risk, to improve endothelial function.507,508 Weight reduction509 and smoking cessation510 are also showed to be helpful for improvement of NO bioavailability.
Calcium channel signaling
Calcium channel blockers (CCBs) are effective drugs to inhibit calcium influx. Based on their selectivity for L-type voltage-dependent transmembrane calcium channels in either the cardiac, vascular, or both tissues, CCBs can be divided into 3 groups: dihydropyridinic agents, phenilalchilaminic agents, and benzothiazepinic agents. Dihydropyridinic agents can block the voltage-dependent L-type calcium channels, which gives it vascular selectivity to preferentially block the calcium channels in VSMCs. Dihydropyridinic CCBs are now a first-line therapeutic option to treat hypertension.511,512,513 Whereas verapamil and diltiazem, the representative drugs of the latter two types, have cardiac selectivity.514 CCBs are known to be vasodilators of small resistance arteries. When administered acutely, they can reduce total peripheral resistance and mean BP, while also increasing cardiac output. However, after chronic administration, cardiac output returns to pretreatment levels, while mean arterial pressure and systemic vascular resistance remain low.
RAAS
Targeting at angiotensin-related receptors
Four groups of drugs are clinically established to inhibit the RAAS: ACE inhibitors (ACEI), ARBs (which block the AT1 receptor), renin inhibitors and mineralocorticoid receptor blockers. Up to now, there are more than 10 types of ACEI available around the world. The main mechanism of ACEI is to limit the formation of Ang II as well as to reduce the catabolism of other vasodilator oligopeptides (such as bradykinin) by ACE. ACEI mediates the activity of the ACE2–Ang (1–7)–MAS1 axis as well.515,516 Long-time application in clinical settings have confirmed the safety and tolerability profiles for these drugs. In addition, there exist about nine ARBs for clinical use. ARBs can lead to an increase in the levels of Ang II, which can serve as a substrate of ACE2. This can result in clinical benefits due to the stimulation of the ACE2/Ang (1–7)/Mas1 axis.515,517 LCZ696 (Sacubatril/Valsartan) combines a neprilysin inhibitor moiety with a valsartan moiety, and is the first agent of the angiotensin-receptor–neprilysin (ARN) inhibitor. It can decrease BP in animals models and healthy human subjects with low incidence of adverse effects, which has been confirmed by several trials.518,519,520,521 Notably, LCZ696 is the first drug to be approved for the treatment of heart failure (HF). Clinical trials have shown that LCZ696 is superior to enalapril (an ACEi agonists) in reducing hospitalizations in worsening HF patients and all-cause mortality in patients with left ventricular ejection fraction (LVEF) ≤40%.522
As for the ACE2/Ang (1–7)/Mas1 axis, Human recombinant ACE2,523 AVE 0991,524 and IRAP inhibitors525 are considered as promising drugs in hypertension treatment. Compound 21 is an AT2 agonist which can alleviate hypertension and target-organ damage.526 To be mentioned, there are currently vaccines under development that target the RAAS. For example, the CYT006-AngGb vaccine, which targets Ang II, has shown promising results in a phase II clinical trial by reducing BP in patients with mild to moderate hypertension without causing serious adverse events.527 The ATRQβ-001 vaccine, which targets the AT1R, has shown success in lowering BP in Ang II-induced hypertensive mice and SHR.528 However, more further studies on antihypertensive vaccines are warranted. However, vaccines may cause pain and require repeated injections,527 while oral medication is mature and painless.
Targeting at renin and MR
In theory, blocking the rate-limiting enzyme, renin, can be more effective in preventing Ang II production compared to other approaches that target different components of the RAAS. Currently, the only available direct renin inhibitor for the treatment of hypertension is Aliskiren, which is an orally active non-peptide drug with high selectivity.529,530,531 However, it did not achieve satisfying effects in clinical trail.242 And another drug, ACT‑077825, lacks evidence to confirm its effect. ACT 178882, the next generation of ACT‑077825, is still under exploration.532,533
The mineralocorticoid receptor antagonist mainly refers to spironolactone, eplerenone, and canrenone. As a potassium-sparing diuretic, the efficacy of spironolactone to treat hypertension has been confirmed by RCTs.534 Eplerenone has a similar antihypertensive efficacy as spironolactone, but with fewer adverse effects. However, the evidence is yet relatively insufficient.535 Canrenone is available in some countries, but there is no large RCT demonstrating its beneficial effect.536,537 Some other drugs, such as finerenone, a nonsteroidal mineralocorticoid receptor antagonist, has presented a promising effect in hypertension treatment.538,539 BR-4628,540 PF-3882845541, and SM-368229542 are nonsteroidal drugs still under exploration at present. Further studies are needed to confirm their safety and efficacy.543 As for drugs targeting at aldosterone synthase, FAD 286A, LCI699 and its second-generation drug are still under development.544 Besides, the expression of CYP11B1 and CYP11B2, which are responsible for the production of aldosterone and cortisol in adrenocortical cells, can be regulated post-transciptionally by dicer-dependent microRNAs (miRNAs), affecting the secretion of these hormones.545
Anti-vascular remodeling therapy
MMPs
Antihypertensive drugs, including ACEI, ARBs, and CCBs, can regulate the MMP activity and concentration.546,547,548,549 Administration combined with atorvastatin for 2 months induced a larger reduction in MMP-9 compared with administration alone in hypercholesterolemic subjects.550 To be noted, there are studies confirming an improvement in MMP reduction after weight loss and appropriate exercise.551,552,553
MMPs inhibitors can be classified into two categories, hydroxamate-based inhibitors and non-hydroxamate MMP inhibitors. Collagen-based peptidomimetic hydroxamates include marimastat, ilomastat, and batimastat. Batimastat is the first MMP inhibitor studied in clinical trials. It is a low-molecular-mass hydroxamate derivative with low water solubility.554 It exerts its inhibitory effect by directly binding to Zn2+ ions in the active site of several MMPs, including MMP1, MMP2, MMP7, and MMP9.555 Non-hydroxamate MMP inhibitors, including rebimastat, tanomastat, etc. Rebimastat is a broad-spectrum MMP inhibitor which contains a thiol zinc-binding group.556 Tanomastat contains a thioether zinc-binding group and a biphenyl deep-pocket-binding segment, and it has been confirmed in trials to be well-tolerated.557 However, no specific MMPs inhibitors have been used to treat vascular remodeling or hypertension. In treatment of cancer, despite the promising preclinical data supporting the use of MMP inhibitors as anticancer drugs, it failed to achieve the desired results in clinical settings.558 Similarly, more evidence is needed for the utilization of MMPs inhibitors in the treatment of hypertension.
AGEs
Levels of AGEs can be reduced by comsuming a diet low in AGEs, which involves reducing the intake of glucose, red meat, butter, cream, and other sweetened fatty foods but increasing the proportion of grains, vegetables, fruits, and milk in the diet.559 From the perspective of AGE formation, there are drugs such as aminoguanidine,560 vitamins,561 ACEI,562 metformin,563 acidic ingredients,128 and pomegranate that can suppress its production.564 Animal studies and clinical trials proved that, alagebrium and ALT-711 could degrade AGE.564,565,566,567 RAGE expression can be suppressed by numerous agents, such as statins, ACEI and ARBs.568 Besides, statins, ACEI, ARBs, antidiabetic drugs, and systemic administration of recombinant sRAGE have been reported to elevate the levels of both sRAGE and esRAGE.569
Calcification
An effective measure to prevent calcification in murine models is the administration of exogenous pyrophosphate.570,571,572 Moreover, it has been shown that inhibition of TNAP (tissue-nonspecific alkaline phosphatase)573,574,575 and exogenous administration of ENPP1576 can also prevent vascular calcification. Nevertheless, the effects of these treatments still need to be further discussed in humans. SNF472 is an intravenous formulation of myo-inositol hexaphosphate. It can inhibit the formation and growth of hydroxyapatite crystals through a novel pathway, which is the final common step in the pathophysiology of vascular calcification, selectively and directly.577,578 Ferroptosis has emerged as a potential therapeutic target for anti-calcification intervention. Metformin can exert anti-ferroptotic effects and attenuate hyperlipidemia-associated vascular calcification.579 Moreover, recent evidence suggests that autophagy may have a direct protect effect against vascular calcification.580
There are also some other potential drugs or treatment targets. For example, Valporic acid, an inducer of autophagy, has been demonstrated to inhibit VSMC calcification in vitro.581 Rapamycin has been shown to alleviate vascular calcification in DBA/2 mice with diabetes.582 XBP1u (unspliced X-box binding protein 1), an endogenous inhibitor, can promote β-catenin ubiquitination degradation and attenuate vascular calcification.583 Suppressing activation of NF-κB, zinc can potentially protect against phosphate-induced arterial calcification. Furthermore, there is evidence to suggest that higher intake of dietary zinc is related to a reduced risk of severe abdominal aortic calcification.584
Anti-oxidative stress therapy
Evidence is mounting that antioxidants may paly a crucial role in the management of hypertension. They have also been shown to improve endothelial function, attenuate vascular remodeling, and lower arterial stiffness in some models.585,586 Antioxidants compounds such as vitamins E (or α-tocopherol) and vitamins C (or ascorbic acid), polyphenols and some clinical hypertensive drugs may have antioxidative pleiotropic effects. Vitamins E is a promising drug with its potenial as an antioxidant. Several clinical trials have demonstrated its important role in prevention of hypertension.587,588 Vitamins C is a potent water-soluble antioxidant which may improve vasodilation response in hypertension by increasing eNOS activity and reducing ROS levels.589,590,591
Polyphenols have been shown to have antihypertensive effects and to improve endothelial function, which may be due to its ability to inhibit ROS generating enzymes and to enhance GSH.592,593 RAAS inhibitors such as ACEI and ARBs can effectively lower NADPH oxidase levels. In addition, treatment with ACEI and ARBs has been associated with an increase in superoxide dismutase activity.594,595 Antioxidant effect of dihydropyridine CCBs can contribute to the prevention and medication of endothelial dysfunction as well. Nifedipine and nicardipine have been demonstrated to prevent ROS-induced endothelial dysfunction directly and improve endogenous antioxidants in cultured cell lines.596 Benidipine has a protective effect on human endothelial cells via reducing oxidative damage induced by ox-LDL which triggers ROS generation.597 There are also some drugs that have shown antioxidant and antihypertensive effects in studies, such as Genistein,598 N-acetylcysteine(NAC),599 and Allopurinol.600,601 Nevertheless, their functions need to be further elucidated.
Anti-inflammation
RAAS-suppressing drugs have been shown to possess potent anti-inflammatory effects. It is noteworthy that, such anti-inflammatory effects may be unrelated to their BP-lowering effects and instead may be due to their ability to directly counteract the pro-inflammatory effects induced by Ang II.602 Statins may have anti-inflammatory effects and can lead to a small decrease in systolic BP in patients with hypercholesterolemea, by reducing pro-inflammatory cytokines levels.603 Some immunosuppressant drugs have potential as a treatment for hypertension, too. Mycophenolate mofetil has been found to reduce BP in SHR,604 and in Dahl salt-sensitive rats.605,606 Lifestyle also affects BP by alleviating inflammation. Mediterranean diet is closely related to anti-inflammatory effects and endothelial function.607 A regular and moderate aerobic physical exercise can also present anti-inflammatory effects.608 However, whether anti-inflammation can be used to treat hypertension clinically it is still doubtful. Nonsteroidal anti-inflammatory drugs can lead to an increase in BP by promoting sodium retention.609,610 Furthermore, Canakinumab, the anti-IL-1β monoclonal antibody, reduced inflammatory factors including IL-1β, IL-6, hs-CRP in patients with atherosclerotic disease, but it did not reduce the incidence of hypertension nor down-regulated BP at 3, 6, or 12 months.364,611,612,613,614 However, study suggested that plasma IL-1β concentration elevated in hypertensive patients, no significant change was observed in its receptors levels. And it indicated that, without IL-1β-related indicators of cellular immune activation, the reduction in plasma IL-1β level alone might be insufficient to reflect the levels of other inmmune activatioin.615 In addition, inflammation in hypertensive patients is considered to be low-level and chronic inflammatory stimuli. The longest follow-up duration in the CANTOS study was 12 months. It is rather unclear when vascular remodeling occurs in patients with hypertension. The 1-year period seems to be relatively short, so the follow-up time of this study may be responsible for the contradiction. These may be the reasons why anti-IL-1β failed to reduce BP. In all, specific anti-inflammatory drugs did not achieve significant antihypertensive effects yet in clinical studies. Whether hypertension can be treated with anti-inflammatory drugs remains controversial.616
Sympathetic nervous system
Clinically, it is one of the important means to control BP by restricting the effect of released NE, with drugs competitively binding to adrenergic receptors. Selective α1-adrenoceptor antagonists mainly include prazosin, terazosin, and doxazosin. α2-adrenergic agonists, such as clonidine, can stimulate α2-adrenoceptor in the brainstem and subsequently reduce sympathetic outflow from the CNS, leading to a decrease in BP.617 Nonselective α-adrenergic antagonists, phentolamine and phenoxybenzamine, were primarily administered parenterally for the management of hypertensive crisis.618 The reduction in plasma NE is directly associated with the hypotensive effect. Clonidine can lower BP via cardiac output and total peripheral resistance. Moxonidine and rilmenidine, which are imidazoline-1 receptor agonists, have been shown to be equally effective as other classes of antihypertensive agents, with their mechanism of action involving a reduction in sympathetic outflow from the CNS. In addition, their side effects like sedation, dry mouth, and rebound hypertension, were not commonly observed in studies.619,620,621 β-adrenergic receptor antagonists, like propranolol, can lower BP by decreasing myocardial contractility, heart rate, and cardiac output. Moreover, β-adrenergic receptor antagonists can improve the prognosis of chronic HF, coronary heart disease and other cardiovascular diseases. Therefore, β-adrenergic receptor antagonists are often used as first-line drugs for hypertension complicated with chronic HF and coronary heart disease.622
Intervening sympathetic activity by invasive means can also achieve the purpose of controlling BP. Surgical interventions such as thoracic sympathectomy were already abandoned due to their high mortality rate and relatively high incidence of complications.623 Recent treatments for hypertension have focused on device-based approaches, such as renal denervation and baroreflex activation therapy, to modify the SNS. Although renal denervation seemed fail to cure resistant hypertension, based on the sobering results from SYMPLICITY-HTN 3,624 it remains a promising therapeutic method with huge potential.625 Baroreflex activation therapy is also considered as an alternative choice for resistant hypertension.626
Lifestyle interventions can reduce SNA and BP in hypertensive patients as well. Neurogenic components contribute much to obesity-related hypertension.627 Exercise training and/or low caloric intake can lead to weight loss and down-regulate SNA.628,629 The effects of exercise involve up-regulating central antioxidants concentrations, reducing pro-oxidant levels, and increasing central nitric oxide synthase activity.630 Chronic psychosocial stress is also associated with highly activated SNS and abnormal BP.631 Stress reduction measures are supposed to be effective in reducing SNA and lowering BP in hypertensives. Besides, device-guided, home-based slow and deep breathing training has been shown to effectively reduce BP in patients with hypertension as well.632,633,634
Biomarkers
Biomarkers can be used to predict disease development, identify disease, define disease severity, and assess prognosis and treatment effect.635 The application of biomarkers in clinical settings contributes greatly to disease monitoring and prognosis prediction. For example, tradional markers like CRP can be used to predict the incidence of hypertension,636 sortlin is able to predict the onset of vascular dysfunction and hypertension,637 APCs and Klotho can be used to identify salt-sensitive hypertension,463,638 platelets and circulating CD34-positive cells can be important indicators of vicious cyclical activity between hypertension and endothelial dysfunction,639 and high-sensitivity cardiac troponin T or NT-proBNP can better guide patients in BP control after being incorporated into risk assessment algorithms.640 As for urinary microalbumin, it is not only applied to BP monitoring, but also to the hypertension-related risk of cardiovascular diseases and mortality assessment.641
Except predicting the initiation and progression of diseases, it is also promising to determine potential treatment targets in the biomarkers-related signaling pathways. Sortlin is a member of the vacuolar protein sorting 10 (VPS10P) family of receptors.642 It was recently reported to induce endothelial dysfunction of mesenteric arteries via activating NOX2 (NADPH oxidase 2) isoform, which could be prevented by ASMase (acid sphingomyelinase) or sphingosine kinase 1 knockdow.637 Addtionally, patients with hypertension, especially those with uncontrolled BP, exhibited an increase in plasma ASMase activity and plasma sortilin concentration.637 Similarly, some other newly discovered circulating proteins (such as Sphingosine-1-Phosphate,643 Klotho644) and some small molecules (such as non-coding RNAs,645,646 microvesicles647) can function as predicting biomarkers, as well as potential treatment targets.
Conclusion and perspectives
An updated Mosaic Theory has been proposed to explain the pathogenesis of hypertension. According to this theory, hypertension is believed to be a response to various combinations of traits and stressors.648 The new Mosaic Theory emphasizes other factors such as oxidative stress, sympathetic activation, inflammation, genetics, microbiome, renal mechanisms, and salt intake, in addition to vascular fuction.648 Although the interaction of these factors results in a complex pathogenesis of hypertension, resembling a network, vascular function is undoubtedly the direct cause of BP elevation. BP control in clinical practice is mainly based on targeting vascular function, such as RAAS inhibitors, CCBs, nitrates, etc. On the contrary, the therapeutic potentials of other factors, except renal function, have not been well elucidated in clinical use or in large clinical studies.
In vascular function, the interaction network formed by molecular pathways is also complex. We divide the molecular pathways that act on blood vessels into two categories (Fig. 9a) One indirectly affects vascular sympathetic activity, such as RAAS, immunity, and redox signaling. The other directly affects vascular functions, such as calcium signaling, NO-(NOsGC)-cGMP, vascular remodeling, etc. The former type of interaction has a complex network and mainly induces vasoconstriction by regulating the direct pathway, for example, sympathetic disorders mainly trigger vasoconstriction by activating calcium channels, RAAS activates calcium channels and triggers vascular remodeling.
Molecular pathways and therapeutic strategies in vascular function and hypertension. a Molecular pathways that act on blood vessels. b Therapeutic strategies targeting blood vessels. (1), (2), and (3) in the signaling pathways section represent the specific intervention involving each pathway according to current evidence
Long-term control of BP is currently the main strategy for treating hypertension and reducing target organ damage in hypertension. In therapeutic strategies targeting blood vessels, there are mainly three ways (Fig. 9b): lifestyle interventions, pharmacological therapy, and invasive interventions. Lifestyle interventions, especially diet and exercise, can affect vascular function and BP in almost all pathways. Llifestyle modification is recommended as the first line of antihypertensive treatment.649,650 Drug therapy is the basis of BP control in clinical work. RAAS inhibitors, and CCBs are first-line antihypertensive drugs; β-blockers and α-adrenergic antagonists play important roles in the treatment of sympathetic-induced hypertension; Nitrates are commonly used in hypertensive emergencies and sub-emergency. Invasive intervention brings new perspective on the treatment of resistant hypertension. As for the utilization of molecular pathways concerning vascular functions in guiding the treatment of hypertension, the efficacy of calcium signaling, NO-(NOsGC)-cGMP, RAAS, and sympathetic activity have been clearly demonstrated in clinical practice. Nevertheless, drugs targeting vascular remodeling, redox signaling, and immunity are still under studying.
For the next research direction, translating basic study findings on vascular remodeling, redox signaling, and immunity to clinical applications is a priority. Especially vascular remodeling. From the current study, vascular remodeling is difficult to reverse, and it may be closely related to refractory hypertension. Since the state and function of cells (especially secretory function) directly affect the ECM, the elucidation of cell identity and the rewriting of cell fate may be crucial to make a breakthrough in vascular remodeling. Therefore, completing vascular cell maps in different pathological conditions should be one important direction in further studies. On the other hand, although current antihypertensive drugs are effective, many people with hypertension still struggle to achieve target BP levels,651 and the optimal BP goal also remains controversial.652,653,654 The importance of reducing cardiovascular events should be emphasized in BP control.655 Therefore, achieving BP targets while simultaneously preventing cardiovascular events is the main goal in hypertension treatment. As discussed above, searching for suitable biomarkers and forming new diagnosis and treatment protocols is promising to save the problem (Fig. 10). Urinary microalbumin is a successful case of biomarkers applied to monitoring BP control as well as predicting cardiovascular events,641 it is now a classic indicator widely accepted by clinical guidelines for hypertension. Utilization of high-throughput sequencing and machine learning are cutting-edge methods to further mine the role of biomarkers.656,657
It is worth mentioning that hypertension has been defined as a disease for over a century. However, modern medicine tends to view hypertension as a risk factor for vascular disease rather than a disease in itself. The essence of controlling BP is to reduce cardiovascular events, similar to controlling blood lipids and glucose. We need to be aware that the molecular mechanism of hypertension is essentially a molecular mechanism of vascular dysfunction and/or vascular volume. Understanding this is particularly important for in-depth research on the signaling pathways of hypertension.
In conclusion, our review demonstrated the signaling pathways involved in the vascular function and hypertension, and summarized therapeutic methods of hypertension which target at vascular function. In addition, we raised unresolved questions in vascular function and hypertension, and presented our own perspectives. It is expected to enlighten other researchers on the future research directions.
References
Mills, K. T., Stefanescu, A. & He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 16, 223–237 (2020).
Zhou, B. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 398, 957–980 (2021).
Sudharsanan, N. et al. Variation in the proportion of adults in need of blood pressure-lowering medications by hypertension care guideline in low- and middle-income countries: a cross-sectional study of 1 037 215 individuals from 50 nationally representative surveys. Circulation 143, 991–1001 (2021).
Wenceslau, C. F. et al. Guidelines for the measurement of vascular function and structure in isolated arteries and veins. Am. J. Physiol. Heart Circ. Physiol. 321, H77–h111 (2021).
Augustin, H. G. & Koh, G. Y. Organotypic vasculature: from descriptive heterogeneity to functional pathophysiology. Science 357, eaal2379 (2017).
Nagy, N. et al. Inhibition of hyaluronan synthesis accelerates murine atherosclerosis: novel insights into the role of hyaluronan synthesis. Circulation 122, 2313–2322 (2010).
Dogné, S., Flamion, B. & Caron, N. Endothelial glycocalyx as a shield against diabetic vascular complications: involvement of hyaluronan and hyaluronidases. Arterioscler Thromb. Vasc. Biol. 38, 1427–1439 (2018).
Krüger-Genge, A., Blocki, A., Franke, R. P. & Jung, F. Vascular endothelial cell biology: an update. Int. J. Mol. Sci. 20, 4411 (2019).
Rafii, S., Butler, J. M. & Ding, B. S. Angiocrine functions of organ-specific endothelial cells. Nature 529, 316–325 (2016).
Ambrosino, P. et al. Mechanisms and clinical implications of endothelial dysfunction in arterial hypertension. J. Cardiovasc. Dev. Dis. 9, 136 (2022).
Davis, G. E. & Senger, D. R. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ. Res. 97, 1093–1107 (2005).
Durgin, B. G. & Straub, A. C. Redox control of vascular smooth muscle cell function and plasticity. Lab. Investig. 98, 1254–1262 (2018).
Dinardo, C. L. et al. Variation of mechanical properties and quantitative proteomics of VSMC along the arterial tree. Am. J. Physiol. Heart Circ. Physiol. 306, H505–16 (2014).
Lacolley, P., Regnault, V., Segers, P. & Laurent, S. Vascular smooth muscle cells and arterial stiffening: relevance in development, aging, and disease. Physiol. Rev. 97, 1555–1617 (2017).
Pauly, R. R. et al. Experimental models that mimic the differentiation and dedifferentiation of vascular cells. Circulation 86, Iii68–73 (1992).
Majesky, M. W. Vascular development. Arterioscler Thromb. Vasc. Biol. 38, e17–e24 (2018).
Ribeiro-Silva, J. C., Nolasco, P., Krieger, J. E. & Miyakawa, A. A. Dynamic crosstalk between vascular smooth muscle cells and the aged extracellular matrix. Int. J. Mol. Sci. 22, 10175 (2021).
Majesky, M. W. Adventitia and perivascular cells. Arterioscler Thromb. Vasc. Biol. 35, e31–e35 (2015).
Stenmark, K. R. et al. The adventitia: essential regulator of vascular wall structure and function. Annu. Rev. Physiol. 75, 23–47 (2013).
Rizzoni, D. et al. Prognostic significance of small-artery structure in hypertension. Circulation 108, 2230–2235 (2003).
De Ciuceis, C. et al. Structural alterations of subcutaneous small-resistance arteries may predict major cardiovascular events in patients with hypertension. Am. J. Hypertens. 20, 846–852 (2007).
Rizzoni, D. et al. Morning rise of blood pressure and subcutaneous small resistance artery structure. J. Hypertens. 25, 1698–1703 (2007).
Mathiassen, O. N. et al. Small artery structure is an independent predictor of cardiovascular events in essential hypertension. J. Hypertens. 25, 1021–1026 (2007).
Buus, N. H. et al. Small artery structure during antihypertensive therapy is an independent predictor of cardiovascular events in essential hypertension. J. Hypertens. 31, 791–797 (2013).
Touyz, R. M. et al. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 114, 529–539 (2018).
Briones, A. M., Arribas, S. M. & Salaices, M. Role of extracellular matrix in vascular remodeling of hypertension. Curr. Opin. Nephrol. Hypertens. 19, 187–194 (2010).
Zanoli, L. et al. Vascular consequences of inflammation: a position statement from the ESH Working Group on Vascular Structure and Function and the ARTERY Society. J. Hypertens. 38, 1682–1698 (2020).
Hai, C. M. & Murphy, R. A. Ca2+, crossbridge phosphorylation, and contraction. Annu. Rev. Physiol. 51, 285–298 (1989).
Kuo, I. Y. & Ehrlich, B. E. Signaling in muscle contraction. Cold Spring Harb. Perspect. Biol. 7, a006023 (2015).
Ottolini, M. & Sonkusare, S. K. The calcium signaling mechanisms in arterial smooth muscle and endothelial cells. Compr. Physiol. 11, 1831–1869 (2021).
Hill-Eubanks, D. C., Werner, M. E., Heppner, T. J. & Nelson, M. T. Calcium signaling in smooth muscle. Cold Spring Harb. Perspect. Biol. 3, a004549 (2011).
Cribbs, L. L. T.-type Ca2+ channels in vascular smooth muscle: multiple functions. Cell Calcium 40, 221–230 (2006).
Weiss, S., Oz, S., Benmocha, A. & Dascal, N. Regulation of cardiac L-type Ca2+ channel CaV1.2 via the β-adrenergic-cAMP-protein kinase A pathway: old dogmas, advances, and new uncertainties. Circ. Res. 113, 617–631 (2013).
Lee, D. L., Webb, R. C. & Jin, L. Hypertension and RhoA/Rho-kinase signaling in the vasculature. Hypertension 44, 796–799 (2004).
Uehata, M. et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389, 990–994 (1997).
Lincoln, T. M. Myosin phosphatase regulatory pathways. Circ. Res. 100, 10–12 (2007).
Soloviev, A. I. & Bershtein, S. A. The contractile apparatus in vascular smooth muscle cells of spontaneously hypertensive rats possess increased calcium sensitivity: the possible role of protein kinase C. J. Hypertens. 10, 131–136 (1992).
Etienne-Manneville, S. & Hall, A. Rho GTPases in cell biology. Nature 420, 629–635 (2002).
Cherfils, J. & Zeghouf, M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol. Rev. 93, 269–309 (2013).
Ishizaki, T. et al. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 15, 1885–1893 (1996).
Matsui, T. et al. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 15, 2208–2216 (1996).
Shimokawa, H., Sunamura, S. & Satoh, K. RhoA/Rho-kinase in the cardiovascular system. Circ. Res. 118, 352–366 (2016).
Kimura, K. et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273, 245–248 (1996).
Kureishi, Y. et al. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J. Biol. Chem. 272, 12257–12260 (1997).
Deng, J. T., Bhaidani, S., Sutherland, C., MacDonald, J. A. & Walsh, M. P. Rho-associated kinase and zipper-interacting protein kinase, but not myosin light chain kinase, are involved in the regulation of myosin phosphorylation in serum-stimulated human arterial smooth muscle cells. PLoS ONE 14, e0226406 (2019).
Noma, K. et al. Smoking, endothelial function, and Rho-kinase in humans. Arterioscler Thromb. Vasc. Biol. 25, 2630–2635 (2005).
Noma, K. et al. Smoking activates rho-kinase in smooth muscle cells of forearm vasculature in humans. Hypertension 41, 1102–1105 (2003).
Lee, M. W. & Severson, D. L. Signal transduction in vascular smooth muscle: diacylglycerol second messengers and PKC action. Am. J. Physiol. 267, C659–78 (1994).
Steinberg, S. F. Structural basis of protein kinase C isoform function. Physiol. Rev. 88, 1341–1378 (2008).
Pucci, M. L. et al. Vascular responsiveness to nitric oxide synthesis inhibition in hypertensive rats. Hypertension 23, 744–751 (1994).
Bruschi, G., Bruschi, M. E., Capelli, P., Regolisti, G. & Borghetti, A. Increased sensitivity to protein kinase C activation in aortas of spontaneously hypertensive rats. J. Hypertens. Suppl. 6, S248–51 (1988).
Dimopoulos, G. J., Semba, S., Kitazawa, K., Eto, M. & Kitazawa, T. Ca2+-dependent rapid Ca2+ sensitization of contraction in arterial smooth muscle. Circ. Res. 100, 121–129 (2007).
Panza, J. A., Casino, P. R., Kilcoyne, C. M. & Quyyumi, A. A. Role of endothelium-derived nitric oxide in the abnormal endothelium-dependent vascular relaxation of patients with essential hypertension. Circulation 87, 1468–1474 (1993).
Panza, J. A., Quyyumi, A. A., Brush, J. E. Jr. & Epstein, S. E. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N. Engl. J. Med. 323, 22–27 (1990).
Münzel, T. et al. Physiology and pathophysiology of vascular signaling controlled by guanosine 3’,5’-cyclic monophosphate-dependent protein kinase [corrected]. Circulation 108, 2172–2183 (2003).
Li, Q., Youn, J. Y. & Cai, H. Mechanisms and consequences of endothelial nitric oxide synthase dysfunction in hypertension. J. Hypertens. 33, 1128–1136 (2015).
Gauthier, C. et al. The negative inotropic effect of beta3-adrenoceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. J. Clin. Invest. 102, 1377–1384 (1998).
Szöcs, K. et al. Increased superoxide production in nitrate tolerance is associated with NAD(P)H oxidase and aldehyde dehydrogenase 2 downregulation. J. Mol. Cell Cardiol. 42, 1111–1118 (2007).
Buckley, B. J., Mirza, Z. & Whorton, A. R. Regulation of Ca(2+)-dependent nitric oxide synthase in bovine aortic endothelial cells. Am. J. Physiol. 269, C757–C765 (1995).
McDuffie, J. E., Coaxum, S. D. & Maleque, M. A. 5-Hydroxytryptamine evokes endothelial nitric oxide synthase activation in bovine aortic endothelial cell cultures. Proc. Soc. Exp. Biol. Med. 221, 386–390 (1999).
Awolesi, M. A., Widmann, M. D., Sessa, W. C. & Sumpio, B. E. Cyclic strain increases endothelial nitric oxide synthase activity. Surgery 116, 439–444 (1994). discussion 444-5.
Mollnau, H. et al. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ. Res. 90, e58–e65 (2002).
Lundberg, J. O., Weitzberg, E. & Gladwin, M. T. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Disco. 7, 156–167 (2008).
Bescós, R., Sureda, A., Tur, J. A. & Pons, A. The effect of nitric-oxide-related supplements on human performance. Sports Med. 42, 99–117 (2012).
Huang, P. L. et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 377, 239–242 (1995).
Leo, F. et al. Red Blood Cell and Endothelial eNOS Independently Regulate Circulating Nitric Oxide Metabolites and Blood Pressure. Circulation 144, 870–889 (2021).
Straub, A. C. et al. Endothelial cell expression of haemoglobin α regulates nitric oxide signalling. Nature 491, 473–477 (2012).
Förstermann, U. & Sessa, W. C. Nitric oxide synthases: regulation and function. Eur. Heart J. 33, 829–837 (2012). 837a-837d.
Rochette, L. et al. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: possible therapeutic targets? Pharm. Ther. 140, 239–257 (2013).
Feil, R. & Kemp-Harper, B. cGMP signalling: from bench to bedside. EMBO Rep. 7, 149–153 (2006).
Surks, H. K. et al. Regulation of Myosin Phosphatase by a Specific Interaction with cGMP- Dependent Protein Kinase Iα. Science 286, 1583–1587 (1999).
Weber, S. et al. Rescue of cGMP kinase I knockout mice by smooth muscle–specific expression of either isozyme. Circ. Res. 101, 1096–1103 (2007).
Feil, R. et al. Functional reconstitution of vascular smooth muscle cells with cGMP-dependent protein kinase I isoforms. Circ. Res. 90, 1080–1086 (2002).
Surks, H. K. cGMP-dependent protein kinase I and smooth muscle relaxation. Circ. Res. 101, 1078–1080 (2007).
Begum, N., Sandu, O. A., Ito, M., Lohmann, S. M. & Smolenski, A. Active Rho kinase (ROK-alpha) associates with insulin receptor substrate-1 and inhibits insulin signaling in vascular smooth muscle cells. J. Biol. Chem. 277, 6214–6222 (2002).
Intengan, H. D., Deng, L. Y., Li, J. S. & Schiffrin, E. L. Mechanics and composition of human subcutaneous resistance arteries in essential hypertension. Hypertension 33, 569–574 (1999).
Arribas, S. M., Hinek, A. & González, M. C. Elastic fibres and vascular structure in hypertension. Pharm. Ther. 111, 771–791 (2006).
Chirinos, J. A., Segers, P., Hughes, T. & Townsend, R. Large-artery stiffness in health and disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 74, 1237–1263 (2019).
Harvey, A., Montezano, A. C. & Touyz, R. M. Vascular biology of ageing—Implications in hypertension. J. Mol. Cell Cardiol. 83, 112–121 (2015).
Lakatta, E. G. The reality of aging viewed from the arterial wall. Artery Res. 7, 73–80 (2013).
Yasmin et al. Matrix Metalloproteinase-9 (MMP-9), MMP-2, and Serum Elastase Activity Are Associated With Systolic Hypertension and Arterial Stiffness. Arterioscler Thromb. Vasc. Biol. 25, 372–378 (2005).
Basalyga, D. M. et al. Elastin degradation and calcification in an abdominal aorta injury model: role of matrix metalloproteinases. Circulation 110, 3480–3487 (2004).
Basalyga, D. M. et al. Elastin degradation and calcification in an abdominal aorta injury model. Circulation 110, 3480–3487 (2004).
Struewing, I. T., Durham, S. N., Barnett, C. D. & Mao, C. D. Enhanced endothelial cell senescence by lithium-induced matrix metalloproteinase-1 expression. J. Biol. Chem. 284, 17595–17606 (2009).
Agrinier, N. et al. Prognostic value of serum PIIINP, MMP1 and TIMP1 levels in hypertensive patients: a community-based prospective cohort study. Fundam. Clin. Pharmacol. 27, 572–580 (2013).
Morillas, P. et al. Circulating biomarkers of collagen metabolism in arterial hypertension: relevance of target organ damage. J. Hypertens. 31, 1611–1617 (2013).
Belo, V. A., Parente, J. M., Tanus-Santos, J. E. & Castro, M. M. Matrix metalloproteinase (MMP)-2 decreases calponin-1 levels and contributes to arterial remodeling in early hypertension. Biochem. Pharm. 118, 50–58 (2016).
Wang, M. et al. Angiotensin II activates matrix metalloproteinase type II and mimics age-associated carotid arterial remodeling in young rats. Am. J. Pathol. 167, 1429–1442 (2005).
Ceron, C. S. et al. Time course involvement of matrix metalloproteinases in the vascular alterations of renovascular hypertension. Matrix Biol. 31, 261–270 (2012).
Djurić, T., Živković, M., Stanković, A., Mečanin, S. & Alavantić, D. Endothelial NOS G894 T and MMP-3 5A/6A gene polymorphisms and hypertension in Serbian population. J. Clin. Lab Anal. 19, 241–246 (2005).
Medley, T. L., Kingwell, B. A., Gatzka, C. D., Pillay, P. & Cole, T. J. Matrix metalloproteinase-3 genotype contributes to age-related aortic stiffening through modulation of gene and protein expression. Circ. Res. 92, 1254–1261 (2003).
Onal, I. K. et al. Serum levels of MMP-9 and TIMP-1 in primary hypertension and effect of antihypertensive treatment. Eur. J. Intern. Med. 20, 369–372 (2009).
Lacerda, L., Faria, A. P., Fontana, V., Moreno, H. & Sandrim, V. Role of MMP-2 and MMP-9 in resistance to drug therapy in patients with resistant hypertension. Arq. Bras. Cardiol. 105, 168–175 (2015).
Cui, N., Hu, M. & Khalil, R. A. Biochemical and biological attributes of matrix metalloproteinases. Prog. Mol. Biol. Transl. Sci. 147, 1–73 (2017).
Nagase, H., Visse, R. & Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 69, 562–573 (2006).
Cerofolini, L., Fragai, M. & Luchinat, C. Mechanism and Inhibition of Matrix Metalloproteinases. Curr. Med Chem. 26, 2609–2633 (2019).
Laronha, H. & Caldeira, J. Structure and function of human matrix metalloproteinases. Cells 9, 1076 (2020).
Berg, G., Barchuk, M. & Miksztowicz, V. Behavior of metalloproteinases in adipose tissue, liver and arterial wall: an update of extracellular matrix remodeling. Cells 8, 158 (2019).
Jiang, L. et al. Calpain-1 regulation of matrix metalloproteinase 2 activity in vascular smooth muscle cells facilitates age-associated aortic wall calcification and fibrosis. Hypertension 60, 1192–1199 (2012).
Sasaki, T. et al. Matrix metalloproteinase-2 deficiency impairs aortic atherosclerotic calcification in ApoE-deficient mice. Atherosclerosis 227, 43–50 (2013).
Harvey, A., Montezano, A. C., Lopes, R. A., Rios, F. & Touyz, R. M. Vascular fibrosis in aging and hypertension: molecular mechanisms and clinical implications. Can. J. Cardiol. 32, 659–668 (2016).
Wang, M., Kim, S. H., Monticone, R. E. & Lakatta, E. G. Matrix metalloproteinases promote arterial remodeling in aging, hypertension, and atherosclerosis. Hypertension 65, 698–703 (2015).
Abdalvand, A., Morton, J. S., Bourque, S. L., Quon, A. L. & Davidge, S. T. Matrix metalloproteinase enhances big-endothelin-1 constriction in mesenteric vessels of pregnant rats with reduced uterine blood flow. Hypertension 61, 488–493 (2013).
Fernandez-Patron, C. et al. Vascular matrix metalloproteinase-2-dependent cleavage of calcitonin gene-related peptide promotes vasoconstriction. Circ. Res. 87, 670–676 (2000).
Nagareddy, P. R. et al. Inhibition of matrix metalloproteinase-2 improves endothelial function and prevents hypertension in insulin-resistant rats. Br. J. Pharm. 165, 705–715 (2012).
Rodrigues, S. F., Tran, E. D., Fortes, Z. B. & Schmid-Schönbein, G. W. Matrix metalloproteinases cleave the beta2-adrenergic receptor in spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 299, H25–35 (2010).
Kalogeris, T. J. & Korthuis, R. J. Vascular receptors as new substrates for matrix metalloproteinases in hypertension and other inflammatory states. Am. J. Physiol. Heart Circ. Physiol. 299, H13–5 (2010).
Li, K., Tay, F. R. & Yiu, C. K. Y. The past, present and future perspectives of matrix metalloproteinase inhibitors. Pharm. Ther. 207, 107465 (2020).
Cui, Y. et al. Platelet-derived growth factor-BB induces matrix metalloproteinase-2 expression and rat vascular smooth muscle cell migration via ROCK and ERK/p38 MAPK pathways. Mol. Cell Biochem. 393, 255–263 (2014).
Wang, Y. et al. Inhibitory effects of cycloastragenol on abdominal aortic aneurysm and its related mechanisms. Br. J. Pharm. 176, 282–296 (2019).
Sun, H. J. et al. Salusin-β promotes vascular smooth muscle cell migration and intimal hyperplasia after vascular injury via ROS/NFκB/MMP-9 pathway. Antioxid. Redox Signal 24, 1045–1057 (2016).
Stone, J. D. et al. AMP-activated protein kinase inhibits vascular smooth muscle cell proliferation and migration and vascular remodeling following injury. Am. J. Physiol. Heart Circ. Physiol. 304, H369–81 (2013).
Endo, H., Owada, S., Inagaki, Y., Shida, Y. & Tatemichi, M. Glucose starvation induces LKB1-AMPK-mediated MMP-9 expression in cancer cells. Sci. Rep. 8, 10122 (2018).
Lim, W. W. et al. Inhibition of IL11 signaling reduces aortic pathology in murine marfan syndrome. Circ. Res. 130, 728–740 (2022).
Wang, Y. et al. Involvement of macrophage-derived exosomes in abdominal aortic aneurysms development. Atherosclerosis 289, 64–72 (2019).
Chang, S. et al. Histone deacetylase 7 maintains vascular integrity by repressing matrix metalloproteinase 10. Cell 126, 321–334 (2006).
Bierhaus, A., Hofmann, M. A., Ziegler, R. & Nawroth, P. P. AGEs and their interaction with AGE-receptors in vascular disease and diabetes mellitus. I. The AGE concept. Cardiovasc. Res. 37, 586–600 (1998).
Chaudhuri, J. et al. The role of advanced glycation end products in aging and metabolic diseases: bridging association and causality. Cell Metab. 28, 337–352 (2018).
Vlassara, H. & Striker, G. E. Advanced glycation endproducts in diabetes and diabetic complications. Endocrinol. Metab. Clin. North Am. 42, 697–719 (2013).
McNair, E., Qureshi, M., Prasad, K. & Pearce, C. Atherosclerosis and the hypercholesterolemic AGE-RAGE axis. Int. J. Angiol. 25, 110–116 (2016).
Prasad, K., Dhar, I. & Caspar-Bell, G. Role of advanced glycation end products and its receptors in the pathogenesis of cigarette smoke-induced cardiovascular disease. Int. J. Angiol. 24, 75–80 (2015).
Kiefer, A. S. et al. Methylglyoxal concentrations differ in standard and washed neonatal packed red blood cells. Pediatr. Res. 75, 409–414 (2014).
Sousa Silva, M., Gomes, R. A., Ferreira, A. E., Ponces Freire, A. & Cordeiro, C. The glyoxalase pathway: the first hundred years… and beyond. Biochem. J. 453, 1–15 (2013).
Koschinsky, T. et al. Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc. Natl Acad. Sci. USA 94, 6474–6479 (1997).
Uribarri, J. et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 110, 911–16.e12 (2010).
Uribarri, J. et al. Diet-derived advanced glycation end products are major contributors to the body’s AGE pool and induce inflammation in healthy subjects. Ann. N. Y Acad. Sci. 1043, 461–466 (2005).
Lin, R. Y. et al. Dietary glycotoxins promote diabetic atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 168, 213–220 (2003).
Ramful, D. et al. Citrus fruit extracts reduce advanced glycation end products (AGEs)- and H2O2-induced oxidative stress in human adipocytes. J. Agric Food Chem. 58, 11119–11129 (2010).
Liu, W. et al. Pomegranate phenolics inhibit formation of advanced glycation endproducts by scavenging reactive carbonyl species. Food Funct. 5, 2996–3004 (2014).
Schmidt, A. M. et al. Receptor for advanced glycation end products (AGEs) has a central role in vessel wall interactions and gene activation in response to circulating AGE proteins. Proc. Natl Acad. Sci. USA 91, 8807–8811 (1994).
Goldin, A., Beckman, J. A., Schmidt, A. M. & Creager, M. A. Advanced Glycation End Products. Circulation 114, 597–605 (2006).
Tam, X. H. et al. Enhanced expression of receptor for advanced glycation end-products is associated with low circulating soluble isoforms of the receptor in Type 2 diabetes. Clin. Sci. 120, 81–89 (2011).
McNulty, M., Mahmud, A. & Feely, J. Advanced glycation end-products and arterial stiffness in hypertension. Am. J. Hypertens. 20, 242–247 (2007).
Gelžinský, J. et al. Serum biomarkers, skin autofluorescence and other methods. Which parameter better illustrates the relationship between advanced glycation end products and arterial stiffness in the general population? Hypertens. Res. 44, 518–527 (2021).
Birukov, A., Cuadrat, R., Polemiti, E., Eichelmann, F. & Schulze, M. B. Advanced glycation end-products, measured as skin autofluorescence, associate with vascular stiffness in diabetic, pre-diabetic and normoglycemic individuals: a cross-sectional study. Cardiovasc. Diabetol. 20, 110 (2021).
Dimitriadis, K. et al. Soluble receptor for advanced glycation end-product levels are related to albuminuria and arterial stiffness in essential hypertension. Nutr. Metab. Cardiovasc. Dis. 23, 382–388 (2013).
Geroldi, D. et al. Decreased plasma levels of soluble receptor for advanced glycation end-products in patients with essential hypertension. J. Hypertens. 23, 1725–1729 (2005).
Colhoun, H. M. et al. Total soluble and endogenous secretory receptor for advanced glycation end products as predictive biomarkers of coronary heart disease risk in patients with type 2 diabetes: an analysis from the CARDS trial. Diabetes 60, 2379–2385 (2011).
Prasad, K. & Mishra, M. Do advanced glycation end products and its receptor play a role in pathophysiology of hypertension? Int. J. Angiol. 26, 1–11 (2017).
Schmidt, A. M. et al. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J. Clin. Invest. 96, 1395–1403 (1995).
Tanaka, S., Avigad, G., Brodsky, B. & Eikenberry, E. F. Glycation induces expansion of the molecular packing of collagen. J. Mol. Biol. 203, 495–505 (1988).
Striker, L. J. & Striker, G. E. Administration of AGEs in vivo induces extracellular matrix gene expression. Nephrol. Dial. Transpl. 11, 62–65 (1996). Suppl 5.
Reiser, K., McCormick, R. J. & Rucker, R. B. Enzymatic and nonenzymatic cross-linking of collagen and elastin. FASEB J. 6, 2439–2449 (1992).
Ramasamy, R., Yan, S. F. & Schmidt, A. M. Receptor for AGE (RAGE): signaling mechanisms in the pathogenesis of diabetes and its complications. Ann. N. Y Acad. Sci. 1243, 88–102 (2011).
Yan, S. F., Ramasamy, R. & Schmidt, A. M. The RAGE axis: a fundamental mechanism signaling danger to the vulnerable vasculature. Circ. Res. 106, 842–853 (2010).
Schmidt, A. M., Yan, S. D., Wautier, J. L. & Stern, D. Activation of receptor for advanced glycation end products: a mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ. Res. 84, 489–497 (1999).
Schmidt, A. M., Yan, S. D., Yan, S. F. & Stern, D. M. The biology of the receptor for advanced glycation end products and its ligands. Biochim. Biophys. Acta 1498, 99–111 (2000).
Tóbon-Velasco, J. C., Cuevas, E. & Torres-Ramos, M. A. Receptor for AGEs (RAGE) as mediator of NF-kB pathway activation in neuroinflammation and oxidative stress. CNS Neurol. Disord. Drug Targets 13, 1615–1626 (2014).
Wautier, M.-P. et al. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am. J. Physiol. Endocrinol. Metab. 280, E685–E694 (2001).
Ott, C. et al. Role of advanced glycation end products in cellular signaling. Redox Biol. 2, 411–429 (2014).
Hofmann, M. A. et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell 97, 889–901 (1999).
D’Agati, V. & Schmidt, A. M. RAGE and the pathogenesis of chronic kidney disease. Nat. Rev. Nephrol. 6, 352–360 (2010).
Farmer, D. G. & Kennedy, S. RAGE, vascular tone and vascular disease. Pharm. Ther. 124, 185–194 (2009).
Harja, E. et al. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE-/- mice. J. Clin. Invest 118, 183–194 (2008).
Hori, O. et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J. Biol. Chem. 270, 25752–25761 (1995).
Geroldi, D., Falcone, C. & Emanuele, E. Soluble receptor for advanced glycation end products: from disease marker to potential therapeutic target. Curr. Med Chem. 13, 1971–1978 (2006).
Shroff, R. C. & Shanahan, C. M. The vascular biology of calcification. Semin Dial. 20, 103–109 (2007).
Kalra, S. S. & Shanahan, C. M. Vascular calcification and hypertension: cause and effect. Ann. Med. 44, S85–92 (2012).
Lanzer, P. et al. Medial arterial calcification: JACC state-of-the-art review. J. Am. Coll. Cardiol. 78, 1145–1165 (2021).
Dao, H. H., Essalihi, R., Bouvet, C. & Moreau, P. Evolution and modulation of age-related medial elastocalcinosis: impact on large artery stiffness and isolated systolic hypertension. Cardiovasc Res. 66, 307–317 (2005).
Blacher, J., Guerin, A. P., Pannier, B., Marchais, S. J. & London, G. M. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 38, 938–942 (2001).
Niederhoffer, N. et al. Calcification of medial elastic fibers and aortic elasticity. Hypertension 29, 999–1006 (1997).
Van den Bergh, G., Opdebeeck, B., D’Haese, P. C. & Verhulst, A. The vicious cycle of arterial stiffness and arterial media calcification. Trends Mol. Med. 25, 1133–1146 (2019).
Proudfoot, D. & Shanahan, C. M. Biology of calcification in vascular cells: intima versus media. Herz 26, 245–251 (2001).
Li, N. et al. Vascular adventitia calcification and its underlying mechanism. PLoS ONE 10, e0132506 (2015).
Hunt, J. L. et al. Bone formation in carotid plaques: a clinicopathological study. Stroke 33, 1214–1219 (2002).
Jeffcoate, W. J., Rasmussen, L. M., Hofbauer, L. C. & Game, F. L. Medial arterial calcification in diabetes and its relationship to neuropathy. Diabetologia 52, 2478–2488 (2009).
Villa-Bellosta, R. New insights into endogenous mechanisms of protection against arterial calcification. Atherosclerosis 306, 68–74 (2020).
Giachelli, C. M. The emerging role of phosphate in vascular calcification. Kidney Int. 75, 890–897 (2009).
Voelkl, J. et al. Signaling pathways involved in vascular smooth muscle cell calcification during hyperphosphatemia. Cell Mol. Life Sci. 76, 2077–2091 (2019).
Ketteler, M., Brandenburg, V., Jahnen-Dechent, W., Westenfeld, R. & Floege, J. Do not be misguided by guidelines: the calcium x phosphate product can be a Trojan horse. Nephrol. Dial. Transpl. 20, 673–677 (2005).
Shanahan, C. M., Crouthamel, M. H., Kapustin, A. & Giachelli, C. M. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ. Res. 109, 697–711 (2011).
Schlieper, G., Schurgers, L., Brandenburg, V., Reutelingsperger, C. & Floege, J. Vascular calcification in chronic kidney disease: an update. Nephrol. Dial. Transpl. 31, 31–39 (2016).
Lanzer, P. et al. Medial vascular calcification revisited: review and perspectives. Eur. Heart J. 35, 1515–1525 (2014).
Johnson, R. C., Leopold, J. A. & Loscalzo, J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ. Res. 99, 1044–1059 (2006).
Addison, W. N., Azari, F., Sørensen, E. S., Kaartinen, M. T. & McKee, M. D. Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J. Biol. Chem. 282, 15872–15883 (2007).
Moochhala, S. H. Extracellular pyrophosphate in the kidney: how does it get there and what does it do? Nephron Physiol. 120, p33–8 (2012).
Roberts, F., Zhu, D., Farquharson, C. & Macrae, V. E. ENPP1 in the regulation of mineralization and beyond. Trends Biochem. Sci. 44, 616–628 (2019).
Eytan, O. et al. Cole disease results from mutations in ENPP1. Am. J. Hum. Genet 93, 752–757 (2013).
Chen, I. P., Luxmi, R., Kanaujiya, J., Hao, Z. & Reichenberger, E. J. Craniometaphyseal dysplasia mutations in ANKH negatively affect human induced pluripotent stem cell differentiation into osteoclasts. Stem Cell Rep. 9, 1369–1376 (2017).
Ho, A. M., Johnson, M. D. & Kingsley, D. M. Role of the mouse ank gene in control of tissue calcification and arthritis. Science 289, 265–270 (2000).
Williams, C. J. The role of ANKH in pathologic mineralization of cartilage. Curr. Opin. Rheumatol. 28, 145–151 (2016).
Paloian, N. J. & Giachelli, C. M. A current understanding of vascular calcification in CKD. Am. J. Physiol. Ren. Physiol. 307, F891–900 (2014).
Goettsch, C. et al. Sortilin mediates vascular calcification via its recruitment into extracellular vesicles. J. Clin. Invest. 126, 1323–1336 (2016).
Blaser, M. C. & Aikawa, E. Roles and regulation of extracellular vesicles in cardiovascular mineral metabolism. Front. Cardiovasc. Med. 5, 187 (2018).
Demer, L. L. & Tintut, Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation 117, 2938–2948 (2008).
Kapustin, A. N. et al. Calcium regulates key components of vascular smooth muscle cell-derived matrix vesicles to enhance mineralization. Circ. Res. 109, e1–12 (2011).
Proudfoot, D. et al. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ. Res. 87, 1055–1062 (2000).
Clarke, M. C. et al. Chronic apoptosis of vascular smooth muscle cells accelerates atherosclerosis and promotes calcification and medial degeneration. Circ. Res. 102, 1529–1538 (2008).
Alesutan, I. et al. Augmentation of phosphate-induced osteo-/chondrogenic transformation of vascular smooth muscle cells by homoarginine. Cardiovasc. Res. 110, 408–418 (2016).
Qiu, C. et al. Vitamin K2 inhibits rat vascular smooth muscle cell calcification by restoring the Gas6/Axl/Akt anti-apoptotic pathway. Mol. Cell Biochem. 433, 149–159 (2017).
Son, B. K. et al. Gas6/Axl-PI3K/Akt pathway plays a central role in the effect of statins on inorganic phosphate-induced calcification of vascular smooth muscle cells. Eur. J. Pharm. 556, 1–8 (2007).
Son, B. K. et al. Statins protect human aortic smooth muscle cells from inorganic phosphate-induced calcification by restoring Gas6-Axl survival pathway. Circ. Res. 98, 1024–1031 (2006).
Lau, W. L., Festing, M. H. & Giachelli, C. M. Phosphate and vascular calcification: emerging role of the sodium-dependent phosphate co-transporter PiT-1. Thromb. Haemost. 104, 464–470 (2010).
Cui, L., Bai, Y., Zhang, J., Zhang, S. & Xu, J. Effects of extracellular acid stimulation on rat vascular smooth muscle cell in Gas6/Axl or PI3K/Akt signaling pathway. Clin. Exp. Hypertens. 38, 451–456 (2016).
Xu, M., Liu, L., Song, C., Chen, W. & Gui, S. Ghrelin improves vascular autophagy in rats with vascular calcification. Life Sci. 179, 23–29 (2017).
Son, B. K. et al. Adiponectin antagonizes stimulatory effect of tumor necrosis factor-alpha on vascular smooth muscle cell calcification: regulation of growth arrest-specific gene 6-mediated survival pathway by adenosine 5’-monophosphate-activated protein kinase. Endocrinology 149, 1646–1653 (2008).
Ma, W. Q. et al. Restoring mitochondrial biogenesis with metformin attenuates β-GP-induced phenotypic transformation of VSMCs into an osteogenic phenotype via inhibition of PDK4/oxidative stress-mediated apoptosis. Mol. Cell Endocrinol. 479, 39–53 (2019).
Voelkl, J. et al. Zinc inhibits phosphate-induced vascular calcification through TNFAIP3-mediated suppression of NF-κB. J. Am. Soc. Nephrol. 29, 1636–1648 (2018).
Alesutan, I. et al. Involvement of vascular aldosterone synthase in phosphate-induced osteogenic transformation of vascular smooth muscle cells. Sci. Rep. 7, 2059 (2017).
Chen, N. X. & Moe, S. M. Pathophysiology of vascular calcification. Curr. Osteoporos. Rep. 13, 372–380 (2015).
Voelkl, J. et al. Spironolactone ameliorates PIT1-dependent vascular osteoinduction in klotho-hypomorphic mice. J. Clin. Invest 123, 812–822 (2013).
Leibrock, C. B. et al. NH4Cl treatment prevents tissue calcification in klotho deficiency. J. Am. Soc. Nephrol. 26, 2423–2433 (2015).
Xu, Z. et al. SOX9 and myocardin counteract each other in regulating vascular smooth muscle cell differentiation. Biochem. Biophys. Res.Commun. 422, 285–290 (2012).
Alesutan, I. et al. Inhibition of phosphate-induced vascular smooth muscle cell osteo-/chondrogenic signaling and calcification by bafilomycin A1 and methylamine. Kidney Blood Press Res. 40, 490–499 (2015).
Speer, M. Y., Li, X., Hiremath, P. G. & Giachelli, C. M. Runx2/Cbfa1, but not loss of myocardin, is required for smooth muscle cell lineage reprogramming toward osteochondrogenesis. J. Cell Biochem. 110, 935–947 (2010).
Sun, Y. et al. Smooth muscle cell-specific runx2 deficiency inhibits vascular calcification. Circ. Res. 111, 543–552 (2012).
Lee, H. L., Woo, K. M., Ryoo, H. M. & Baek, J. H. Tumor necrosis factor-alpha increases alkaline phosphatase expression in vascular smooth muscle cells via MSX2 induction. Biochem. Biophys. Res. Commun. 391, 1087–1092 (2010).
Alencar, G. F. et al. Stem cell pluripotency genes Klf4 and Oct4 regulate complex SMC phenotypic changes critical in late-stage atherosclerotic lesion pathogenesis. Circulation 142, 2045–2059 (2020).
Yoshida, T., Yamashita, M. & Hayashi, M. Kruppel-like factor 4 contributes to high phosphate-induced phenotypic switching of vascular smooth muscle cells into osteogenic cells. J. Biol. Chem. 287, 25706–25714 (2012).
Zhu, L. et al. Hyperhomocysteinemia induces vascular calcification by activating the transcription factor RUNX2 via Krüppel-like factor 4 up-regulation in mice. J. Biol. Chem. 294, 19465–19474 (2019).
Leibrock, C. B. et al. Acetazolamide sensitive tissue calcification and aging of klotho-hypomorphic mice. J. Mol. Med. 94, 95–106 (2016).
Shanahan, C. M. et al. Medial localization of mineralization-regulating proteins in association with Mönckeberg’s sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation 100, 2168–2176 (1999).
Forrester, S. J. et al. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol. Rev. 98, 1627–1738 (2018).
Povlsen, A. L., Grimm, D., Wehland, M., Infanger, M. & Krüger, M. The vasoactive mas receptor in essential hypertension. J. Clin. Med 9, 267 (2020).
Santos, R. A. et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc. Natl Acad. Sci. USA 100, 8258–8263 (2003).
Santos, R. A. S. et al. The ACE2/angiotensin-(1-7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1-7). Physiol. Rev. 98, 505–553 (2018).
Te Riet, L., van Esch, J. H., Roks, A. J., van den Meiracker, A. H. & Danser, A. H. Hypertension: renin-angiotensin-aldosterone system alterations. Circ. Res. 116, 960–975 (2015).
Becher, U. M., Endtmann, C., Tiyerili, V., Nickenig, G. & Werner, N. Endothelial damage and regeneration: the role of the renin-angiotensin-aldosterone system. Curr. Hypertens. Rep. 13, 86–92 (2011).
Li, Y., Yan, Z., Chaudhry, K. & Kazlauskas, A. The renin-angiotensin-aldosterone system (RAAS) is one of the effectors by which vascular endothelial growth factor (VEGF)/anti-VEGF controls the endothelial cell barrier. Am. J. Pathol. 190, 1971–1981 (2020).
Sun, Y., Ramires, F. J. & Weber, K. T. Fibrosis of atria and great vessels in response to angiotensin II or aldosterone infusion. Cardiovasc Res. 35, 138–147 (1997).
Ferrario, C. M. et al. Angiotensin (1-12) in humans with normal blood pressure and primary hypertension. Hypertension 77, 882–890 (2021).
Azushima, K., Morisawa, N., Tamura, K. & Nishiyama, A. Recent research advances in renin-angiotensin-aldosterone system receptors. Curr. Hypertens. Rep. 22, 22 (2020).
Balakumar, P. et al. Unraveling the differentially articulated axes of the century-old renin-angiotensin-aldosterone system: potential therapeutic implications. Cardiovasc. Toxicol. 22, 246–253 (2022).
Basu, S. et al. Notch transcriptional control of vascular smooth muscle regulatory gene expression and function. J. Biol. Chem. 288, 11191–11202 (2013).
Alenina, N., Xu, P., Rentzsch, B., Patkin, E. L. & Bader, M. Genetically altered animal models for Mas and angiotensin-(1-7). Exp. Physiol. 93, 528–537 (2008).
Bader, M. A. C. E. 2 angiotensin-(1–7), and Mas: the other side of the coin. Pflug. Arch. 465, 79–85 (2013).
Patel, S. K. et al. From gene to protein-experimental and clinical studies of ACE2 in blood pressure control and arterial hypertension. Front Physiol. 5, 227 (2014).
Durand, M. J. et al. Vascular actions of angiotensin 1-7 in the human microcirculation: novel role for telomerase. Arterioscler Thromb. Vasc. Biol. 36, 1254–1262 (2016).
Beyer, A. M. et al. Critical role for telomerase in the mechanism of flow-mediated dilation in the human microcirculation. Circ. Res. 118, 856–866 (2016).
Ocaranza, M. P. et al. Rho kinase inhibition activates the homologous angiotensin-converting enzyme-angiotensin-(1-9) axis in experimental hypertension. J. Hypertens. 29, 706–715 (2011).
Siragy, H. M., Jaffa, A. A. & Margolius, H. S. Bradykinin B2 receptor modulates renal prostaglandin E2 and nitric oxide. Hypertension 29, 757–762 (1997).
Abadir, P. M., Carey, R. M. & Siragy, H. M. Angiotensin AT2 receptors directly stimulate renal nitric oxide in bradykinin B2-receptor–null mice. Hypertension 42, 600–604 (2003).
Tsutsumi, Y. et al. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J. Clin. Invest. 104, 925–935 (1999).
Chai, S. Y. et al. The angiotensin IV/AT4 receptor. Cell Mol. Life Sci. 61, 2728–2737 (2004).
Ichihara, A. & Yatabe, M. S. The (pro)renin receptor in health and disease. Nat. Rev. Nephrol. 15, 693–712 (2019).
Sakoda, M. et al. (Pro)renin receptor-mediated activation of mitogen-activated protein kinases in human vascular smooth muscle cells. Hypertens Res. 30, 1139–1146 (2007).
Kurauchi-Mito, A. et al. Significant roles of the (pro)renin receptor in integrity of vascular smooth muscle cells. Hypertens. Res. 37, 830–835 (2014).
Krop, M., Lu, X., Danser, A. H. & Meima, M. E. The (pro)renin receptor. A decade of research: what have we learned? Pflug. Arch. 465, 87–97 (2013).
Cruciat, C. M. et al. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science 327, 459–463 (2010).
Sihn, G., Rousselle, A., Vilianovitch, L., Burckle, C. & Bader, M. Physiology of the (pro)renin receptor: Wnt of change? Kidney Int. 78, 246–256 (2010).
Parving, H. H. et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N. Engl. J. Med. 367, 2204–2213 (2012).
McCurley, A. et al. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat. Med. 18, 1429–1433 (2012).
Koenig, J. B. & Jaffe, I. Z. Direct role for smooth muscle cell mineralocorticoid receptors in vascular remodeling: novel mechanisms and clinical implications. Curr. Hypertens. Rep. 16, 427 (2014).
Freinbichler, W. et al. Highly reactive oxygen species: detection, formation, and possible functions. Cell Mol. Life Sci. 68, 2067–2079 (2011).
Wu, R. et al. Redox signaling, mitochondrial metabolism, epigenetics and redox active phytochemicals. Free Radic. Biol. Med. 179, 328–336 (2022).
Forman, H. J., Ursini, F. & Maiorino, M. An overview of mechanisms of redox signaling. J. Mol. Cell Cardiol. 73, 2–9 (2014).
Zhang, J. et al. ROS and ROS-mediated cellular signaling. Oxid. Med. Cell Longev. 2016, 4350965 (2016).
Griendling, K. K. et al. Oxidative stress and hypertension. Circ. Res. 128, 993–1020 (2021).
Majzunova, M., Dovinova, I., Barancik, M. & Chan, J. Y. Redox signaling in pathophysiology of hypertension. J. Biomed. Sci. 20, 69 (2013).
Lee, M. Y. & Griendling, K. K. Redox signaling, vascular function, and hypertension. Antioxid. Redox Signal 10, 1045–1059 (2008).
Touyz, R. M. & Briones, A. M. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertens. Res. 34, 5–14 (2011).
Kemble, D. J. & Sun, G. Direct and specific inactivation of protein tyrosine kinases in the Src and FGFR families by reversible cysteine oxidation. Proc. Natl Acad. Sci. USA 106, 5070–5075 (2009).
Paulsen, C. E. & Carroll, K. S. Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem. Biol. 5, 47–62 (2010).
Burgoyne, J. R. et al. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science 317, 1393–1397 (2007).
Lyle, A. N. & Griendling, K. K. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology 21, 269–280 (2006).
García-Redondo, A. B. et al. c-Src, ERK1/2 and Rho kinase mediate hydrogen peroxide-induced vascular contraction in hypertension: role of TXA2, NAD(P)H oxidase and mitochondria. J. Hypertens. 33, 77–87 (2015).
Liu, Q. et al. The association between oxidative stress, activator protein-1, inflammatory, total antioxidant status and artery stiffness and the efficacy of olmesartan in elderly patients with mild-to-moderate essential hypertension. Clin. Exp. Hypertens. 38, 365–369 (2016).
Ferroni, P., Basili, S., Paoletti, V. & Davì, G. Endothelial dysfunction and oxidative stress in arterial hypertension. Nutr. Metab. Cardiovasc. Dis. 16, 222–233 (2006).
Gang, C. et al. Puerarin suppresses angiotensin II-induced cardiac hypertrophy by inhibiting NADPH oxidase activation and oxidative stress-triggered AP-1 signaling pathways. J. Pharm. Pharm. Sci. 18, 235–248 (2015).
Chen, B., Lu, Y., Chen, Y. & Cheng, J. The role of Nrf2 in oxidative stress-induced endothelial injuries. J. Endocrinol. 225, R83–99 (2015).
Thannickal, V. J. & Fanburg, B. L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 279, L1005–28 (2000).
Bou-Teen, D. et al. Mitochondrial ROS and mitochondria-targeted antioxidants in the aged heart. Free Radic. Biol. Med. 167, 109–124 (2021).
Zorov, D. B., Juhaszova, M. & Sollott, S. J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 94, 909–950 (2014).
Xie, N. et al. NAD+ metabolism: pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target Ther. 5, 227 (2020).
Schmidt, H. H. et al. Antioxidants in translational medicine. Antioxid. Redox Signal 23, 1130–1143 (2015).
Schönfeld, P., Dymkowska, D. & Wojtczak, L. Acyl-CoA-induced generation of reactive oxygen species in mitochondrial preparations is due to the presence of peroxisomes. Free Radic. Biol. Med. 47, 503–509 (2009).
Liu, Q. et al. A Fenton reaction at the endoplasmic reticulum is involved in the redox control of hypoxia-inducible gene expression. Proc. Natl Acad. Sci. USA 101, 4302–4307 (2004).
Youle, R. J. & van der Bliek, A. M. Mitochondrial fission, fusion, and stress. Science 337, 1062–1065 (2012).
van der Bliek, A. M., Shen, Q. & Kawajiri, S. Mechanisms of mitochondrial fission and fusion. Cold Spring Harb. Perspect. Biol. 5, a011072 (2013).
Aon, M. A., Cortassa, S. & O’Rourke, B. Percolation and criticality in a mitochondrial network. Proc. Natl Acad. Sci. USA 101, 4447–4452 (2004).
Zhang, Y., Murugesan, P., Huang, K. & Cai, H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: novel therapeutic targets. Nat. Rev. Cardiol. 17, 170–194 (2020).
Hilenski, L. L., Clempus, R. E., Quinn, M. T., Lambeth, J. D. & Griendling, K. K. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb. Vasc. Biol. 24, 677–683 (2004).
El-Benna, J., Dang, P. M., Gougerot-Pocidalo, M. A., Marie, J. C. & Braut-Boucher, F. p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases. Exp. Mol. Med. 41, 217–225 (2009).
Fukui, T. et al. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circ. Res. 80, 45–51 (1997).
Zalba, G. et al. Vascular NADH/NADPH oxidase is involved in enhanced superoxide production in spontaneously hypertensive rats. Hypertension 35, 1055–1061 (2000).
Beswick, R. A., Dorrance, A. M., Leite, R. & Webb, R. C. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension 38, 1107–1111 (2001).
Landmesser, U. & Harrison, D. G. Oxidative stress and vascular damage in hypertension. Coron. Artery Dis. 12, 455–461 (2001).
Rajagopalan, S. et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Inves.t 97, 1916–1923 (1996).
Lavoie, J. L. & Sigmund, C. D. Minireview: overview of the renin-angiotensin system—an endocrine and paracrine system. Endocrinology 144, 2179–2183 (2003).
Soe, N. N. et al. Cyclophilin A is required for angiotensin II-induced p47phox translocation to caveolae in vascular smooth muscle cells. Arterioscler Thromb. Vasc. Biol. 33, 2147–2153 (2013).
Basset, O. et al. NADPH oxidase 1 deficiency alters caveolin phosphorylation and angiotensin II-receptor localization in vascular smooth muscle. Antioxid. Redox Signal 11, 2371–2384 (2009).
Niethammer, P., Grabher, C., Look, A. T. & Mitchison, T. J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 459, 996–999 (2009).
Colavitti, R. et al. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J. Biol. Chem. 277, 3101–3108 (2002).
Sundaresan, M., Yu, Z. X., Ferrans, V. J., Irani, K. & Finkel, T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270, 296–299 (1995).
Ren, J., Bi, Y., Sowers, J. R., Hetz, C. & Zhang, Y. Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases. Nat. Rev. Cardiol. 18, 499–521 (2021).
Young, C. N. Endoplasmic reticulum stress in the pathogenesis of hypertension. Exp. Physiol. 102, 869–884 (2017).
Cunard, R. Endoplasmic reticulum stress, a driver or an innocent bystander in endothelial dysfunction associated with hypertension? Curr. Hypertens. Rep. 19, 64 (2017).
Ochoa, C. D., Wu, R. F. & Terada, L. S. ROS signaling and ER stress in cardiovascular disease. Mol. Asp. Med. 63, 18–29 (2018).
Szasz, T., Thakali, K., Fink, G. D. & Watts, S. W. A comparison of arteries and veins in oxidative stress: producers, destroyers, function, and disease. Exp. Biol. Med (Maywood) 232, 27–37 (2007).
Ali, S. S., Ahsan, H., Zia, M. K., Siddiqui, T. & Khan, F. H. Understanding oxidants and antioxidants: classical team with new players. J. Food Biochem. 44, e13145 (2020).
Zhang, Y. et al. Role of selenoproteins in redox regulation of signaling and the antioxidant system: a review. Antioxid. (Basel) 9, 383 (2020).
Ahmad, K. A. et al. Antioxidant therapy for management of oxidative stress induced hypertension. Free Radic. Res. 51, 428–438 (2017).
Redón, J. et al. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension 41, 1096–1101 (2003).
Sáez, G. T. et al. Factors related to the impact of antihypertensive treatment in antioxidant activities and oxidative stress by-products in human hypertension. Am. J. Hypertens. 17, 809–816 (2004).
Zhou, L. et al. Reduction in extracellular superoxide dismutase activity in African-American patients with hypertension. Free Radic. Biol. Med. 41, 1384–1391 (2006).
Zhou, X. J., Vaziri, N. D., Wang, X. Q., Silva, F. G. & Laszik, Z. Nitric oxide synthase expression in hypertension induced by inhibition of glutathione synthase. J. Pharm. Exp. Ther. 300, 762–767 (2002).
Welch, W. J. et al. Role of extracellular superoxide dismutase in the mouse angiotensin slow pressor response. Hypertension 48, 934–941 (2006).
Rodriguez-Iturbe, B. et al. Association of mitochondrial SOD deficiency with salt-sensitive hypertension and accelerated renal senescence. J. Appl Physiol. 102, 255–260 (2007).
Nandi, A., Yan, L. J., Jana, C. K. & Das, N. Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxid. Med. Cell Longev. 2019, 9613090 (2019).
Iqbal, H. et al. Anti-inflammatory, anti-oxidant and cardio-protective properties of novel fluorophenyl benzimidazole in L-NAME-induced hypertensive rats. Eur. J. Pharm. 929, 175132 (2022).
Zhang, J. et al. Neohesperidin protects angiotensin II-induced hypertension and vascular remodeling. Front Pharm. 13, 890202 (2022).
Batista, G. M. S. et al. Ascorbic acid inhibits vascular remodeling induced by mental stress in overweight/obese men. Life Sci. 250, 117554 (2020).
Caillon, A. & Schiffrin, E. L. Role of inflammation and immunity in hypertension: recent epidemiological, laboratory, and clinical evidence. Curr. Hypertens. Rep. 18, 21 (2016).
Rodriguez-Iturbe, B., Pons, H. & Johnson, R. J. Role of the immune system in hypertension. Physiol. Rev. 97, 1127–1164 (2017).
Caillon, A., Paradis, P. & Schiffrin, E. L. Role of immune cells in hypertension. Br. J. Pharm. 176, 1818–1828 (2019).
Tellides, G. & Pober, J. S. Inflammatory and immune responses in the arterial media. Circ. Res. 116, 312–322 (2015).
Sharma, A. et al. Oxidative stress and NLRP3-inflammasome activity as significant drivers of diabetic cardiovascular complications: therapeutic implications. Front. Physiol. 9, 114 (2018).
Jaiswal, S. et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 377, 111–121 (2017).
Kanashiro, A. et al. The role of neutrophils in neuro-immune modulation. Pharm. Res. 151, 104580 (2020).
Vida, G. et al. 2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system. Faseb j. 25, 4476–4485 (2011).
Abais-Battad, J. M., Dasinger, J. H., Fehrenbach, D. J. & Mattson, D. L. Novel adaptive and innate immunity targets in hypertension. Pharm. Res. 120, 109–115 (2017).
Xiao, L. & Harrison, D. G. Inflammation in hypertension. Can. J. Cardiol. 36, 635–647 (2020).
Zhang, R. M., McNerney, K. P., Riek, A. E. & Bernal-Mizrachi, C. Immunity and hypertension. Acta Physiol. 231, e13487 (2021).
Grüneboom, A. et al. Imaging innate immunity. Immunol. Rev. 306, 293–303 (2022).
O’Neill, L. A. J., Golenbock, D. & Bowie, A. G. The history of Toll-like receptors—redefining innate immunity. Nat. Rev. Immunol. 13, 453–460 (2013).
Kumar, H., Kawai, T. & Akira, S. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 388, 621–625 (2009).
Vijay, K. Toll-like receptors in immunity and inflammatory diseases: past, present, and future. Int. Immunopharmacol. 59, 391–412 (2018).
Kawai, T. & Akira, S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 21, 317–337 (2009).
De Batista, P. R. et al. Toll-like receptor 4 upregulation by angiotensin II contributes to hypertension and vascular dysfunction through reactive oxygen species production. PLoS ONE 9, e104020 (2014).
Eissler, R. et al. Hypertension augments cardiac Toll-like receptor 4 expression and activity. Hypertens. Res. 34, 551–558 (2011).
Marketou, M. E. et al. TLR2 and TLR4 gene expression in peripheral monocytes in nondiabetic hypertensive patients: the effect of intensive blood pressure-lowering. J. Clin. Hypertens. 14, 330–335 (2012).
Bomfim, G. F. et al. Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin. Sci. (Lond.) 122, 535–543 (2012).
Bomfim, G. F. et al. Toll-like receptor 4 inhibition reduces vascular inflammation in spontaneously hypertensive rats. Life Sci. 122, 1–7 (2015).
Lee, G. L. et al. TLR4-activated MAPK-IL-6 axis regulates vascular smooth muscle cell function. Int. J. Mol. Sci. 17, 1394 (2016).
Song, Y. et al. TLR4/NF-κB/Ceramide signaling contributes to Ox-LDL-induced calcification of human vascular smooth muscle cells. Eur. J. Pharm. 794, 45–51 (2017).
Carrillo-Sepulveda, M. A., Spitler, K., Pandey, D., Berkowitz, D. E. & Matsumoto, T. Inhibition of TLR4 attenuates vascular dysfunction and oxidative stress in diabetic rats. J. Mol. Med (Berl.) 93, 1341–1354 (2015).
McCarthy, C. G. et al. Circulating mitochondrial DNA and Toll-like receptor 9 are associated with vascular dysfunction in spontaneously hypertensive rats. Cardiovasc. Res. 107, 119–130 (2015).
Rodrigues, F. L. et al. Toll-like receptor 9 plays a key role in the autonomic cardiac and baroreflex control of arterial pressure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 308, R714–23 (2015).
Takahashi, M. NLRP3 inflammasome as a key driver of vascular disease. Cardiovasc Res. 118, 372–385 (2022).
Toldo, S. et al. Targeting the NLRP3 inflammasome in cardiovascular diseases. Pharm. Ther. 236, 108053 (2022).
Tai, G. J. et al. NLRP3 inflammasome links vascular senescence to diabetic vascular lesions. Pharm. Res. 178, 106143 (2022).
Sun, H. J. et al. NLRP3 inflammasome activation contributes to VSMC phenotypic transformation and proliferation in hypertension. Cell Death Dis. 8, e3074 (2017).
Zhang, X. et al. NLRP3 inflammasome is involved in calcium-sensing receptor-induced aortic remodeling in SHRs. Mediators Inflamm. 2019, 6847087 (2019).
Guzik, T. J. et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med. 204, 2449–2460 (2007).
Mattson, D. L. et al. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R407–14 (2013).
Rudemiller, N., Lund, H., Jacob, H. J., Geurts, A. M. & Mattson, D. L. CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension 63, 559–564 (2014).
Korshunov, V. A., Daul, M., Massett, M. P. & Berk, B. C. Axl mediates vascular remodeling induced by deoxycorticosterone acetate-salt hypertension. Hypertension 50, 1057–1062 (2007).
Batchu, N. et al. Role of Axl in T-lymphocyte survival in salt-dependent hypertension. Arterioscler Thromb. Vasc. Biol. 36, 1638–1646 (2016).
Trott, D. W. et al. Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension 64, 1108–1115 (2014).
Chan, C. T. et al. Obligatory role for B cells in the development of angiotensin II-dependent hypertension. Hypertension 66, 1023–1033 (2015).
Crowley, S. D. et al. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1089–R1097 (2010).
Sundgren, N. C. et al. IgG receptor FcγRIIB plays a key role in obesity-induced hypertension. Hypertension 65, 456–462 (2015).
Madhur, M. S. et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55, 500–507 (2010).
Benson, L. N. et al. The IFNγ-PDL1 pathway enhances CD8T-DCT interaction to promote hypertension. Circ. Res. 130, 1550–1564 (2022).
Majeed, B. et al. Interleukin-2/anti-interleukin-2 immune complex expands regulatory T cells and reduces angiotensin II-induced aortic stiffening. Int. J. Hypertens. 2014, 126365 (2014).
Lamb, F. S., Choi, H., Miller, M. R. & Stark, R. J. TNFα and reactive oxygen signaling in vascular smooth muscle cells in hypertension and atherosclerosis. Am. J. Hypertens. 33, 902–913 (2020).
Asadikaram, G. et al. The study of the serum level of IL-4, TGF-β, IFN-γ, and IL-6 in overweight patients with and without diabetes mellitus and hypertension. J. Cell Biochem. 120, 4147–4157 (2019).
Dale, B. L. et al. Critical role of Interleukin 21 and T follicular helper cells in hypertension and vascular dysfunction. JCI Insight 5, e129278 (2019).
Ye, J. et al. Interleukin 22 promotes blood pressure elevation and endothelial dysfunction in angiotensin II–treated mice. J. Am. Heart Assoc. 6, e005875 (2017).
Ferreira, L. M. R., Muller, Y. D., Bluestone, J. A. & Tang, Q. Next-generation regulatory T cell therapy. Nat. Rev. Drug Disco. 18, 749–769 (2019).
Raffin, C., Vo, L. T. & Bluestone, J. A. T(reg) cell-based therapies: challenges and perspectives. Nat. Rev. Immunol. 20, 158–172 (2020).
Chapman, N. M., Boothby, M. R. & Chi, H. Metabolic coordination of T cell quiescence and activation. Nat. Rev. Immunol. 20, 55–70 (2020).
Meng, X. et al. Regulatory T cells in cardiovascular diseases. Nat. Rev. Cardiol. 13, 167–179 (2016).
Cui, C. et al. CD4(+) T-cell endogenous cystathionine γ lyase-hydrogen sulfide attenuates hypertension by sulfhydrating liver kinase B1 to promote T regulatory cell differentiation and proliferation. Circulation 142, 1752–1769 (2020).
Viel, E. C., Lemarié, C. A., Benkirane, K., Paradis, P. & Schiffrin, E. L. Immune regulation and vascular inflammation in genetic hypertension. Am. J. Physiol. Heart Circ. Physiol. 298, H938–44 (2010).
Kasal, D. A. et al. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension 59, 324–330 (2012).
Kassan, M., Galan, M., Partyka, M., Trebak, M. & Matrougui, K. Interleukin-10 released by CD4(+)CD25(+) natural regulatory T cells improves microvascular endothelial function through inhibition of NADPH oxidase activity in hypertensive mice. Arterioscler Thromb. Vasc. Biol. 31, 2534–2542 (2011).
Barhoumi, T. et al. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension 57, 469–476 (2011).
Belanger, K. M. et al. Greater T regulatory cells in females attenuate DOCA-salt-induced increases in blood pressure versus males. Hypertension 75, 1615–1623 (2020).
Vongpatanasin, W. et al. C-reactive protein causes downregulation of vascular angiotensin subtype 2 receptors and systolic hypertension in mice. Circulation 115, 1020–1028 (2007).
Ridker, P. M. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ. Res. 118, 145–156 (2016).
Campese, V. M., Ye, S. & Zhong, H. Downregulation of neuronal nitric oxide synthase and interleukin-1beta mediates angiotensin II-dependent stimulation of sympathetic nerve activity. Hypertension 39, 519–524 (2002).
Rothman, A. M. et al. Effects of interleukin-1β inhibition on blood pressure, incident hypertension, and residual inflammatory risk: a secondary analysis of CANTOS. Hypertension 75, 477–482 (2020).
Scott-Solomon, E., Boehm, E. & Kuruvilla, R. The sympathetic nervous system in development and disease. Nat. Rev. Neurosci. 22, 685–702 (2021).
Malpas, S. C. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol. Rev. 90, 513–557 (2010).
Grassi, G., Mark, A. & Esler, M. The sympathetic nervous system alterations in human hypertension. Circ. Res. 116, 976–990 (2015).
Parati, G. & Esler, M. The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur. Heart J. 33, 1058–1066 (2012).
Grassi, G. et al. Sympathetic and reflex alterations in systo-diastolic and systolic hypertension of the elderly. J. Hypertens. 18, 587–593 (2000).
Grassi, G., Cattaneo, B. M., Seravalle, G., Lanfranchi, A. & Mancia, G. Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension 31, 68–72 (1998).
Smith, P. A., Graham, L. N., Mackintosh, A. F., Stoker, J. B. & Mary, D. A. Sympathetic neural mechanisms in white-coat hypertension. J. Am. Coll. Cardiol. 40, 126–132 (2002).
Greenwood, J. P., Scott, E. M., Stoker, J. B. & Mary, D. A. Hypertensive left ventricular hypertrophy: relation to peripheral sympathetic drive. J. Am. Coll. Cardiol. 38, 1711–1717 (2001).
Grassi, G., Colombo, M., Seravalle, G., Spaziani, D. & Mancia, G. Dissociation between muscle and skin sympathetic nerve activity in essential hypertension, obesity, and congestive heart failure. Hypertension 31, 64–67 (1998).
Schlaich, M. P. et al. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and Angiotensin neuromodulation. Hypertension 43, 169–175 (2004).
Greenwood, J. P., Stoker, J. B. & Mary, D. A. Single-unit sympathetic discharge: quantitative assessment in human hypertensive disease. Circulation 100, 1305–1310 (1999).
Miyajima, E. et al. Muscle sympathetic nerve activity in renovascular hypertension and primary aldosteronism. Hypertension 17, 1057–1062 (1991).
Johansson, M. et al. Increased sympathetic nerve activity in renovascular hypertension. Circulation 99, 2537–2542 (1999).
Schobel, H. P., Fischer, T., Heuszer, K., Geiger, H. & Schmieder, R. E. Preeclampsia—a state of sympathetic overactivity. N. Engl. J. Med 335, 1480–1485 (1996).
Nilsson, H., Ljung, B., Sjöblom, N. & Wallin, B. G. The influence of the sympathetic impulse pattern on contractile responses of rat mesenteric arteries and veins. Acta Physiol. Scand. 123, 303–309 (1985).
Macefield, V. G. & Wallin, B. G. Physiological and pathophysiological firing properties of single postganglionic sympathetic neurons in humans. J. Neurophysiol. 119, 944–956 (2018).
Bylund, D. B. Subtypes of alpha 1- and alpha 2-adrenergic receptors. FASEB J. 6, 832–839 (1992).
Civantos Calzada, B. & Aleixandre de Artiñano, A. Alpha-adrenoceptor subtypes. Pharm. Res. 44, 195–208 (2001).
Docherty, J. R. Subtypes of functional alpha1-adrenoceptor. Cell Mol. Life Sci. 67, 405–417 (2010).
Sporkova, A., Perez-Rivera, A. & Galligan, J. J. Interaction between alpha(1)- and alpha(2)-adrenoreceptors contributes to enhanced constrictor effects of norepinephrine in mesenteric veins compared to arteries. Eur. J. Pharm. 643, 239–246 (2010).
Stull, J. T., Gallagher, P. J., Herring, B. P. & Kamm, K. E. Vascular smooth muscle contractile elements. Cellular regulation. Hypertension 17, 723–732 (1991).
Somlyo, A. P. & Somlyo, A. V. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 83, 1325–1358 (2003).
Aburto, T. K., Lajoie, C. & Morgan, K. G. Mechanisms of signal transduction during alpha 2-adrenergic receptor-mediated contraction of vascular smooth muscle. Circ. Res. 72, 778–785 (1993).
Eelen, G., Treps, L., Li, X. & Carmeliet, P. Basic and therapeutic aspects of angiogenesis updated. Circ. Res. 127, 310–329 (2020).
Mack, J. J. & Iruela-Arispe, M. L. NOTCH regulation of the endothelial cell phenotype. Curr. Opin. Hematol. 25, 212–218 (2018).
Kiriakidis, S. et al. Factor-inhibiting HIF-1 (FIH-1) is required for human vascular endothelial cell survival. FASEB J. 29, 2814–2827 (2015).
Wilhelm, K. et al. FOXO1 couples metabolic activity and growth state in the vascular endothelium. Nature 529, 216–220 (2016).
Andrade, J. et al. Control of endothelial quiescence by FOXO-regulated metabolites. Nat. Cell Biol. 23, 413–423 (2021).
Alvandi, Z. & Bischoff, J. Endothelial-mesenchymal transition in cardiovascular disease. Arterioscler Thromb. Vasc. Biol. 41, 2357–2369 (2021).
Kovacic, J. C. et al. Endothelial to mesenchymal transition in cardiovascular disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 73, 190–209 (2019).
Pardali, E., Sanchez-Duffhues, G. & Gomez-Puerto, M. C. & Ten Dijke, P. TGF-β-induced endothelial-mesenchymal transition in fibrotic diseases. Int. J. Mol. Sci. 18, 2157 (2017).
Son, M., Oh, S., Jang, J. T., Son, K. H. & Byun, K. Pyrogallol-phloroglucinol-6 6-bieckol on attenuates high-fat diet-induced hypertension by modulating endothelial-to-mesenchymal transition in the aorta of mice. Oxid. Med. Cell Longev. 2021, 8869085 (2021).
Tang, R. N. et al. Effects of angiotensin II receptor blocker on myocardial endothelial-to-mesenchymal transition in diabetic rats. Int. J. Cardiol. 162, 92–99 (2013).
Ma, J., Liu, T. & Dong, X. Advanced glycation end products of bovine serum albumin-induced endothelial-to-mesenchymal transition in cultured human and monkey endothelial cells via protein kinase B signaling cascades. Mol. Vis. 16, 2669–2679 (2010).
Sánchez-Duffhues, G. et al. Inflammation induces endothelial-to-mesenchymal transition and promotes vascular calcification through downregulation of BMPR2. J. Pathol. 247, 333–346 (2019).
Zhang, K., Kan, H., Mao, A., Geng, L. & Ma, X. Single-cell analysis of salt-induced hypertensive mouse aortae reveals cellular heterogeneity and state changes. Exp. Mol. Med. 53, 1866–1876 (2021).
Gomez, D. & Owens, G. K. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc. Res. 95, 156–164 (2012).
Chen, P. Y. et al. Smooth muscle cell reprogramming in aortic aneurysms. Cell Stem Cell 26, 542–557.e11 (2020).
Bennett, M. R., Sinha, S. & Owens, G. K. Vascular smooth muscle cells in atherosclerosis. Circ. Res. 118, 692–702 (2016).
Pan, H. et al. Single-cell genomics reveals a novel cell state during smooth muscle cell phenotypic switching and potential therapeutic targets for atherosclerosis in mouse and human. Circulation 142, 2060–2075 (2020).
Yap, C., Mieremet, A., de Vries, C. J. M., Micha, D. & de Waard, V. Six shades of vascular smooth muscle cells illuminated by KLF4 (Krüppel-like factor 4). Arterioscler Thromb. Vasc. Biol. 41, 2693–2707 (2021).
Wang, W. et al. Essential role of Smad3 in angiotensin II-induced vascular fibrosis. Circ. Res. 98, 1032–1039 (2006).
Ruiz-Ortega, M., Rodríguez-Vita, J., Sanchez-Lopez, E., Carvajal, G. & Egido, J. TGF-beta signaling in vascular fibrosis. Cardiovasc. Res. 74, 196–206 (2007).
Wu, J. et al. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J. Clin. Invest. 126, 1607 (2016).
Hu, H. H. et al. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem. Biol. Interact. 292, 76–83 (2018).
Goumans, M. J. & Ten Dijke, P. TGF-β signaling in control of cardiovascular function. Cold Spring Harb. Perspect. Biol. 10, a022210 (2018).
Leask, A. & Abraham, D. J. TGF-beta signaling and the fibrotic response. FASEB J. 18, 816–827 (2004).
Verrecchia, F. & Mauviel, A. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J. Invest. Dermatol. 118, 211–215 (2002).
Abreu, J. G., Ketpura, N. I., Reversade, B. & De Robertis, E. M. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat. Cell Biol. 4, 599–604 (2002).
Ihn, H. Pathogenesis of fibrosis: role of TGF-beta and CTGF. Curr. Opin. Rheumatol. 14, 681–685 (2002).
Kaikita, K. et al. Plasminogen activator inhibitor-1 deficiency prevents hypertension and vascular fibrosis in response to long-term nitric oxide synthase inhibition. Circulation 104, 839–844 (2001).
Samarakoon, R. & Higgins, P. J. Integration of non-SMAD and SMAD signaling in TGF-beta1-induced plasminogen activator inhibitor type-1 gene expression in vascular smooth muscle cells. Thromb. Haemost. 100, 976–983 (2008).
Li, J. H. et al. Advanced glycation end products activate Smad signaling via TGF-beta-dependent and independent mechanisms: implications for diabetic renal and vascular disease. FASEB J. 18, 176–178 (2004).
O’Dowd, B. F. et al. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene 136, 355–360 (1993).
Tatemoto, K. et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Biophys. Res. Commun. 251, 471–476 (1998).
Kleinz, M. J. & Davenport, A. P. Immunocytochemical localization of the endogenous vasoactive peptide apelin to human vascular and endocardial endothelial cells. Regul. Pept. 118, 119–125 (2004).
Kleinz, M. J., Skepper, J. N. & Davenport, A. P. Immunocytochemical localisation of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul. Pept. 126, 233–240 (2005).
Pope, G. R., Roberts, E. M., Lolait, S. J. & O’Carroll, A. M. Central and peripheral apelin receptor distribution in the mouse: species differences with rat. Peptides 33, 139–148 (2012).
Mughal, A., Sun, C. & OʼRourke, S. T. Apelin reduces nitric oxide-induced relaxation of cerebral arteries by inhibiting activation of large-conductance, calcium-activated K channels. J. Cardiovasc. Pharm. 71, 223–232 (2018).
Tatemoto, K. et al. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul. Pept. 99, 87–92 (2001).
Japp, A. G. et al. Vascular effects of apelin in vivo in man. J. Am. Coll. Cardiol. 52, 908–913 (2008).
Jia, Y. X. et al. Apelin activates L-arginine/nitric oxide synthase/nitric oxide pathway in rat aortas. Peptides 28, 2023–2029 (2007).
Xie, H. et al. Lowered circulating apelin is significantly associated with an increased risk for hypertension: A meta-analysis. Clin. Exp. Hypertens. 39, 435–440 (2017).
Mohammadi, M. et al. Apelin as a candidate for hypertension management; a systematic review and meta-analysis on animal studies. Arch. Acad. Emerg. Med. 10, e90 (2022).
Siddiquee, K., Hampton, J., McAnally, D., May, L. & Smith, L. The apelin receptor inhibits the angiotensin II type 1 receptor via allosteric trans-inhibition. Br. J. Pharm. 168, 1104–1117 (2013).
Sun, X. et al. Non-activated APJ suppresses the angiotensin II type 1 receptor, whereas apelin-activated APJ acts conversely. Hypertens. Res. 34, 701–706 (2011).
Siddiquee, K. et al. Apelin protects against angiotensin II-induced cardiovascular fibrosis and decreases plasminogen activator inhibitor type-1 production. J. Hypertens. 29, 724–731 (2011).
Sato, T. et al. Apelin is a positive regulator of ACE2 in failing hearts. J. Clin. Invest. 123, 5203–5211 (2013).
Zhong, J. C. et al. Targeting the apelin pathway as a novel therapeutic approach for cardiovascular diseases. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 1942–1950 (2017).
Han, X., Zhang, D. L., Yin, D. X., Zhang, Q. D. & Liu, W. H. Apelin-13 deteriorates hypertension in rats after damage of the vascular endothelium by ADMA. Can. J. Physiol. Pharm. 91, 708–714 (2013).
Maguire, J. J., Kleinz, M. J., Pitkin, S. L. & Davenport, A. P. [Pyr1]apelin-13 identified as the predominant apelin isoform in the human heart: vasoactive mechanisms and inotropic action in disease. Hypertension 54, 598–604 (2009).
Chng, S. C., Ho, L., Tian, J. & Reversade, B. ELABELA: a hormone essential for heart development signals via the apelin receptor. Dev. Cell 27, 672–680 (2013).
Pauli, A. et al. Toddler: an embryonic signal that promotes cell movement via Apelin receptors. Science 343, 1248636 (2014).
Li, Y. et al. Declined circulating Elabela levels in patients with essential hypertension and its association with impaired vascular function: A preliminary study. Clin. Exp. Hypertens. 42, 239–243 (2020).
Song, J., Tang, J., Zhang, Z., Liu, Y. & Zhong, J. Targeting the elabela/apelin-apelin receptor axis as a novel therapeutic approach for hypertension. Chin. Med. J. 135, 1019–1026 (2022).
Mughal, A. & O’Rourke, S. T. Vascular effects of apelin: mechanisms and therapeutic potential. Pharm. Ther. 190, 139–147 (2018).
Couvineau, P., Llorens-Cortes, C. & Iturrioz, X. Elabela/Toddler and apelin bind differently to the apelin receptor. Faseb j. 34, 7989–8000 (2020).
Dong, H., Jiang, Y., Triggle, C. R., Li, X. & Lytton, J. Novel role for K+-dependent Na+/Ca2+ exchangers in regulation of cytoplasmic free Ca2+ and contractility in arterial smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 291, H1226–35 (2006).
Cai, X. & Lytton, J. Molecular cloning of a sixth member of the K+-dependent Na+/Ca2+ exchanger gene family, NCKX6. J. Biol. Chem. 279, 5867–5876 (2004).
Blaustein, M. P. & Lederer, W. J. Sodium/calcium exchange: its physiological implications. Physiol. Rev. 79, 763–854 (1999).
Lytton, J., Li, X. F., Dong, H. & Kraev, A. K+-dependent Na+/Ca2+ exchangers in the brain. Ann. N. Y Acad. Sci. 976, 382–393 (2002).
Iwamoto, T. et al. Salt-sensitive hypertension is triggered by Ca2+ entry via Na+/Ca2+ exchanger type-1 in vascular smooth muscle. Nat. Med 10, 1193–1199 (2004).
Moore, E. D. et al. Coupling of the Na+/Ca2+ exchanger, Na+/K+ pump and sarcoplasmic reticulum in smooth muscle. Nature 365, 657–660 (1993).
Jaitovich, A. & Bertorello, A. M. Salt, Na+,K+-ATPase and hypertension. Life Sci. 86, 73–78 (2010).
Hamlyn, J. M. et al. A circulating inhibitor of (Na+ + K+)ATPase associated with essential hypertension. Nature 300, 650–652 (1982).
Dora, K. A., Gallagher, N. T., McNeish, A. & Garland, C. J. Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ. Res. 102, 1247–1255 (2008).
Edwards, G., Dora, K. A., Gardener, M. J., Garland, C. J. & Weston, A. H. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 396, 269–272 (1998).
Blaustein, M. P. et al. The pump, the exchanger, and endogenous ouabain: signaling mechanisms that link salt retention to hypertension. Hypertension 53, 291–298 (2009).
Shen, H. et al. The role of Na(+), K(+)-ATPase in the hypoxic vasoconstriction in isolated rat basilar artery. Vasc. Pharm. 81, 53–60 (2016).
Matchkov, V. V. et al. Interaction between Na+/K+-pump and Na+/Ca2+-exchanger modulates intercellular communication. Circ. Res. 100, 1026–1035 (2007).
Hangaard, L. et al. Na-K-ATPase regulates intercellular communication in the vascular wall via cSrc kinase-dependent connexin43 phosphorylation. Am. J. Physiol. Cell Physiol. 312, C385–C397 (2017).
Pitzer, A. L., Van Beusecum, J. P., Kleyman, T. R. & Kirabo, A. ENaC in salt-sensitive hypertension: kidney and beyond. Curr. Hypertens. Rep. 22, 69 (2020).
Kusche-Vihrog, K., Callies, C., Fels, J. & Oberleithner, H. The epithelial sodium channel (ENaC): Mediator of the aldosterone response in the vascular endothelium? Steroids 75, 544–549 (2010).
Jia, G. et al. Epithelial sodium channel in aldosterone-induced endothelium stiffness and aortic dysfunction. Hypertension 72, 731–738 (2018).
Martinez-Lemus, L. A. et al. Amiloride improves endothelial function and reduces vascular stiffness in female mice fed a western diet. Front. Physiol. 8, 456 (2017).
Tarjus, A. et al. The endothelial αENaC contributes to vascular endothelial function in vivo. PLoS One 12, e0185319 (2017).
Takahashi, H., Yoshika, M., Komiyama, Y. & Nishimura, M. The central mechanism underlying hypertension: a review of the roles of sodium ions, epithelial sodium channels, the renin-angiotensin-aldosterone system, oxidative stress and endogenous digitalis in the brain. Hypertens. Res. 34, 1147–1160 (2011).
Hoorn, E. J., Gritter, M., Cuevas, C. A. & Fenton, R. A. Regulation of the renal NaCl cotransporter and its role in potassium homeostasis. Physiol. Rev. 100, 321–356 (2020).
Pitzer, A. et al. DC ENaC-dependent inflammasome activation contributes to salt-sensitive hypertension. Circ. Res. 131, 328–344 (2022).
Barbaro, N. R. et al. Dendritic cell amiloride-sensitive channels mediate sodium-induced inflammation and hypertension. Cell Rep. 21, 1009–1020 (2017).
Liakos, C. I. et al. Blood pressure-lowering effect of newer antihyperglycemic agents (SGLT-2 inhibitors, GLP-1 receptor agonists, and DPP-4 inhibitors). Am. J. Cardiovasc. Drugs 21, 123–137 (2021).
Mazidi, M., Rezaie, P., Gao, H. K. & Kengne, A. P. Effect of sodium-glucose cotransport-2 inhibitors on blood pressure in people with type 2 diabetes mellitus: a systematic review and meta-analysis of 43 randomized control trials with 22 528 patients. J. Am. Heart Assoc. 6, e004007 (2017).
Weber, M. A. et al. Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Diabetes Endocrinol. 4, 211–220 (2016).
Weber, M. A. et al. Effects of dapagliflozin on blood pressure in hypertensive diabetic patients on renin-angiotensin system blockade. Blood Press 25, 93–103 (2016).
List, J. F., Woo, V., Morales, E., Tang, W. & Fiedorek, F. T. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 32, 650–657 (2009).
Lambers Heerspink, H. J., de Zeeuw, D., Wie, L., Leslie, B. & List, J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes. Metab. 15, 853–862 (2013).
Tikkanen, I. et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care 38, 420–428 (2015).
Lovshin, J. A. & Gilbert, R. E. Are SGLT2 inhibitors reasonable antihypertensive drugs and renoprotective? Curr. Hypertens. Rep. 17, 551 (2015).
Takeshige, Y. et al. A sodium-glucose co-transporter 2 inhibitor empagliflozin prevents abnormality of circadian rhythm of blood pressure in salt-treated obese rats. Hypertens. Res. 39, 415–422 (2016).
Cefalu, W. T. et al. Effects of canagliflozin on body weight and relationship to HbA1c and blood pressure changes in patients with type 2 diabetes. Diabetologia 58, 1183–1187 (2015).
DeFronzo, R. A., Norton, L. & Abdul-Ghani, M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat. Rev. Nephrol. 13, 11–26 (2017).
Zelniker, T. A. & Braunwald, E. Mechanisms of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC state-of-the-art review. J. Am. Coll. Cardiol. 75, 422–434 (2020).
Chilton, R. et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes. Metab. 17, 1180–1193 (2015).
Striepe, K. et al. Effects of the selective sodium-glucose cotransporter 2 inhibitor empagliflozin on vascular function and central hemodynamics in patients with type 2 diabetes mellitus. Circulation 136, 1167–1169 (2017).
Cherney, D. Z. et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc. Diabetol. 13, 28 (2014).
Park, S. H. et al. Empagliflozin improved systolic blood pressure, endothelial dysfunction and heart remodeling in the metabolic syndrome ZSF1 rat. Cardiovasc. Diabetol. 19, 19 (2020).
Oelze, M. et al. The sodium-glucose co-transporter 2 inhibitor empagliflozin improves diabetes-induced vascular dysfunction in the streptozotocin diabetes rat model by interfering with oxidative stress and glucotoxicity. PLoS ONE 9, e112394 (2014).
Li, C. et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol. 18, 15 (2019).
Wan, N., Rahman, A., Hitomi, H. & Nishiyama, A. The effects of sodium-glucose cotransporter 2 inhibitors on sympathetic nervous activity. Front. Endocrinol. 9, 421 (2018).
Kusaka, H., Koibuchi, N., Hasegawa, Y., Ogawa, H. & Kim-Mitsuyama, S. Empagliflozin lessened cardiac injury and reduced visceral adipocyte hypertrophy in prediabetic rats with metabolic syndrome. Cardiovasc Diabetol. 15, 157 (2016).
Dampney, R. A. Functional organization of central pathways regulating the cardiovascular system. Physiol. Rev. 74, 323–364 (1994).
Guyenet, P. G. The sympathetic control of blood pressure. Nat. Rev. Neurosci. 7, 335–346 (2006).
Hirooka, Y. Sympathetic activation in hypertension: importance of the central nervous system. Am. J. Hypertens. 33, 914–926 (2020).
Barman, S. M. 2019 Ludwig Lecture: rhythms in sympathetic nerve activity are a key to understanding neural control of the cardiovascular system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 318, R191–R205 (2020).
Zubcevic, J. et al. Functional neural-bone marrow pathways: implications in hypertension and cardiovascular disease. Hypertension 63, e129–39 (2014).
Zubcevic, J. et al. Altered inflammatory response is associated with an impaired autonomic input to the bone marrow in the spontaneously hypertensive rat. Hypertension 63, 542–550 (2014).
Jun, J. Y. et al. Brain-mediated dysregulation of the bone marrow activity in angiotensin II-induced hypertension. Hypertension 60, 1316–1323 (2012).
de Git, K. C. & Adan, R. A. Leptin resistance in diet-induced obesity: the role of hypothalamic inflammation. Obes. Rev. 16, 207–224 (2015).
Hall, J. E., do Carmo, J. M., da Silva, A. A., Wang, Z. & Hall, M. E. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ. Res. 116, 991–1006 (2015).
Ataei Ataabadi, E. et al. Nitric oxide-cGMP signaling in hypertension: current and future options for pharmacotherapy. Hypertension 76, 1055–1068 (2020).
Wang, A. et al. Statins attenuate cholesterol-induced ROS via inhibiting NOX2/NOX4 and mitochondrial pathway in collecting ducts of the kidney. BMC Nephrol. 23, 184 (2022).
Lim, S. & Barter, P. Antioxidant effects of statins in the management of cardiometabolic disorders. J. Atheroscler. Thromb. 21, 997–1010 (2014).
Burger, D. et al. Endothelial microparticle formation by angiotensin II is mediated via Ang II receptor type I/NADPH oxidase/ Rho kinase pathways targeted to lipid rafts. Arterioscler Thromb. Vasc. Biol. 31, 1898–1907 (2011).
Wu, Y., Ding, Y., Ramprasath, T. & Zou, M. H. Oxidative stress, GTPCH1, and endothelial nitric oxide synthase uncoupling in hypertension. Antioxid. Redox Signal 34, 750–764 (2021).
Wallerath, T. et al. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 106, 1652–1658 (2002).
Xia, N., Förstermann, U. & Li, H. Effects of resveratrol on eNOS in the endothelium and the perivascular adipose tissue. Ann. N. Y Acad. Sci. 1403, 132–141 (2017).
Kass, D. A., Takimoto, E., Nagayama, T. & Champion, H. C. Phosphodiesterase regulation of nitric oxide signaling. Cardiovasc Res. 75, 303–314 (2007).
Golshiri, K., Ataei Ataabadi, E., Portilla Fernandez, E. C., Jan Danser, A. H. & Roks, A. J. M. The importance of the nitric oxide-cGMP pathway in age-related cardiovascular disease: focus on phosphodiesterase-1 and soluble guanylate cyclase. Basic Clin. Pharm. Toxicol. 127, 67–80 (2020).
Samidurai, A. et al. Role of phosphodiesterase 1 in the pathophysiology of diseases and potential therapeutic opportunities. Pharm. Ther. 226, 107858 (2021).
Gilotra, N. A. et al. Acute hemodynamic effects and tolerability of phosphodiesterase-1 inhibition with ITI-214 in human systolic heart failure. Circ. Heart Fail 14, e008236 (2021).
Lundberg, J. O., Weitzberg, E. & Gladwin, M. T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Disco. 7, 156–167 (2008).
Corti, R., Flammer, A. J., Hollenberg, N. K. & Lüscher, T. F. Cocoa and cardiovascular health. Circulation 119, 1433–1441 (2009).
Ribeiro, F., Alves, A. J., Duarte, J. A. & Oliveira, J. Is exercise training an effective therapy targeting endothelial dysfunction and vascular wall inflammation? Int. J. Cardiol. 141, 214–221 (2010).
Barton, M. Prevention and endothelial therapy of coronary artery disease. Curr. Opin. Pharm. 13, 226–241 (2013).
Esposito, K. et al. Endothelial microparticles correlate with endothelial dysfunction in obese women. J. Clin. Endocrinol. Metab. 91, 3676–3679 (2006).
Delgado, G. E. et al. Influence of smoking and smoking cessation on biomarkers of endothelial function and their association with mortality. Atherosclerosis 292, 52–59 (2020).
Pepine, C. J. et al. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. Jama 290, 2805–2816 (2003).
Whelton, P. K. et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of The American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 71, 2199–2269 (2018).
Williams, B. et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 39, 3021–3104 (2018).
Hansson, L. et al. Randomised trial of effects of calcium antagonists compared with diuretics and beta-blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazem (NORDIL) study. Lancet 356, 359–365 (2000).
Schefe, J. H. et al. A novel signal transduction cascade involving direct physical interaction of the renin/prorenin receptor with the transcription factor promyelocytic zinc finger protein. Circ. Res. 99, 1355–1366 (2006).
Sun, Y., Danser, A. H. J. & Lu, X. (Pro)renin receptor as a therapeutic target for the treatment of cardiovascular diseases?. Pharmacol. Res. 125, 48–56 (2017).
Nguyen, G. Renin/prorenin receptors. Kidney Int. 69, 1503–1506 (2006).
Ruilope, L. M. et al. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet 375, 1255–1266 (2010).
Gu, J. et al. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi). J. Clin. Pharm. 50, 401–414 (2010).
Kario, K. et al. Efficacy and safety of LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor, in Asian patients with hypertension: a randomized, double-blind, placebo-controlled study. Hypertension 63, 698–705 (2014).
Supasyndh, O. et al. Efficacy and safety of sacubitril/valsartan (LCZ696) compared with olmesartan in elderly Asian patients (≥65 years) with systolic hypertension. Am. J. Hypertens. 30, 1163–1169 (2017).
McDonagh, T. A. et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail 24, 4–131 (2022).
Gheblawi, M. et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 126, 1456–1474 (2020).
Cunha, T. M. et al. The nonpeptide ANG-(1-7) mimic AVE 0991 attenuates cardiac remodeling and improves baroreflex sensitivity in renovascular hypertensive rats. Life Sci. 92, 266–275 (2013).
Li, X. C., Zhang, J. & Zhuo, J. L. The vasoprotective axes of the renin-angiotensin system: physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharm. Res. 125, 21–38 (2017).
Meng, W. et al. Identification of a hydroxypyrimidinone compound (21) as a potent APJ receptor agonist for the potential treatment of heart failure. J. Med. Chem. 64, 18102–18113 (2021).
Tissot, A. C. et al. Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: a double-blind, randomised, placebo-controlled phase IIa study. Lancet 371, 821–827 (2008).
Chen, X. et al. Effectiveness and safety of a therapeutic vaccine against angiotensin II receptor type 1 in hypertensive animals. Hypertension 61, 408–416 (2013).
Brown, M. J. Aliskiren. Circulation 118, 773–784 (2008).
Alshahrani, S. Aliskiren—a promising antioxidant agent beyond hypertension reduction. Chem. Biol. Interact. 326, 109145 (2020).
Romero, C. A., Orias, M. & Weir, M. R. Novel RAAS agonists and antagonists: clinical applications and controversies. Nat. Rev. Endocrinol. 11, 242–252 (2015).
Dingemanse, J., Cavallaro, M. & Eydeler, U. Single-dose pharmacokinetics of the renin inhibitor ACT-077825 in elderly and young subjects of both sexes. Pharmacology 94, 135–142 (2014).
Dingemanse, J. & Nicolas, L. Drug-drug interaction study of ACT-178882, a new renin inhibitor, and diltiazem in healthy subjects. Clin. Drug Investig. 33, 207–213 (2013).
Fagard, R. H., Celis, H., Thijs, L. & Wouters, S. Regression of left ventricular mass by antihypertensive treatment: a meta-analysis of randomized comparative studies. Hypertension 54, 1084–1091 (2009).
Zannad, F. et al. Eplerenone in patients with systolic heart failure and mild symptoms. N. Engl. J. Med. 364, 11–21 (2011).
Armanini, D., Sabbadin, C., Donà, G., Clari, G. & Bordin, L. Aldosterone receptor blockers spironolactone and canrenone: two multivalent drugs. Expert Opin. Pharmacother. 15, 909–912 (2014).
Kolkhof, P. & Bärfacker, L. 30 YEARS OF THE MINERALOCORTICOID RECEPTOR: Mineralocorticoid receptor antagonists: 60 years of research and development. J. Endocrinol. 234, T125–T140 (2017).
Pitt, B. et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N. Engl. J. Med. 385, 2252–2263 (2021).
Kolkhof, P. et al. Effects of finerenone combined with empagliflozin in a model of hypertension-induced end-organ damage. Am. J. Nephrol. 52, 642–652 (2021).
Fagart, J. et al. A new mode of mineralocorticoid receptor antagonism by a potent and selective nonsteroidal molecule. J. Biol. Chem. 285, 29932–29940 (2010).
Meyers, M. J. et al. Discovery of (3S,3aR)-2-(3-chloro-4-cyanophenyl)-3-cyclopentyl-3,3a,4,5-tetrahydro-2H-benzo[g]indazole-7-carboxylic acid (PF-3882845), an orally efficacious mineralocorticoid receptor (MR) antagonist for hypertension and nephropathy. J. Med. Chem. 53, 5979–6002 (2010).
Nariai, T. et al. Antihypertensive and cardiorenal protective effects of SM-368229, a novel mineralocorticoid receptor antagonist, in aldosterone/salt-treated rats. Pharmacology 89, 44–52 (2012).
Pitt, B. et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur. Heart J. 34, 2453–2463 (2013).
Lenzini, L., Zanotti, G., Bonchio, M. & Rossi, G. P. Aldosterone synthase inhibitors for cardiovascular diseases: A comprehensive review of preclinical, clinical and in silico data. Pharm. Res. 163, 105332 (2021).
Robertson, S. et al. MicroRNA-24 is a novel regulator of aldosterone and cortisol production in the human adrenal cortex. Hypertension 62, 572–578 (2013).
Hopps, E. & Caimi, G. Matrix metalloproteases as a pharmacological target in cardiovascular diseases. Eur. Rev. Med. Pharm. Sci. 19, 2583–2589 (2015).
Fontana, V. et al. Comprehensive evaluation of the effects of enalapril on matrix metalloproteinases levels in hypertension. Cardiovasc. Drugs Ther. 26, 511–519 (2012).
Uzui, H. et al. Effects of combination therapy with olmesartan and azelnidipine on serum osteoprotegerin in patients with hypertension. J. Cardiovasc. Pharm. Ther. 19, 304–309 (2014).
Ceron, C. S. et al. Spironolactone and hydrochlorothiazide exert antioxidant effects and reduce vascular matrix metalloproteinase-2 activity and expression in a model of renovascular hypertension. Br. J. Pharm. 160, 77–87 (2010).
Ercan, E. et al. Atorvastatin treatment decreases inflammatory and proteolytic activity in patients with hypercholesterolemia. Kardiol. Pol. 60, 454–458 (2004).
Madsen, E. L. et al. Long-term weight loss decreases the nontraditional cardiovascular risk factors interleukin-18 and matrix metalloproteinase-9 in obese subjects. Metabolism 58, 946–953 (2009).
Ress, C. et al. Influence of significant weight loss on serum matrix metalloproteinase (MMP)-7 levels. Eur. Cytokine Netw. 21, 65–70 (2010).
Winchester, L., Veeranki, S., Givvimani, S. & Tyagi, S. C. Exercise mitigates the adverse effects of hyperhomocysteinemia on macrophages, MMP-9, skeletal muscle, and white adipocytes. Can. J. Physiol. Pharm. 92, 575–582 (2014).
Macaulay, V. M. et al. Phase I study of intrapleural batimastat (BB-94), a matrix metalloproteinase inhibitor, in the treatment of malignant pleural effusions. Clin. Cancer Res. 5, 513–520 (1999).
Acharya, M. R., Venitz, J., Figg, W. D. & Sparreboom, A. Chemically modified tetracyclines as inhibitors of matrix metalloproteinases. Drug Resist Updat. 7, 195–208 (2004).
Naglich, J. G. et al. Inhibition of angiogenesis and metastasis in two murine models by the matrix metalloproteinase inhibitor, BMS-275291. Cancer Res. 61, 8480–8485 (2001).
Lutz, J. et al. Inhibition of matrix metalloproteinases during chronic allograft nephropathy in rats. Transplantation 79, 655–661 (2005).
Vandenbroucke, R. E. & Libert, C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat. Rev. Drug Disco. 13, 904–927 (2014).
Deng, R. et al. Glucose-derived AGEs promote migration and invasion of colorectal cancer by up-regulating Sp1 expression. Biochim. Biophys. Acta Gen. Subj. 1861, 1065–1074 (2017).
Brownlee, M., Vlassara, H., Kooney, A., Ulrich, P. & Cerami, A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science 232, 1629–1632 (1986).
Weng, S. et al. Vitamin D deficiency induces high blood pressure and accelerates atherosclerosis in mice. PLoS One 8, e54625 (2013).
Sebeková, K. et al. Effects of ramipril in nondiabetic nephropathy: improved parameters of oxidatives stress and potential modulation of advanced glycation end products. J. Hum. Hypertens. 17, 265–270 (2003).
Diamanti-Kandarakis, E. et al. Effect of metformin administration on plasma advanced glycation end product levels in women with polycystic ovary syndrome. Metabolism 56, 129–134 (2007).
Ito, H. et al. Ellagitannin oligomers and a neolignan from pomegranate arils and their inhibitory effects on the formation of advanced glycation end products. Food Chem. 152, 323–330 (2014).
Vaitkevicius, P. V. et al. A cross-link breaker has sustained effects on arterial and ventricular properties in older rhesus monkeys. Proc. Natl Acad. Sci. USA 98, 1171–1175 (2001).
Toprak, C. & Yigitaslan, S. Alagebrium and complications of diabetes mellitus. Eurasia. J. Med. 51, 285–292 (2019).
Forbes, J. M. et al. Advanced glycation end product interventions reduce diabetes-accelerated atherosclerosis. Diabetes 53, 1813–1823 (2004).
Lanati, N., Emanuele, E., Brondino, N. & Geroldi, D. Soluble RAGE-modulating drugs: state-of-the-art and future perspectives for targeting vascular inflammation. Curr. Vasc. Pharm. 8, 86–92 (2010).
Prasad, K. & Tiwari, S. Therapeutic interventions for advanced glycation-end products and its receptor-mediated cardiovascular disease. Curr. Pharm. Des. 23, 937–943 (2017).
Villa-Bellosta, R. et al. Defective extracellular pyrophosphate metabolism promotes vascular calcification in a mouse model of Hutchinson-Gilford progeria syndrome that is ameliorated on pyrophosphate treatment. Circulation 127, 2442–2451 (2013).
Pomozi, V. et al. Pyrophosphate supplementation prevents chronic and acute calcification in ABCC6-deficient mice. Am. J. Pathol. 187, 1258–1272 (2017).
de Oliveira, R. B. et al. Peritoneal delivery of sodium pyrophosphate blocks the progression of pre-existing vascular calcification in uremic apolipoprotein-E knockout mice. Calcif. Tissue Int. 97, 179–192 (2015).
Tani, T. et al. Inhibition of tissue-nonspecific alkaline phosphatase protects against medial arterial calcification and improves survival probability in the CKD-MBD mouse model. J. Pathol. 250, 30–41 (2020).
Ziegler, S. G. et al. Ectopic calcification in pseudoxanthoma elasticum responds to inhibition of tissue-nonspecific alkaline phosphatase. Sci. Transl. Med. 9, eaal1669 (2017).
Li, Q. et al. Inhibition of tissue-nonspecific alkaline phosphatase attenuates ectopic mineralization in the Abcc6(-/-) mouse model of PXE but not in the Enpp1 mutant mouse models of GACI. J. Invest. Dermatol. 139, 360–368 (2019).
Albright, R. A. et al. ENPP1-Fc prevents mortality and vascular calcifications in rodent model of generalized arterial calcification of infancy. Nat. Commun. 6, 10006 (2015).
Ferrer, M. D. et al. A novel pharmacodynamic assay to evaluate the effects of crystallization inhibitors on calcium phosphate crystallization in human plasma. Sci. Rep. 7, 6858 (2017).
Ferrer, M. D. et al. Characterization of SNF472 pharmacokinetics and efficacy in uremic and non-uremic rats models of cardiovascular calcification. PLoS ONE 13, e0197061 (2018).
Ma, W. Q., Sun, X. J., Zhu, Y. & Liu, N. F. Metformin attenuates hyperlipidaemia-associated vascular calcification through anti-ferroptotic effects. Free Radic. Biol. Med 165, 229–242 (2021).
Phadwal, K., Feng, D., Zhu, D. & MacRae, V. E. Autophagy as a novel therapeutic target in vascular calcification. Pharm. Ther. 206, 107430 (2020).
Dai, X. Y. et al. Phosphate-induced autophagy counteracts vascular calcification by reducing matrix vesicle release. Kidney Int. 83, 1042–1051 (2013).
Frauscher, B. et al. Autophagy protects from uremic vascular media calcification. Front. Immunol. 9, 1866 (2018).
Yang, L. et al. Unspliced XBP1 counteracts β-catenin to inhibit vascular calcification. Circ. Res. 130, 213–229 (2022).
Nakatani, S., Mori, K., Shoji, T. & Emoto, M. Association of zinc deficiency with development of CVD events in patients with CKD. Nutrients 13, 1680 (2021).
Castrejón-Téllez, V. et al. Effect of a resveratrol/quercetin mixture on the reversion of hypertension induced by a short-term exposure to high sucrose levels near weaning and a long-term exposure that leads to metabolic syndrome in rats. Int. J. Mol. Sci. 21, 2231 (2020).
Prysyazhna, O. et al. Blood pressure-lowering by the antioxidant resveratrol is counterintuitively mediated by oxidation of cGMP-dependent protein kinase. Circulation 140, 126–137 (2019).
Huang, J., Weinstein, S. J., Yu, K., Männistö, S. & Albanes, D. Relationship between serum alpha-tocopherol and overall and cause-specific mortality. Circ. Res. 125, 29–40 (2019).
Li, Z., Chen, J. & Zhang, D. Association between dietary carotenoid intakes and hypertension in adults: National Health and Nutrition Examination Survey 2007-2014. J. Hypertens. 37, 2371–2379 (2019).
Ulker, S., McKeown, P. P. & Bayraktutan, U. Vitamins reverse endothelial dysfunction through regulation of eNOS and NAD(P)H oxidase activities. Hypertension 41, 534–539 (2003).
Taddei, S., Virdis, A., Ghiadoni, L., Magagna, A. & Salvetti, A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation 97, 2222–2229 (1998).
Duffy, S. J. et al. Effect of ascorbic acid treatment on conduit vessel endothelial dysfunction in patients with hypertension. Am. J. Physiol. Heart Circ. Physiol. 280, H528–34 (2001).
Rodrigo, R., Gil, D., Miranda-Merchak, A. & Kalantzidis, G. Antihypertensive role of polyphenols. Adv. Clin. Chem. 58, 225–254 (2012).
Ward, N. C. et al. The combination of vitamin C and grape-seed polyphenols increases blood pressure: a randomized, double-blind, placebo-controlled trial. J. Hypertens. 23, 427–434 (2005).
Sies, H. & Jones, D. P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 21, 363–383 (2020).
Griendling, K. K., Sorescu, D. & Ushio-Fukai, M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ. Res. 86, 494–501 (2000).
Mak, I. T., Boehme, P. & Weglicki, W. B. Antioxidant effects of calcium channel blockers against free radical injury in endothelial cells. Correlation of protection with preservation of glutathione levels. Circ. Res. 70, 1099–1103 (1992).
Matsubara, M. & Hasegawa, K. Benidipine, a dihydropyridine-calcium channel blocker, prevents lysophosphatidylcholine-induced injury and reactive oxygen species production in human aortic endothelial cells. Atherosclerosis 178, 57–66 (2005).
Cho, H. Y. et al. Comparative effect of genistein and daidzein on the expression of MCP-1, eNOS, and cell adhesion molecules in TNF-α-stimulated HUVECs. Nutr. Res. Pr. 5, 381–388 (2011).
Barrios, V., Calderón, A., Navarro-Cid, J., Lahera, V. & Ruilope, L. M. N-acetylcysteine potentiates the antihypertensive effect of ACE inhibitors in hypertensive patients. Blood Press 11, 235–239 (2002).
Feig, D. I., Soletsky, B. & Johnson, R. J. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. Jama 300, 924–932 (2008).
Mazzali, M. et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 38, 1101–1106 (2001).
Di Raimondo, D. et al. Effects of ACE-inhibitors and angiotensin receptor blockers on inflammation. Curr. Pharm. Des. 18, 4385–4413 (2012).
Dinh, Q. N., Drummond, G. R., Sobey, C. G. & Chrissobolis, S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed. Res. Int. 2014, 406960 (2014).
Rodríguez-Iturbe, B. et al. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am. J. Physiol. Ren. Physiol. 282, F191–201 (2002).
Tian, N. et al. Immune suppression prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Am. J. Physiol. Heart Circ. Physiol. 292, H1018–25 (2007).
Mattson, D. L., James, L., Berdan, E. A. & Meister, C. J. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 48, 149–156 (2006).
Yubero-Serrano, E. M. et al. Mediterranean diet and endothelial function in patients with coronary heart disease: An analysis of the CORDIOPREV randomized controlled trial. PLoS Med. 17, e1003282 (2020).
Di Francescomarino, S., Sciartilli, A., Di Valerio, V., Di Baldassarre, A. & Gallina, S. The effect of physical exercise on endothelial function. Sports Med. 39, 797–812 (2009).
MacIntyre, I. M. et al. Regular acetaminophen use and blood pressure in people with hypertension: the PATH-BP trial. Circulation 145, 416–423 (2022).
Spence, J. D., Grosser, T. & FitzGerald, G. A. Acetaminophen, nonsteroidal anti-inflammatory drugs, and hypertension. Hypertension 79, 1922–1926 (2022).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Everett, B. M. et al. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation 139, 1289–1299 (2019).
Ridker, P. M. et al. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet 391, 319–328 (2018).
Ridker, P. M. et al. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur. Heart J. 39, 3499–3507 (2018).
Dalekos, G. N., Elisaf, M. S., Papagalanis, N., Tzallas, C. & Siamopoulos, K. C. Elevated interleukin-1 beta in the circulation of patients with essential hypertension before any drug therapy: a pilot study. Eur. J. Clin. Invest. 26, 936–939 (1996).
Madhur, M. S. et al. Hypertension: do inflammation and immunity hold the key to solving this epidemic? Circ. Res. 128, 908–933 (2021).
Zhang, F. & Steinberg, S. F. S49G and R389G polymorphisms of the β1-adrenergic receptor influence signaling via the cAMP-PKA and ERK pathways. Physiol. Genomics 45, 1186–1192 (2013).
Sorota, S. The sympathetic nervous system as a target for the treatment of hypertension and cardiometabolic diseases. J. Cardiovasc Pharm. 63, 466–476 (2014).
Sica, D. A. Centrally acting antihypertensive agents: an update. J. Clin. Hypertens. 9, 399–405 (2007).
Fenton, C., Keating, G. M. & Lyseng-Williamson, K. A. Moxonidine: a review of its use in essential hypertension. Drugs 66, 477–496 (2006).
Morris, S. T. & Reid, J. L. Moxonidine: a review. J. Hum. Hypertens. 11, 629–635 (1997).
Laurent, S. Antihypertensive drugs. Pharm. Res. 124, 116–125 (2017).
Hashmonai, M., Cameron, A. E., Licht, P. B., Hensman, C. & Schick, C. H. Thoracic sympathectomy: a review of current indications. Surg. Endosc. 30, 1255–1269 (2016).
Bhatt, D. L. et al. A controlled trial of renal denervation for resistant hypertension. N. Engl. J. Med. 370, 1393–1401 (2014).
Azizi, M. et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet 397, 2476–2486 (2021).
Wallbach, M. et al. Effects of baroreflex activation therapy on ambulatory blood pressure in patients with resistant hypertension. Hypertension 67, 701–709 (2016).
Mancia, G. et al. The sympathetic nervous system and the metabolic syndrome. J. Hypertens. 25, 909–920 (2007).
Straznicky, N. E. et al. Sympathetic neural adaptation to hypocaloric diet with or without exercise training in obese metabolic syndrome subjects. Diabetes 59, 71–79 (2010).
Trombetta, I. C. et al. Weight loss improves neurovascular and muscle metaboreflex control in obesity. Am. J. Physiol. Heart Circ. Physiol. 285, H974–82 (2003).
Zucker, I. H. et al. Exercise training and sympathetic regulation in experimental heart failure. Exerc Sport Sci. Rev. 32, 107–111 (2004).
Lucini, D., Di Fede, G., Parati, G. & Pagani, M. Impact of chronic psychosocial stress on autonomic cardiovascular regulation in otherwise healthy subjects. Hypertension 46, 1201–1206 (2005).
Oneda, B., Ortega, K. C., Gusmão, J. L., Araújo, T. G. & Mion, D. Jr. Sympathetic nerve activity is decreased during device-guided slow breathing. Hypertens. Res. 33, 708–712 (2010).
Viskoper, R. et al. Nonpharmacologic treatment of resistant hypertensives by device-guided slow breathing exercises. Am. J. Hypertens. 16, 484–487 (2003).
Anderson, D. E., McNeely, J. D. & Windham, B. G. Regular slow-breathing exercise effects on blood pressure and breathing patterns at rest. J. Hum. Hypertens. 24, 807–813 (2010).
Vasan, R. S. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation 113, 2335–2362 (2006).
Sesso, H. D. et al. C-reactive protein and the risk of developing hypertension. Jama 290, 2945–2951 (2003).
Di Pietro, P. et al. Targeting the ASMase/S1P pathway protects from sortilin-evoked vascular damage in hypertension. J. Clin. Invest 132, e146343 (2022).
Citterio, L. et al. Klotho gene in human salt-sensitive hypertension. Clin. J. Am. Soc. Nephrol. 15, 375–383 (2020).
Shimizu, Y. et al. Platelets and circulating CD34-positive cells as an indicator of the activity of the vicious cycle between hypertension and endothelial dysfunction in elderly Japanese men. Atherosclerosis 259, 26–31 (2017).
Pandey, A. et al. Incorporation of biomarkers into risk assessment for allocation of antihypertensive medication according to the 2017 ACC/AHA high blood pressure guideline: a pooled cohort analysis. Circulation 140, 2076–2088 (2019).
Ibsen, H. et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study. Hypertension 45, 198–202 (2005).
Goettsch, C., Kjolby, M. & Aikawa, E. Sortilin and its multiple roles in cardiovascular and metabolic diseases. Arterioscler Thromb. Vasc. Biol. 38, 19–25 (2018).
Jujic, A. et al. Plasma S1P (sphingosine-1-phosphate) links to hypertension and biomarkers of inflammation and cardiovascular disease: findings from a translational investigation. Hypertension 78, 195–209 (2021).
Drew, D. A. et al. Soluble Klotho and Incident Hypertension. Clin. J. Am. Soc. Nephrol. 16, 1502–1511 (2021).
Zhang, J. R. & Sun, H. J. MiRNAs, lncRNAs, and circular RNAs as mediators in hypertension-related vascular smooth muscle cell dysfunction. Hypertens. Res. 44, 129–146 (2021).
Jusic, A. & Devaux, Y. Noncoding RNAs in hypertension. Hypertension 74, 477–492 (2019).
Lazaridis, A. et al. A study of endothelial and platelet microvesicles across different hypertension phenotypes. J. Hum. Hypertens. 36, 561–569 (2022).
Harrison, D. G., Coffman, T. M. & Wilcox, C. S. Pathophysiology of hypertension: the mosaic theory and beyond. Circ. Res. 128, 847–863 (2021).
Unger, T. et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension 75, 1334–1357 (2020).
Cuspidi, C., Tadic, M., Grassi, G. & Mancia, G. Treatment of hypertension: the ESH/ESC guidelines recommendations. Pharm. Res. 128, 315–321 (2018).
Wang, Z. et al. Status of hypertension in China: results from the China Hypertension Survey, 2012-2015. Circulation 137, 2344–2356 (2018).
Lewis, C. E. et al. Final report of a trial of intensive versus standard blood-pressure control. N. Engl. J. Med. 384, 1921–1930 (2021).
Zhang, W. et al. Trial of intensive blood-pressure control in older patients with hypertension. N. Engl. J. Med. 385, 1268–1279 (2021).
Flack, J. M. & Adekola, B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc Med. 30, 160–164 (2020).
Rahimi, K. et al. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet 397, 1625–1636 (2021).
Xiong, T. et al. Single-cell sequencing analysis and multiple machine learning methods identified G0S2 and HPSE as novel biomarkers for abdominal aortic aneurysm. Front. Immunol. 13, 907309 (2022).
Bauer, Y. et al. Identifying early pulmonary arterial hypertension biomarkers in systemic sclerosis: machine learning on proteomics from the DETECT cohort. Eur. Respir. J. 57, 2002591 (2021).
Sandner, P. et al. Soluble GC stimulators and activators: past, present and future. Br. J. Pharmacol. https://doi.org/10.1111/bph.15698 (2021).
Tocci, G. et al. How to improve effectiveness and adherence to antihypertensive drug therapy: central role of dihydropyridinic calcium channel blockers in hypertension. High. Blood Press Cardiovasc. Prev. 25, 25–34 (2018).
Pitt, B. Diversity of calcium antagonists. Clin. Ther. 19, 3–17 (1997). Suppl A.
Benjamin, M. M. & Khalil, R. A. Matrix metalloproteinase inhibitors as investigative tools in the pathogenesis and management of vascular disease. Exp. Suppl. 103, 209–279 (2012).
Pohl, M., Sakurai, H., Bush, K. T. & Nigam, S. K. Matrix metalloproteinases and their inhibitors regulate in vitro ureteric bud branching morphogenesis. Am. J. Physiol. Ren. Physiol. 279, F891–900 (2000).
Honda, H. et al. Olmesartan medoxomil is associated with decreased plasma AGEs, pentosidine, and N-(epsilon)-carboxymethyl-lysine levels in hemodialysis patients. Clin. Exp. Hypertens. 34, 17–23 (2012).
Doggrell, S. A. ALT-711 decreases cardiovascular stiffness and has potential in diabetes, hypertension and heart failure. Expert Opin. Investig. Drugs 10, 981–983 (2001).
Susic, D., Varagic, J., Ahn, J. & Frohlich, E. D. Cardiovascular and renal effects of a collagen cross-link breaker (ALT 711) in adult and aged spontaneously hypertensive rats. Am. J. Hypertens. 17, 328–333 (2004).
Breuss, J. M., Atanasov, A. G. & Uhrin, P. Resveratrol and its effects on the vascular system. Int. J. Mol. Sci. 20, 1523 (2019).
Barrios, V., Escobar, C., Calderon, A. & Lahera, V. N-acetylcysteine for the prevention of atrial fibrillation: beyond its antioxidant effect. Eur. Heart J. 29, 2822–2823 (2008).
Desiniotis, A. & Kyprianou, N. Advances in the design and synthesis of prazosin derivatives over the last ten years. Expert Opin. Ther. Targets 15, 1405–1418 (2011).
Crassous, P. A., Denis, C., Paris, H. & Sénard, J. M. Interest of alpha2-adrenergic agonists and antagonists in clinical practice: background, facts and perspectives. Curr. Top. Med Chem. 7, 187–194 (2007).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81900404, No. 81970355).
Author information
Authors and Affiliations
Contributions
All authors have read and approved the article. Concept and design: J.M. Drafting of the manuscript: J.M., Y.N.L., X.Y.Y., X.H.Z., and Q.T.M. Revision of the manuscript for important intellectual content: K.L., X.Z., R.Y.Y., R.F.S., Z.Q.W., Q.T.M., and X.P.C. Obtained funding: J.M. and X.P.C. Supervision: Q.T.M. and X.P.C.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ma, J., Li, Y., Yang, X. et al. Signaling pathways in vascular function and hypertension: molecular mechanisms and therapeutic interventions. Sig Transduct Target Ther 8, 168 (2023). https://doi.org/10.1038/s41392-023-01430-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-023-01430-7